Abstract
Mammographic density (MD), a strong marker of breast cancer risk, is influenced by genetic, environmental, and hormonal factors. Cadmium, a persistent and widespread environmental pollutant, has been associated with risk of breast cancer, and laboratory evidence suggests cadmium is a carcinogen in the breast. We investigated the hypothesis that cadmium exposure is associated with higher MD. In a cross-sectional study of MD and urinary cadmium concentration, percentage MD (MD%) and Breast Imaging-Reporting and Data Systems (BI-RADS®) density category were determined from screening mammograms of 190 premenopausal women ages 40–45 years. Women completed a health questionnaire, and the cadmium content of spot urine samples was measured with inductively coupled plasma mass spectrometry and corrected for urine creatinine. Urinary cadmium concentrations are thought to reflect exposure to cadmium during a period of 20–30 years. Multivariable linear regression and logistic regression were used to estimate the strength of association between urinary cadmium and mammographic breast density. Adjusted mean MD% among women in the upper tertile of creatinine-corrected urinary cadmium was 4.6% higher (95% CI: –2.3 to 11.6%) than in women in the lowest cadmium tertile. Each twofold increase in urinary cadmium was associated with higher odds of MD% in the upper tertile (OR: 1.29, 95% CI: 0.82–2.02) or a BI-RADS category rating of “extremely dense” (OR: 1.75, 95% CI: 1.14–2.70). Stronger associations were observed among nulliparous women, and current or former smokers. Exposure to cadmium may be associated with increased breast density in premenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammographic density (MD) is a strong marker of breast cancer risk [1], although the etiological relationship between MD and breast cancer remains unclear [2]. MD has been proposed as an “intermediate phenotype” of breast cancer that is largely determined by genetics and reflects cumulative exposure to sex steroid hormones and other breast mitogens [3]. However, the association between circulating levels of endogenous sex steroid hormones and MD is weak [4–6], and the increase in breast cancer risk associated with hormone therapy or endogenous hormones is likely independent of MD [4, 7]. Notably, environmental exposures may also change MD; active cigarette smoking decreases MD [8], alcohol consumption may increase MD [9], and some dietary factors may also play a role [10].
Cadmium is a heavy metal that has been widely dispersed in the environment as a result of industry and agriculture [11, 12]. Non-occupationally exposed persons are mainly exposed to cadmium by smoking tobacco or through eating contaminated cereals, certain vegetables, and shellfish [12–14]. Cadmium, once ingested, is inefficiently excreted and accumulates primarily in the liver and kidneys, and has also been measured in breast tissue samples [12].
Preliminary epidemiologic evidence suggests that cadmium exposure may increase risk of invasive breast cancer [15–17] and endometrial cancer [18]. Laboratory evidence indicates that cadmium may have estrogenic activity in rats [19, 20] and epithelial breast cell lines [21–24], and additional recent evidence highlights nonhormonal carcinogenic effects of cadmium on breast epithelial cells in vitro [25]. Cadmium can also induce cellular oxidative stress [26], and biomarkers of oxidative stress have been associated with MD [27–29].
The incomplete understanding of the variability in MD, the putative carcinogenic actions of cadmium, and epidemiologic and laboratory evidence supporting the hypothesis that cadmium is a breast carcinogen motivated us to investigate whether cadmium exposure is associated with MD.
Materials and methods
Study population and recruitment
The recruitment, exclusion criteria, and clinical protocols of the Equol, Breast, and Bone (EBB) study have been detailed [30, 31]. Briefly, premenopausal women aged 40–45 years were recruited from a mammographic screening program within Group Health (GH), an integrated healthcare system in Washington State. Women were sampled from the GH population based on Breast Imaging and Reporting Data System (BI-RADS®—American College of Radiology) category recorded at the time of eligibility review to achieve similar numbers of women across BI-RADS categories. For this purpose, BI-RADS ratings of 1 (almost entirely fat) and 2 (scattered density) were combined. Peri- and postmenopausal women, women with a history of breast cancer, women using hormone therapy or oral contraceptives, and, consistent with other goals of the EBB study, women with certain digestive conditions or using antibiotics were excluded [30, 31]. Study procedures were approved by the Fred Hutchinson Cancer Research Center and GH Institutional Review Boards.
