Abstract
Resveratrol is a natural polyphenolic compound and has been shown to exhibit cardio-protective as well as anti-neoplastic effects on various types of cancers. However, the exact mechanism of its anti-tumor effect is not clearly defined. Resveratrol has been shown to have strong hypolipidemic effect on normal adipocytes and as hyper-lipogenesis is a hallmark of cancer cell physiology, the effect of resveratrol on lipid synthesis in cancer stem-like cells (CD24−/CD44+/ESA+) that were isolated from both ER+ and ER− breast cancer cell lines was examined. The authors found that resveratrol significantly reduced the cell viability and mammosphere formation followed by inducing apoptosis in cancer stem-like cells. This inhibitory effect of resveratrol is accompanied by a significant reduction in lipid synthesis which is caused by the down-regulation of the fatty acid synthase (FAS) gene followed by up-regulation of pro-apoptotic genes, DAPK2 and BNIP3. The activation of apoptotic pathway in the cancer stem-like cells was suppressed by TOFA and by Fumonisin B1, suggesting that resveratrol-induced apoptosis is indeed through the modulation of FAS-mediated cell survival signaling. Importantly, resveratrol was able to significantly suppress the growth of cancer stem-like cells in an animal model of xenograft without showing apparental toxicity. Taken together, the results of this study indicate that resveratrol is capable of inducing apoptosis in the cancer stem-like cells through suppression of lipogenesis by modulating FAS expression, which highlights a novel mechanism of anti-tumor effect of resveratrol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resveratrol (3,4′,5-trihydroxystilbene) is a phytochemical which is abundantly found in natural foods including grapes, red wine, berries, and peanuts [1]. It shows a wide spectrum of pharmacological effects and is considered to reduce the risk of cardiovascular disorders and cancer [2–4]. The cardio-protective effect of resveratrol has been extensively studied in various pre-clinical models, and it has been shown that the strong anti-oxidant activity of resveratrol contributes to the protective effect through intracellular redox signaling [5, 6]. The other potential mechanism of cardio-protection of resveratrol is due to its hypolipidemic effect, which is supported by several epidemiological data also known as French paradox (low-risk coronary heart disease despite high-fat diet) [7]. Indeed, resveratrol has been shown to significantly reduce the basal and insulin-induced lipogenesis from glucose in freshly isolated adipocytes, and the results of in vivo studies also showed that resveratrol was capable of suppressing the expression of genes related to lipid metabolism in animals [8].
The anti-tumor effect of resveratrol has been shown in many types of cancers using various animal models, and resveratrol has been shown to modulate various steps of tumorigenesis such as initiation, progression, and metastasis [9, 10]. Jang et al. reported that resveratrol showed anti-cancer effect on melanoma by blocking the expression of cyclooxygenase [11]. Resveratrol also reduced the incidence of carcinogen-induced mammary tumor through down-regulation of NF-kappaB, COX, and matrix metalloprotease-9 expression [12]. In prostate cancer, transgenic adenocarcinoma of mouse prostate mouse fed with resveratrol as diet significantly reduced the onset of prostate cancer and exhibited a decrease in insulin-like growth factor 1 and phosphorylated-extracellular regulating kinase 1 (ERK1) [13]. Therefore, resveratrol has both preventive and therapeutic effects on cancers in these pre-clinical tests. Resveratrol appears to show its anti-tumor effect by inducing apoptosis through modulation of various signaling pathways; however, the exact underlying molecular mechanism is yet to be understood. The objectives of this study are to clarify the mechanism by which resveratrol exerts its anti-tumor effect through modulation of lipogenesis in breast cancer stem-like cells and to define a potential target for chemoprevention of breast cancer.
It has long been recognized that up-regulation of lipid metabolism is a hallmark of cancers because rapidly growing tumor cells require lipid as a source for membrane synthesis as well as for energy supply [14]. Fatty acid synthase (FAS) is a key enzyme for lipogenesis and the expression of this gene is highly up-regulated in various types of cancers while it is undetectable in normal cells [15–19]. Importantly, blocking the FAS expression results in cell growth arrest and induction of apoptosis [20]. The authors also previously reported that inhibition of FAS results in accumulation of malonyl-CoA and ceramide followed by activation of pro-apoptotic genes such as DAPK2, BNIP3 and TRAIL [21]. Therefore, FAS is considered to be an ideal target for chemotherapy and chemoprevention [22–24]. In this report, it has been shown that resveratrol is indeed capable of suppressing lipid metabolism by blocking the FAS expression followed by induction of apoptosis in cancer stem-like cells that are believed to play critical roles in initiation, progression as well as chemo-resistance of cancers.