Of the 203 EBB study participants [30, 31], the 190 included in this study completed a health questionnaire, provided a urine sample on which cadmium and creatinine analysis were completed, and had MD% measured from screening mammograms.
Data collection
Participants completed a health questionnaire and a study clinic visit during which height and weight were measured, and spot urine samples were provided using standard “clean-catch” procedures. Clinic visits were timed to fall between days 5 and 9 of each woman’s menstrual cycle [31]. Urine samples were aliquoted and frozen at –70°C until analysis. Separate aliquots were assayed for creatinine and heavy metals. (Home-collected soy challenge urine samples also obtained from these participants as described [30] were not used in the present analysis.)
Percentage mammographic density
Percentage mammographic density (MD%) was calculated from the left cranial-caudio view of screening mammograms collected up to 14 months prior to urine and data collection, as described [30]. Mammograms were digitized and read for total area and dense area using Cumulus, a computer-assisted method developed at the University of Toronto [32]. Mammograms were read by a single reader (EJAB), unaware of urine results. MD% is calculated as the proportion of dense area to total area of the breast. A random sample (10%) of mammograms was re-read for quality control; no discrepancies were apparent [30]. Both BI-RADS and MD% were determined without knowledge of urinary cadmium content.
Urinary cadmium and creatinine measurement
A panel of 30 elements including cadmium was quantified by sector field inductively coupled plasma mass spectrometry (SF-ICPMS) on a Thermo-Finnigan Element 2 (Thermo Scientific, Waltham, MA) at the Wisconsin State Laboratory of Hygiene (Madison, WI), following quality control procedures similar to those previously described [33]. Urine was diluted in 2% high-purity nitric acid containing internal normalization standards, and introduced to the plasma with a low-flow Teflon nebulizer interfaced to an ESI FAST auto-sampler. Isotopes were acquired in peak jumping mode. A series of dedicated samples (molybdenum spiked reference materials and spiked samples) were positioned throughout the analytical sequence to address potential molybdenum oxide interference. A typical SF-ICPMS batch included 20 participant samples, 3 standard reference material aliquots (including National Institute of Standards of Technology 2670a), and multiple quality control samples (duplicates, spikes, check standards, and blanks) [33]. The mean of batch-specific reagent blanks was subtracted from the mean of triplicate sample measurements to arrive at the cadmium concentration used for statistical analysis. All participant samples were above the lower limit of quantification of cadmium, 0.008 μg/l. Levels of extractable metals (0.5 M nitric acid for 48 h) in unused urine collection containers and storage/transport vials were also quantified to assess potential contamination biases. Extractable concentrations of cadmium in the collection containers (0.0004 ± 0.0002 μg/l, n = 4) and 1.8 ml storage vials (0.0020 ± 0.0006 μg/l, n = 9) were significantly below the method quantification limit of cadmium in urine and therefore judged to be an insignificant source of error.
Urinary creatinine was assessed using a colorimetric assay [30].
Statistical analysis
Potential confounders identified prior to data analysis were age, parity, body mass index (BMI), education, race/ethnicity, smoking history, and consumption of mineral or multivitamin supplements. Occupation and alcohol intake were additionally considered during revision of this study report. Alcohol intake was estimated from participant food frequency questionnaires. For analysis, these potential confounders were included in regression models as linear continuous variables centered at the mean (age), or parameterized into categories: parity (none, 1–2, 3, or more pregnancies longer than 6 months); BMI (in tertiles); education (no college degree, college degree); race/ethnicity (Asian, non-Asian); smoking history (never, ever); current supplement use (yes, no); daily alcohol consumption (<14 g [approximately one drink]; ≥14 g). Education, supplement use, and alcohol intake were excluded because elimination from the fully adjusted model changed linear regression estimates by less than 10% and did not substantially alter estimate standard errors. Occupation was not considered because no reported occupations were reasonably related to exposure to cadmium.
Prior to heavy metal assays, cadmium was selected as potentially related to MD. Associations between other metals and MD were not examined prior to drafting this report and will be presented elsewhere. Cadmium concentration measured in each spot urine sample was divided by the sample’s creatinine concentration; cadmium concentration is reported as microgram cadmium per gram creatinine (μg/g).
Creatinine-normalized urinary cadmium concentrations were log-transformed. The logarithm of creatinine-corrected cadmium as a linear term, or an indicator of tertile of creatinine-normalized cadmium, was entered in each regression model. The association between MD% as a continuous variable and other variables was estimated using ordinary least squares linear regression, from which least squares adjusted mean MD% was calculated. Logistic regression was used to estimate associations after categorizing MD% into tertiles among all participants, or with BI-RADS category as the outcome, comparing category 4 (extremely dense) to 1, 2, or 3 (heterogeneously dense). For subgroup analyses defined by parity and smoking history, an interaction term was added to the statistical model, equal to the product of log-transformed cadmium and a dichotomous indicator for either nulliparity or ever-smoking. Least-squares adjusted means were calculated based on the linear regression models; for least-squares adjusted means among sub-groups, the model with the appropriate interaction term was used.
Linear regression modeling assumptions were examined by graphical methods. No substantial departure from linearity or normality of the residuals was observed in adjusted models, with or without interaction terms. Similarly, no important departures from homoscedasticity either among all women, or in comparing parous and nulliparous, or ever-smokers and never-smokers. Statistical analyses were completed with Stata version 11 (Stata Corp, College Station, TX).
Results
Percent mammographic density ranged from zero to 88.9% in this sample of women. The creatinine-normalized urinary cadmium concentrations ranged from 0.06 to 1.56 μg/g. Geometric means and the range of values within one geometric standard deviation [34] indicated that Asian women, smokers, women with lower BMI, and women with fewer pregnancies had higher urinary creatinine-corrected cadmium (Table 1). Dietary supplement use was related to slightly lower urinary cadmium, and college education and alcohol intake were not related to urinary cadmium content.
Women of higher parity and women with higher BMI had substantially lower mean MD% (Table 1), whereas women with more education, taking dietary supplements, and who consumed more alcohol had higher MD%. The narrow age range of the participants precluded observation of relations between age and MD% or urinary cadmium.
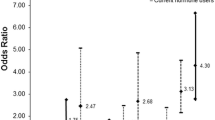
Regression results showed that higher urinary cadmium was associated with higher MD% or BI-RADS rating. Multivariable linear regression of MD% suggested that each twofold increase in creatinine-normalized cadmium was associated with an increase of 1.60 percentage points (95% CI: −2.12–5.32) in MD%, as reflected in increasing estimated adjusted mean MD% in each tertile of cadmium (Table 2). Elevated cadmium was also associated with increased odds of MD% in the uppermost tertile and with a BI-RADS rating of 4 (extremely dense) per twofold increase in creatinine-normalized cadmium (ORs [95%CI]: 1.29 [0.82–2.02] and 1.75 [1.14–2.70], respectively).
The association between cadmium and MD% was stronger among nulliparous women and women with a history of smoking (Table 2), and attenuated among parous women and never-smokers. Similar results were observed with logistic regression analyses repeated on these subgroups (not shown).
Discussion
Overall, the urinary cadmium concentrations measured in this study are comparable to measurements in similar populations, including the National Health and Nutrition Examination Survey [35] and the sample used in a previous study of cadmium and breast cancer risk [15, 33]. Although the spot urine samples analyzed in this study were not obtained with trace metal analysis in mind, they were donated under controlled conditions in a research clinic. The consistency of the urinary cadmium concentrations with comparable cohorts, our quantitative assessment of container blanks, and high accuracy of SF-ICPMS measurements on reference materials indicate that the urinary cadmium measurements appear to be both internally and externally valid.