Materials and methods
Cell culture and reagents
The breast cancer cell lines MCF7 and MDA-MB231 were obtained from American Type Culture Collection (ATCC, VA). MDA-MB231 LM2-4175 (231 LM) was a generous gift from Massagué [25]. 231 LM is the variant of MDA-MB231 which preferentially metastasizes to the lung. MCF10A, which is a spontaneously immortalized, non-tumorigenic epithelial cell line, was also purchased from ATCC. Normal human mammary epithelial cell (HMEC) was purchased from Lonza Inc., MD.
Stable clone of MDA-MB231 cells was established in our lab by the lentivirus infection which constitutively expresses Luciferase gene and designated as MDA-MB231 Luc. MCF7 was cultured in DMEM (ATCC) containing 10% FBS. MDA-MB231, MDA-MB231Luc, and 231 LM were cultured in RPMI (Invitrogen, NY) containing 10% FBS. MCF10A and HMECs were cultured in MEBM media with Bullet kit supplements (Lonza Inc., MD). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Resveratrol, 5-(tetradecyloxy)-2-furoic acid (TOFA), and Fumonisin B1 were purchased from Sigma–Aldrich Co, MO.
Lentivirus production and infection
Lentivector expression system was purchased from System Biosciences (Mountainview, CA). Packaging and production of GFP (control) and FAS-expressing lentiviruses were performed according to the manufacturer’s protocol. The virus culture supernatants were collected, concentrated, and used for infecting the MDA-MB231 cells.
Cell proliferation assay (MTS assay)
Ten thousand cells per well were seeded in 96-well plates and cultured for 48 h. The cells were then treated with or without resveratrol for further 48 h. Cell viability was measured using CellTiter 96 Aqueous one solution Cell Proliferation Assay kit (Promega corp., WI), composed of the novel tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphonyl)-2H-tetrazolium, inner salt (MTS), and an electron-coupling reagent, phenazine ethosulfate, following the manufacturer’s instructions. Absorbance was read at 490 nm using an ELISA reader which was directly proportional to the number of living cells in culture. Cell viability was expressed as a percentage relative to untreated cells.
Measurement of lipid content
Intracellular lipid content was measured using the AdipoRed assay reagent kit (Lonza Inc., MD). AdipoRed reagent is a solution of the hydrophilic stain Nile Red which enables the quantification of intracellular lipid droplets. In brief, 10,000 cells/well were cultured in 96-well plates, and the cells were treated with or without resveratrol for 48 h. The control cells were treated with vehicle only. After 48 h, cells were assayed for the quantification of intracellular lipid content following the manufacturer’s instructions.
In situ apoptosis assay
Cells were cultured on 96-well plates and were treated with different concentrations of resveratrol and further incubated for 48 h. The terminal deoxynucleotidyl transferase, dUTP nick-end labeling assay was performed using In situ Cell Death detection kit/TMR Red (Roche Inc., IN) according to the manufacturer’s instructions. The number of apoptotic cells in each well was counted under a fluorescence microscope and represented in bar diagrams.
Western blotting
Cells after treatment with or without resveratrol were collected and proteins were extracted by homogenizing the pellet in the protein lysis buffer (50 mM Tris–HCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) under reducing conditions. The cell lysate was boiled for 10 min, and the proteins were separated by 10% SDS–PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk and then incubated with antibodies to FAS (0.2 μg/ml, Immuno-biological Laboratories Co., Japan), β-tubulin (1:10000, Upstate Biotechnology, NY), and Actin (1:250, Santa Cruz Biotechnology, CA) at 4°C. The membranes were then incubated with corresponding HRP-conjugated secondary antibodies followed by visualizing bands using ECL Plus Western blotting detection system (Amersham Life Sciences, UK).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the cells using RNeasy mini kit (Qiagen, CA), and they were reverse transcribed with Reverse Transcriptase kit (Fermentas Life Sciences, MD). qRT PCR was carried out using Maxima SYBR Green kit (Fermentas Life Sciences, MD) in DNA engine opticon-2 system (MJ Research, MA), and β-actin was used as an internal control. All the reactions were performed at least in triplicate. The following pairs of primers were used for the gene amplification; for FASN: 5′-CATCCAGATAGGCCTCATAGAC-3′ and 5′-CTCCATGAAGTAGGAGTGGAAG-3′, for p21: 5′-CCGTGGACAGTGAGCAGTT-3′ and 5′-CCAATCTGCGCTTGGAGTGA-3′, for BNIP3: 5′-CCTGGGTAGAACTGCACTTCAGCAAT-3′ and 5′-TTCATGACGCTCGTGTTCCTCATGCT-3′, for DAPK2: 5′-CTTTGATCTCAAGCCAGAAAAC-3′ and 5′-CTCGTAGTTCACAATTTCTGGAG-3′, and for β-actin: 5′-TGAGACCTTCAACACCCCAGCCATG-3′ and 5′-CGTAGATGGGCACAGTGTGGGTG-3′). The thermal cycling conditions comprised an initial denaturation step at 95°C for 2 min followed by 40 cycles of PCR using the following profile: 94°C: 30 s, 57°C: 30 s, and 72°C: 30 s.
siRNA transfection
The authors designed four individual small interfering RNAs (siRNAs) against the FAS gene (sense strand sequences: GAGCGUAUCUGUGAGAAAC, GACGAGAGCACCUUUGAUG, UGACAUCGUCCAUUCGUUU, CCAUGGAGCGUAUCUGUGA) and custom synthesized by Dharmacon Inc. (Lafayette, CO). One siRNA duplex targeting the GFP (green fluorescent protein) gene was also synthesized to use as a negative control in all the experiments. The siRNA was transfected into the breast cancer cells using the Trans-TKO transfection reagent (Mirus Corp., WI) according to the manufacturer’s protocol. The cells were then harvested for real time PCR experiments to determine the mRNA level.
Isolation of cancer stem-like cells from breast cancer cells and mammosphere assay
Breast cancer stem-like cells from MDA-MB231Luc or 231 LM were isolated using the cell surface phenotype CD24−/CD44+/ESA+ by MACS separation columns (Miltenyi Biotec, Germany) [26–29]. Cells were trypsinized and washed with PBS. Biotin-conjugated CD24 (StemCell Technologies, Canada) and allophycocyanin (APC)-conjugated CD44 (BioLegend, CA) antibodies were added to the sample and incubated for 15 min on ice. After washing the sample with PBS, CD24 negative cells were isolated by anti-biotin microbeads (Anti-Biotin MultiSort Kit; Miltenyi Biotec). The cells were then mixed with anti-APC microbeads (Anti-Biotin MultiSort Kit; Miltenyi Biotec) and biotin-conjugated ESA antibody (GeneTex Inc., TX) for 15 min on ice. After washing the cells, CD44 and ESA positive fractions were collected through MACS separation columns. Trypan Blue Solution (Sigma Co, MO) was used for measurement of cell viability. The single cell suspension was cultured in mammosphere forming medium (DMEM/F12 containing B27 supplement; Invitrogen) with or without resveratrol for the following assays.
Immunohistochemistry (IHC) analysis
Formalin-fixed paraffin-embedded breast cancer tissue specimens were obtained from the pathology archives of Iwate Medical School (Iwate, Japan). IHC staining was performed using antibodies to FAS (0.2 μg/ml, Immuno-biological Laboratories Co., Japan) and BNIP (Biocarta, CA). In brief, tumor sections were deparaffinized, rehydrated, and immersed in sodium citrate buffer (pH 6.0) at 90°C water bath for 30 min. After cooling down the sections to room temperature, the slides were dipped in 3% H2O2 for 15 min at room temperature to block endogenous peroxidase activity. After washing with the 0.1% Tween 20/PBS (PBST) solution, the sections were incubated with 2% BSA/PBST for 20 min. The sections were incubated with each primary antibody for 12 h at 4°C and were then processed using EnVision+ System (DAKO, CA) according to the manufacturer’s protocol. Tissue sections were incubated for 1 h with secondary anti-rabbit HRP-conjugated polymer antibody. After three washings, sections were dipped in distilled water for 5 min, subsequently counterstained with hematoxylin and dehydrated with ethanol.
Anti-tumorigenic effect of resveratrol in in vivo
In order to determine the anti-tumor effects of resveratrol in in vivo, cancer stem-like cells isolated from MDA-MB231Luc (1 × 104 cells) was mixed with Matrigel (BD biosciences, MA), and the mixture was injected into the mammary fat pad of 4-week old female nude mice. Resveratrol suspended in PBS (22.4 kg/body weight) was administered to these mice via oral gavage or intraperitoneal (IP) route every 2 days for a period of 4 weeks. The control group received the vehicle only. In vivo imaging was performed to monitor the tumor growth using the IVIS Imaging System (Xenogen, MA). Mice were killed at the endpoint, and tumors were harvested, and the paraffin block sections of tumors were later used for IHC analysis of FAS, DAPK2. and BNIP3.
Statistical analysis
Values were expressed as the mean ± SE of at least three independent experiments. Statistical significance was determined by Student’s t test or one way ANOVA. P values < 0.05 were considered significant. Statistical procedures were carried out using Graphpad prism software.