To measure MD, we used both a qualitative BI-RADS rating and MD%. For this study, we used a qualitative clinic-based BI-RADS rating determined before MD% was measured and not BI-RADS based directly on MD%. Differences in classification of women by MD% and BI-RADS were noted (Table 1). However, previous pooled analysis found that a qualitative BI-RADS rating of 4 was associated with a ~fourfold breast cancer risk compared to a BI-RADS rating of 1 [1]. When we divided study participants into tertiles of MD%, the upper tertile MD% included women with MD% more than 49%; MD% more than 50% is associated with a four-to-fivefold breast cancer risk in comparison to women with very low MD% [1].
Our results suggest that cadmium exposure might be associated with increased mammographic density in premenopausal women. The measured cadmium and MD are in the relevant range of MD to be of epidemiological interest. Furthermore, we observed consistent results whether MD was measured as MD% or qualitative BI-RADS.
An important strength of this study is that by examining the association between cadmium and MD, rather than breast cancer, we avoid potential bias because of breast cancer therapies. Cancer therapies could influence the cadmium content of urine, and thus generate a spurious association with breast cancer. Because women with a history of cancer or use of tamoxifen, raloxifene, or bisphosphonates were excluded from this study, the associations we observed are very unlikely to be because of medications.
The limitations of this study include the modest sample size and the single-spot measurement of urinary cadmium. Spot urine samples are more variable than 24-hour urine collection; to minimize differences in urine dilution between individuals, we normalized cadmium content to creatinine [36]. This common practice is especially appropriate for a homogenous population such as the one in this study [35, 36]. As noted, urine was collected up to 14 months after the screening mammogram was made, and acute exposure to cadmium could have occurred during the intervening time. However, none of the women began smoking in the intervening period, and none reported occupations in which such exposure was likely; overall, the influence of an acute exposure in this period on our results is likely small.
Because of the cross-sectional design, we cannot be certain that urinary cadmium reflects exposure during the time over which MD developed. However, urinary cadmium has been demonstrated to reflect cumulative long-term exposure [12], and the etiologically relevant timeframe during which environmental exposures might influence MD remains unclear [2, 37].
We also observed that adiposity, parity, and current cigarette smoking were strongly related to MD, consistent with previous studies [8, 38], and we selected these and other variables as potential confounders prior to analysis and analytically adjusted. Nonetheless, it is possible that other unknown factors, for example, an underlying physiology, could predispose women to both higher MD and higher urinary cadmium.
Our findings may support the hypothesis that cadmium acts as a “metallo-hormone” or disrupts the activity of endogenous hormones that influence MD. However, evidence that elevated MD is related to higher endogenous sex steroid hormones levels is weak and inconsistent [4], and no association was observed in the EBB study participants examined in this study [5]. Therefore, direct estrogenic actions of cadmium are unlikely to explain an association between cadmium and MD. Beyond sex hormones, direct associations between growth hormone [37], prolactin [39], insulin-like growth factor-I [39–41], and breast density have been reported, mainly in premenopausal women, although evidence is not consistent [7]. Animal and limited human studies suggest that cadmium may influence levels of these hormones in a complex manner [42]. Alternatively, cadmium in breast tissue may increase oxidative stress which, in turn, may influence MD [27–29]. Given the limited current understanding of the etiology of MD and the nature of cadmium’s actions in breast tissue, the mechanisms underlying an association between cadmium and MD, if true, must be the subject of future studies.
We noted two subgroups responsible for much of the association between cadmium and MD: nulliparous women and cigarette smokers. The stronger association among nulliparous women is perhaps consistent with a model in which breast tissue is most susceptible to carcinogens, including cadmium, prior to differentiation induced by pregnancy [43]. Although smoking results in lower MD during active smoking [8], our results suggest that cadmium may act to increase MD as a persistent effect of smoking after cessation. With only eight active smokers in this study, and given the cross-sectional nature of the study, we were unable to further investigate the relationship between smoking duration and timing, cadmium, and MD.
In summary, the results of our study suggest a positive association between urinary cadmium and MD in premenopausal women. This would lend support to the hypothesis that cadmium is associated with breast cancer, potentially as a result of endocrine disruption or other mechanisms. Future studies are needed to reproduce these findings to address the mechanism through which cadmium might be acting to increase MD and to investigate how an association between cadmium and MD relates to an association between cadmium exposure and breast cancer incidence.
References
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15(6):1159–1169
Martin LJ, Boyd NF (2008) Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 10(1):201
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808
Tamimi RM, Byrne C, Colditz GA, Hankinson SE (2007) Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 99(15):1178–1187
Yong M, Atkinson C, Newton KM, Aiello Bowles EJ, Stanczyk FZ, Westerlind KC, Holt VL, Schwartz SM, Leisenring WM, Lampe JW (2009) Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control 20(7):1039–1053
Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH (2007) No relationship between circulating levels of sex steroids and mammographic breast density: the prospect-epic cohort. Breast Cancer Res 9(4):R53
Martin LJ, Minkin S, Boyd NF (2009) Hormone therapy, mammographic density, and breast cancer risk. Maturitas 64(1):20–26
Butler LM, Gold EB, Conroy SM, Crandall CJ, Greendale GA, Oestreicher N, Quesenberry CP Jr, Habel LA (2010) Active, but not passive cigarette smoking was inversely associated with mammographic density. Cancer Causes Control 21(2):301–311
Maskarinec G, Takata Y, Pagano I, Lurie G, Wilkens LR, Kolonel LN (2006) Alcohol consumption and mammographic density in a multiethnic population. Int J Cancer 118(10):2579–2583
Boyd NF, Greenberg C, Lockwood G, Little L, Martin L, Byng J, Yaffe M, Tritchler D (1997) Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian diet and breast cancer prevention study group. J Natl Cancer Inst 89(7):488–496
Elinder CG (1992) Cadmium as an environmental hazard. IARC Sci Publ (118):123–132
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137(1–2):65–83
Olsson IM, Eriksson J, Oborn I, Skerfving S, Oskarsson A (2005) Cadmium in food production systems: a health risk for sensitive population groups. Ambio 34(4–5):344–351
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA (2006) Cadmium exposure and breast cancer risk. J Natl Cancer Inst 98(12):869–873
Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA (2006) Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 7(2):119–126
Cantor KP, Stewart PA, Brinton LA, Dosemeci M (1995) Occupational exposures and female breast cancer mortality in the United States. J Occup Environ Med 37(3):336–348
Akesson A, Julin B, Wolk A (2008) Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res 68(15):6435–6441
Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, Clarke R, Sholler PF, Lirio AA, Foss C, Reiter R, Trock B, Paik S, Martin MB (2003) Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med 9(8):1081–1084
Hofer N, Diel P, Wittsiepe J, Wilhelm M, Degen GH (2009) Dose- and route-dependent hormonal activity of the metalloestrogen cadmium in the rat uterus. Toxicol Lett 191(2–3):123–131
Martin BM, Reiter R, Pham T, Avellanet YR, Camara J, Lahm M, Pentecost E, Pratap K, Gilmore BA, Diverakar S, Dagata RS, Bull JL, Stoica A (2003) Estrogen-like activity of metals in mcf-7 breast cancer cells. Endocrinology 144:2425–2436
Stoica A, Katzenellenbogen BS, Martin MB (2000) Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol 14(4):545–553
Brama M, Gnessi L, Basciani S, Cerulli N, Politi L, Spera G, Mariani S, Cherubini S, d’Abusco AS, Scandurra R, Migliaccio S (2007) Cadmium induces mitogenic signaling in breast cancer cell by an eralpha-dependent mechanism. Mol Cell Endocrinol 264(1–2):102–108
Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC (2010) Cadmium promotes breast cancer cell proliferation by potentiating the interaction between eralpha and c-jun. Mol Endocrinol 24(5):981–992. doi:me.2009-0410[pii]10.1210/me.2009-0410
Benbrahim-Tallaa L, Tokar EJ, Diwan BA, Dill AL, Coppin JF, Waalkes MP (2009) Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ Health Perspect 117(12):1847–1852
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238(3):209–214
Boyd NF, Connelly P, Byng J, Yaffe M, Draper H, Little L, Jones D, Martin LJ, Lockwood G, Tritchler D (1995) Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev 4(7):727–733
Hong CC, Tang BK, Rao V, Agarwal S, Martin L, Tritchler D, Yaffe M, Boyd NF (2004) Cytochrome p450 1a2 (cyp1a2) activity, mammographic density, and oxidative stress: a cross-sectional study. Breast Cancer Res 6(4):R338–R351
Boyd NF, McGuire V (1990) Evidence of lipid peroxidation in premenopausal women with mammographic dysplasia. Cancer Lett 50(1):31–37
Atkinson C, Newton KM, Aiello Bowles EJ, Lehman CD, Stanczyk FZ, Westerlind KC, Li L, Lampe JW (2009) Daidzein-metabolizing phenotypes in relation to mammographic breast density among premenopausal women in the United States. Breast Cancer Res Treat 116(3):587–594
Atkinson C, Newton KM, Bowles EJ, Yong M, Lampe JW (2008) Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am J Clin Nutr 87(3):679–687
Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian national breast screening study. J Natl Cancer Inst 87(9):670–675
McElroy JA, Shafer MM, Hampton JM, Newcomb PA (2007) Predictors of urinary cadmium levels in adult females. Sci Total Environ 382(2–3):214–223
Bland JM, Altman DG (1996) Measurement error proportional to the mean. Bmj 313(7049):106
Paschal DC, Burt V, Caudill SP, Gunter EW, Pirkle JL, Sampson EJ, Miller DT, Jackson RJ (2000) Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Arch Environ Contam Toxicol 38(3):377–383
Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M (2005) Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers 10(2–3):117–126
Boyd N, Martin L, Chavez S, Gunasekara A, Salleh A, Melnichouk O, Yaffe M, Friedenreich C, Minkin S, Bronskill M (2009) Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol 10(6):569–580
Bremnes Y, Ursin G, Bjurstam N, Gram IT (2007) Different measures of smoking exposure and mammographic density in postmenopausal Norwegian women: a cross-sectional study. Breast Cancer Res 9(5):R73
Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S (2002) The association of breast mitogens with mammographic densities. Br J Cancer 87(8):876–882
Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, Gibson L, Hillier SG, Holly JM, Moss S, Dowsett M, Peto J, dos Santos Silva I (2009) Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res 69(16):6490–6499
Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT (2007) Insulin-like growth factor and mammographic density in postmenopausal Norwegian women. Cancer Epidemiol Biomarkers Prev 16(1):57–62
Iavicoli I, Fontana L, Bergamaschi A (2009) The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 12(3):206–223
Rosner B, Colditz GA (1996) Nurses’ health study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst 88(6):359–364
Acknowledgments
This work supported by NIH R01 CA 097366 (Equol, Breast and Bone study), NIH U01 CA 63731 (Group Health Breast Cancer Screening Program), NIH National Cancer Institute Cancer Prevention Training Grant R25 CA094880 (to SVA), and the Breast Cancer Research Program at the Fred Hutchinson Cancer Research Center, through donation by Safeway, Inc.
Conflicts of Interest
Each author declares that he or she has no conflicts of interest related to the content of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adams, S.V., Newcomb, P.A., Shafer, M.M. et al. Urinary cadmium and mammographic density in premenopausal women. Breast Cancer Res Treat 128, 837–844 (2011). https://doi.org/10.1007/s10549-011-1383-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1383-8