Results
Resveratrol suppresses lipogenesis and cell survival by downregulating fatty acid synthase
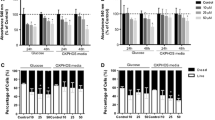
To examine the effect of resveratrol on lipogenesis and cell survival in in vitro culture conditions, three human breast cancer cell lines (MCF7, MDA-MB231, and 231 LM) and two non-tumorigenic and normal breast epithelial cells (MCF10A and HMEC) were chosen. These cells with two different concentration of resveratrol (50 and 100 μM) were treated. This concentration range of resveratrol is used elsewhere [30–34 ]. The authors first confirmed that while resveratrol significantly inhibited the growth and survival of all the breast cancer cell lines, it showed no significant toxicity to MCF10A and HMECs (Fig. 1a), suggesting that while resveratrol causes cytotoxicity mainly in cancer cells, it does not significantly affect normal cells. The amount of lipid synthesis under the same condition was then measured, and it was found that resveratrol indeed significantly blocked lipogenesis in cancer cells while it had no such effect in MCF10A cell, as shown in Fig. 1b. Because FAS is one of the key enzymes that control rate-limiting stage of lipid synthesis, the effect of resveratrol on the expression of FAS in these cell lines by Western blot analysis was examined. As shown in Fig. 1c and d, it was found that resveratrol indeed significantly suppressed the expression of FAS in all the tested breast cancer cell lines at the protein and RNA levels, whereas the normal breast cells had no such effect. These results suggest that suppression of FAS expression and overall lipogenesis is at least one of the mechanisms of the inhibitory effect of resveratrol on tumor cell growth, although several other mechanisms have also been proposed [35, 36].
Resveratrol suppresses cell survival by down-regulating FAS and lipid synthesis. a Three breast cancer cell lines [MCF7, MDA-MB231, and 231 LM] and one non-tumorigenic breast epithelial cell line [MCF10A and HMEC] were cultured in the presence and absence of resveratrol at the indicated concentration for 48 h, and the cell viability was measured by MTS assay. Control wells were treated with ethanol only. b The same three tumor cell lines and MCF10A were cultured in the presence and absence of resveratrol for 48 h, and lipid contents were measured using the AdipoRed kit. c MCF7, MDA-MB231, 231 LM, MCF10A, and HMECs were cultured with or without resveratrol for 48 h, the cells were then harvested, and the amount of FAS was measured by Western blot. d MCF7, MDA-MB231, 231 LM, MCF10A, and HMEC cells were cultured with or without resveratrol for 48 h, mRNA was extracted, and the expression of FAS mRNA was measured by qRT-PCR. All the experiments were performed in triplicate by taking the values as means ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001
Resveratrol induces apoptosis by down-regulating FAS and activating the pro-apoptotic genes
In order to understand how resveratrol suppresses cell growth and survival by modulating the FAS expression, the effect of resveratrol on apoptosis in breast tumor cells was first examined. As shown in Fig. 2a, it was found that resveratrol significantly induced apoptosis in all the tested breast cancer cell lines while it did not affect normal breast epithelial cells MCF10A and HMEC, which is in parallel to the inhibitory effect of resveratrol on FAS expression and lipogenesis. To examine whether the resveratrol-induced apoptosis is mediated by down-regulation of FAS, the authors ectopically expressed FAS in MDA-MB231 cells by infecting the FAS-expressing lentivirus, and cells were treated with or without resveratrol followed by assaying apoptosis. It was found that ectopic expression of FAS significantly blocked the resveratrol-induced apoptosis (Fig. 2b), suggesting that resveratrol indeed induces apoptosis through modulation of FAS expression.
Resveratrol induces apoptosis in breast cancer cells by down-regulating FAS and activating the pro-apoptotic genes. a MCF7, MDA-MB231, 231 LM, MCF10A, and HMECs were cultured in the presence of various concentration of resveratrol for 48 h, and apoptotic index was measured by In situ Cell Death detection kit/TMR red. b MDA-MB231 cells were infected with GFP (Control) or FAS overexpressing lentiviruses. After culturing the cells for 24 h, the cells were treated with or without resveratrol for 48 h, and the apoptotic index was measured using in situ Cell Death detection kit/TMR red. c, d MCF7, MDA-MB231, and 231 LM cells were cultured in the presence of various concentration of resveratrol for 48 h, and the expressions of DAPK2 and BNIP3 were measured using qRT-PCR. e MCF-7 cells were treated with 300 nM of FAS-siRNA or GFP-siRNA for 72 h. The cells were then collected, and mRNAs were extracted and subjected to qRT-PCR to determine the expression of DAPK2 and BNIP3. f MDA-MB231 cells were infected with GFP (Control) or FAS overexpressing lentiviruses. After incubating the cells for 24 h, the cells were treated with or without resveratrol for 48 h. The cells were then collected mRNAs, extracted, and qRT-PCR was performed to measure the expression levels of DAPK2 and BNIP3. g MCF7 and MDA-MB231 cells were cultured with or without resveratrol and in the presence or absence of TOFA [ACC inhibitor] or Fumonisin B1 [ceramide synthase inhibitor] for 48 h followed by assaying DAPK2 mRNA expression by qRT-PCR. h Immunohistochemical analysis of FAS and BNIP3 was performed on 17 tissue specimens from breast cancer patients. Left panels are representative images of IHC and right panel shows relationship between FAS and BNIP3 (P = 0.031)
Next, because it has previously been found that inhibition of FAS induced a series of pro-apoptotic genes such as DAPK2 and BNIP3 [21], the authors tested whether resveratrol too can activate these genes by down-regulating FAS. As shown in Fig. 2c and d, it was found that the treatment of MCF7, MDA-MB231, and MDA-MB231 LM cells with resveratrol significantly up-regulated DAPK2 and BNIP3. It was also confirmed by these findings using siRNA-mediated gene silencing in MCF7 cells by incubating the cells with FAS siRNAs followed by qRT-PCR analysis. As shown in Fig. 2e, specific inhibition of FAS using siRNAs results in the significant up-regulation of pro-apoptotic genes DAPK2 and BNIP3. On the other hand, extopic expression of FAS significantly blocked the resveratrol-induced expression of DAPK2 and BNIP3 (Fig. 2f), indicating that resveratrol induces apoptosis through activation of these pro-apoptotic genes by modulating FAS expression.
As was previously reported by the authors, inhibition of FAS causes accumulation of malonyl-CoA which is synthesized by Acetyl-CoA carboxylase (ACC) from Acetyl-CoA, and this accumulation leads to the inhibition of Carnitine palmitoyl transferase-1 and up-regulation of ceramide followed by induction of the pro-apoptotic genes [21]. Therefore, the effects of TOFA (ACC inhibitor) and Fumonisin B1 (ceramide synthase inhibitor) on the resveratrol-induced expression of DAPK2 gene in two breast tumor cell lines MCF7 and MDA-MB231 were examined. As shown in Fig. 2g, it was found that resveratrol induced the expression of DAPK2 gene in both MCF7 and MDA-MB231; however, both TOFA and Fumonisin B1 significantly blocked the expression of DAPK2 induction by resveratrol, suggesting that the resveratrol-induced apoptosis in these cell lines is indeed mediated through inhibition of FAS. In addition, the results of our immunohistochemical analysis for clinical specimens from breast cancer patients indicate that the expressions of FAS and BNIP3 are inversely correlated (Fig. 2h), which further supports our notion that FAS promotes cell survival by suppressing the DAPK2 and BNIP-mediated apoptotic pathway.
Resveratrol induces apoptosis in tumor stem-like cells by suppressing FAS
According to the recent cancer stem cell theory, which still remains a hypothesis, a tumor arises from a stem cell which has the ability of self-renewal and differentiation and also exhibits resistance to chemotherapy. Therefore, cancer stem cell is considered to be the most rational target for chemoprevention and chemotherapy. To test the efficacy of resveratrol on cancer stem-like cells, the authors first isolated cancer stem-like cells (CD24−/CD44+/ESA+) from MDA-MB231 and tested their tumor-initiating ability in nude mice. As shown in Fig. 3a, the results of a limited dilution analysis indicated that the isolated cancer stem-like cell population was significantly more tumorigenic than the corresponding parental cells, and 50% of the mice that received injection of even 100 cells gave rise to tumor. The authors then isolated cancer stem-like cells from both ER+ (MCF7) and ER− (MDA-MB231) and tested the effect of resveratrol on these cells. As shown in Fig. 3b, when these cells were treated with resveratrol, the amount of lipid synthesis was significantly reduced. The effect of resveratrol on FAS expression in these cells was examined by Western blot and qRT PCR, and it was found that resveratrol strongly suppressed the FAS expression at both protein and mRNA levels (Fig. 3c and d). Next, the effect of resveratrol on mammosphere formation which is characteristic to cancer stem-like cells was examined. As shown in Fig. 3e, it was found that resveratrol treatment significantly decreased the number (left panel) as well as the size of the mammospheres (right upper panel) in dose-dependent manner indicating that resveratrol is capable of suppressing cancer stem-like cell growth. In a parallel experiment, the authors collected the mammospheres in control and treated groups and performed the Western blot analysis for FAS expression. The results obtained showed that FAS was indeed down-regulated in the resveratrol-treated group (Fig. 3e, right lower panel). These data imply that resveratrol suppressed the growth of cancer stem-like cells by down-regulating FAS expression.
Resveratrol induces apoptosis in tumor stem-like cells by the downregulation of FAS. a Tumor stem-like cell population (CD24−/CD44+/ESA+) were isolated from MDA-MB231Luc cells by MACS sorting as described in “Materials and methods” section. Various amounts of cells before sorting (231 parental cells) or after sorting (231 stem cells) were injected into mammary fat pad of nude mice (n = 4), and the tumor incidence was measured by IVIS Imaging system. The results were analyzed by the limited dilution analysis (P < 0.001). b The tumor stem-like cells from MCF7 and MDA-MB231 were isolated and incubated in the presence or absence of resveratrol for 48 h, and the amount of lipid was measured by AdipoRed assay kit. c, d Tumor stem-like cells were isolated from MCF7 and MDA-MB231, they were cultured with or without resveratrol for 48 h, and the expression of FAS was assayed by Western blot and qRT-PCR. e Tumor stem-like cells were isolated from MDA-MB231 cells and were cultured in the mammosphere medium in the presence of various concentrations of resveratrol for 72 h. The number of mammospheres was counted, and the size of the mammospheres was also measured. The mammospheres in each group were then collected to determine the expression of FAS by Western blotting
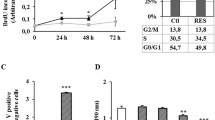
To further clarify the growth inhibitory effect of resveratrol on tumor stem-like cells, the authors isolated and treated MDA-MB231 stem-like cells with or without TOFA and Fumonisin B1 in the presence of resveratrol followed by assaying for apoptosis (Fig. 4a). It was found that resveratrol indeed induced apoptosis in cancer stem-like cells; however, this resveratrol effect was significantly abrogated by both TOFA and Fumonisin B1 treatment, indicating that resveratrol induces apoptosis through the FAS-mediated pathway. The effect of resveratrol on the activation of the pro-apoptotic genes in the cancer stem-like cells was examined, and it was found that resveratrol significantly activated both DAPK2 (Fig. 4b) and BNIP3 (Fig. 4c) mRNA expression. In a parallel experiment, the expression of p21 in the cancer stem-like cells under the same conditions was examined and it was found that p21 was also up-regulated by resveratrol treatment (Fig. 4d). These results strongly suggest that resveratrol suppresses tumor stem-like cells growth by inducing pro-apoptotic genes through down-regulation of FAS expression.
Resveratrol activates pro-apoptotic genes in tumor stem-like cells. a Tumor stem-like cell were isolated from MDA-MB231 cells and treated with or without resveratrol in the presence or absence of TOFA or Fumonisin B1 for 48 h followed by assaying for the apoptotic index by In situ Cell Death detection kit/TMR red. b, c, d Tumor stem-like cells were isolated from MCF7 and MDA-MB231 cells and incubated with various concentrations of resveratrol for 48 h followed by quantifying the mRNA expression of the pro-apoptotic genes DAPK2, BNIP3, and p21 by qRT-PCR
Resveratrol suppresses growth of cancer stem-like cells in vivo
To test the efficacy of resveratrol on cancer stem-like cells in vivo, stem-like cell population from MDA-MB231Luc cells that are “labeled” with the luciferase gene was first isolated. These cells were injected into the mammary fat pad of nude mice as described in “Materials and methods” section. The growth of tumor was then monitored by IVIS Imaging system. As shown in Fig. 5a (left and right panel), administration of resveratrol into the mice either through oral gavage or IP injection significantly suppressed the onset of tumor growth in these animals, indicating that resveratrol is indeed capable of inhibiting the proliferation of cancer stem-like cells in vivo. The weights of mice were not significantly changed among the groups during the course of experiment, suggesting that resveratrol did not show notable adverse effects on these mice (Fig. 5b). At the endpoint, the mice were sacrificed for histological analysis (Fig. 5c). The authors observed that the expression of FAS was remarkably suppressed in the tumors of mice that were treated with resveratrol, while the expression of DAPK2 and BNIP3 was strongly augmented in these animals compared to the mice without the treatment. These results strongly support the notion that resveratrol suppresses cancer stem-like cells growth in mice by down-regulating of FAS and by activating a series of pro-apoptotic genes DAPK2 and BNIP3.
Resveratrol suppressed the growth of tumor stem-like cells in vivo. Tumor stem-like cells were isolated from MDA-MB231Luc cell, and 1 × 104 cells were injected into mammary fat pad of female nude mice (n = 5). They were then treated with resveratrol (22.4 mg/kg/day) through oral gavage or by intraperitoneal injection (IP) every 2 days for a period of 4 weeks. The tumor growth was monitored by injecting luciferin in the mice followed by measuring bioluminescence using IVIS Imaging System. Imaging and quantification of signals were controlled by the acquisition and analysis software living image. a Representative images of mice at day 28 (left panel). Time course of the quantified bioluminescence image (right panel). b The weights of the mice were also measured periodically. c Mice were sacrificed at the end of the experiment, and tumors were harvested and sectioned for immunohistochemical staining. IHC staining of the tumor sections was done to determine the expression of FAS, DAPK2, and BNIP3 proteins
Discussion
The chemo-preventive as well as chemo-therapeutic effects of resveratrol against various types of cancers in pre-clinical testing has been well documented, although actual efficacy on patients is yet to be determined and several clinical trials are currently underway [NCT00721877, NCT00920803, NCT00433576, and NCT00578396]. Resveratrol has a strong anti-oxidant activity and is also capable of inducing apoptosis in cancer cells, and therefore, it is believed to be efficacious at multiple stages of carcinogenesis [37–40]. However, underlining molecular mechanism of its anti-tumor activity is yet to be defined. In this article, a novel action of resveratrol by which it modulates FAS expression and lipid syntheses and thus resulting in the induction of apoptosis in cancer stem-like cells was described. The current cancer stem cell theory predicts that cancer stem cells are responsible for the initiation of tumorigenesis because of their self-renewal ability and that they are also responsible for chemoresistance [41–43]. For this reason, active studies are underway to develop a specific drug to target cancer stem cells. The results obtained above indicate that resveratrol is capable of inducing apoptosis in cancer stem-like cells, and therefore, resveratrol is considered to serve as an effective chemopreventive agent owing to its non-toxicity. Oral intake of this compound is also feasible because resveratrol promptly distributes to various organs and yet maintains their efficacy, suggesting that resveratrol can be taken as a food supplement for the prevention of a variety of cancers [44].
Resveratrol is a multi-functional polyphenolic compound and exhibits various types of biological activities. The strong anti-oxidant activity of resveratrol is believed to act as radical scavenger, and therefore, protect DNA damage which otherwise may lead to tumorigenesis [45]. Resveratrol is also known to exhibit cytotoxic effect on cancer cells by modulating or activating various signaling pathways, including p53/p21, NF-kappaB, Akt, Cox2, IGF-1, and ERK1. as well as Redox signaling [12, 13, 46, 47]. In this report, the authors have revealed that resveratrol is capable of suppressing lipogenesis by inhibiting FAS expression as a novel mechanism of its anti-tumor effect. Our results indicate that blocking FAS by resveratrol indeed caused apoptosis in cancer stem-like cells whereas it has no significant toxicity towards normal cells, and more importantly, resveratrol was able to inhibit growth of cancer stem-like cells by suppressing FAS in our animal model. Up-regulation of FAS has been well documented in a variety of cancers, and it is considered to have a causative effect on tumorigenesis rather than merely a consequence of tumor growth. Overexpression of FAS along with androgen receptors in immortalized, non-transformed human prostate epithelial cells formed invasive adenocarcinomas in immunodeficient mice whereas transgenic expression of FAS in mice resulted in prostate intraepithelial neoplasia [20]. In another study, it was reported that increased lipid synthesis by overexpressing FAS induced a cancer-like phenotype in non-cancerous epithelial cells such as HBL100 and MCF10A by the activation of HER1/HER2 tyrosine receptor kinases [48]. It should be noted that inhibiting FAS expression is known to induce cell death by activating pro-apoptotic signaling. For instance, downregulation of FAS by RNA interference resulted in the significant killing of tumor cells in a panel of mammary carcinoma cell lines (MDA-MB435 and MCF-7) and also in prostate cancer cells (LNCaP and PC-3) by caspase-8-mediated apoptosis [21, 49]. The authors have also previously reported that FAS was highly expressed in tumors of both breast and prostate cancers and the expression was inversely correlated with patient survival [21, 50].The results in this article indicate that resveratrol indeed induced apoptosis in cancer stem-like cells by activating the pro-apoptotic genes DAPK2 and BNIP3 that are generally suppressed by FAS through ceramide synthesis, whereas ectopic expression of FAS rescued the tumor cells from resveratrol-induced apoptotic cell death by down-regulating the expression of these pro-apoptotic genes. In addition, the activation of these genes by resveratrol and concomitant apoptosis were significantly blocked by TOFA and Fumonisin B1, further indicating that the pro-apoptotic effect of resveratrol in tumor stem-like cells is a direct consequence of the ability of resveratrol in down-regulating FAS.
In summary, the authors have shown that resveratrol is able to suppress the growth of cancer stem-like cells through down-regulation of FAS expression and by inducing the pro-apoptotic signaling pathway both in vitro and in a xenograft model of breast cancer. This novel mechanism of resveratrol’s action provides a strong rationale to use this phytochemical for chemoprevention, as well as chemotherapy for breast cancer.
Abbreviations
- FAS:
-
Fatty acid synthase
- ER+:
-
Estrogen receptor positive
- ER−:
-
Estrogen receptor negative
- DAPK2:
-
Death associated kinase 2
- BNIP3:
-
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- ACC:
-
Acetyl-CoA carboxylase
- TOFA:
-
5-(Tetradecyloxy)-2-furoic acid
- qRT-PCR:
-
Quantitative real-time PCR
References
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Pervaiz S (2003) Resveratrol: from grapevines to mammalian biology. FASEB J 17:1975–1985
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421
Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C (2005) Resveratrol and breast cancer risk. Eur J Cancer Prev 14:139–142
Das S, Khan N, Mukherjee S et al (2008) Redox regulation of resveratrol-mediated switching of death signal into survival signal. Free Radic Biol Med 44:82–90
Lin JK, Tsai SH (1999) Chemoprevention of cancer and cardiovascular disease by resveratrol. Proc Natl Sci Counc Repub China B 23:99–106
Renaud S, de Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339:1523–1526
Szkudelska K, Nogowski L, Szkudelski T (2009) Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol 13:17–24
Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL (2007) Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 224:274–283
Bishayee A (2009) Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res 2:409–418
Jang M, Cai L, Udeani GO et al (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Banerjee S, Bueso-Ramos C, Aggarwal BB (2002) Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res 62:4945–4954
Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA (2007) Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 28:1946–1953
Kuhajda FP (2000) Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16:202–208
Alo’ PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U (1996) Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer 77:474–482
Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP (1997) Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res 3:2115–2120
Rashid A, Pizer ES, Moga M et al (1997) Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol 150:201–208
Swinnen JV, Roskams T, Joniau S et al (2002) Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer 98:19–22
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Migita T, Ruiz S, Fornari A et al (2009) Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 101:519–532
Bandyopadhyay S, Zhan R, Wang Y et al (2006) Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res 66:5934–5940
Liu H, Liu Y, Zhang JT (2008) A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther 7:263–270
Furuta E, Pai SK, Zhan R et al (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68:1003–1011
Furuta E, Okuda H, Kobayashi A, Watabe K (2010) Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta 1805:141–152
Minn AJ, Gupta GP, Siegel PM et al (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67(3):1030–1037
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10(2):R2
Li C, Lee CJ, Simeone DM (2009) Identification of human pancreatic cancer stem cells. Methods Mol Biol 568:161–173
Huang TT, Lin HC, Chen CC et al (2010) Resveratrol induces apoptosis of human nasopharyngeal carcinoma cells via activation of multiple apoptotic pathways. J Cell Physiol [Epub ahead of print]
Vanamala J, Reddivari L, Radhakrishnan S, Tarver C (2010) Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 10:238
Lee MH, Choi BY, Kundu JK et al (2009) Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Res 69(18):7449
Boissy P, Andersen TL, Abdallah BM et al (2005) Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res 65(21):9943–9952
Jiang H, Shang X, Wu H et al (2009) Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol 8(1):25–33
Hwang J-T, Kwon DY, Park OJ, Kim (2008) Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr 2(4):323–326. doi:10.1007/s12263-007-0069-7
Lin JN et al (2010) Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J Agric Food Chem 58(3):1584–1592
Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H (2001) Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 7:1466–1473
Bai Y, Mao QQ, Qin J et al (2010) Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci 101:488–493
Gagliano N, Aldini G, Colombo G et al (2010) The potential of resveratrol against human gliomas. Anticancer Drugs 21:140–150
Wang J, He D, Zhang Q, Han Y, Jin S, Qi F (2009) Resveratrol protects against Cisplatin-induced cardiotoxicity by alleviating oxidative damage. Cancer Biother Radiopharm 24:675–680
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737
Hope KJ, Jin L, Dick JE (2004) Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5:738–743
Marx J (2007) Molecular biology. Cancer’s perpetual source? Science 317:1029–1031
Vitrac X, Desmoulière A, Brouillaud B et al (2003) Distribution of (14C)-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci 72:2219–2233
Hamada J, Nakata D, Nakae D et al (2001) Increased oxidative DNA damage in mammary tumor cells by continuous epidermal growth factor stimulation. J Natl Cancer Inst 93:214–219
Whyte L, Huang YY, Torres K, Mehta RG (2007) Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res 67:12007–12017
Zhang J (2006) Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J 397:519–527
Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA (2008) Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif 41:59–85
Knowles LM, Yang C, Osterman A, Smith JW (2008) Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. J Biol Chem 283:31378–31384
Bandyopadhyay S, Pai SK, Watabe M et al (2005) FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene 24:5389–5395
Acknowledgments
This study was supported by the National Institutes of Health [R01CA124650 and R01CA129000], the Department of Defense, and Susan G. Komen Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, P.R., Okuda, H., Watabe, M. et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat 130, 387–398 (2011). https://doi.org/10.1007/s10549-010-1300-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1300-6