Abstract
We conducted a meta-analysis to assess the association between tumor necrosis factor-alpha (TNF-alpha) gene TNFA −308 (G>A), TNFA −238 (G>A), TNFA −857 (C>T), TNFA −863 (C>A), TNFA −1031 (T>C), TNFA −1210 (A>T) polymorphisms and breast cancer(BC) susceptibility. We also performed subgroup analyses based on ethnicity (Caucasian, Asian, and African). An extensive search was performed to identify all case–control studies investigating such association. Thirteen eligible studies, including 10,236 BC patients and 13,143 controls, were identified. No significant association was observed in all genotypes in worldwide populations, but stratification by ethnicity indicated that the TNFA −308 A allele was associated with a decreased risk of BC compared with the G allele in Caucasian individuals (OR = 0.927, 95%CI = 0.879–0.978). Similar results were obtained when the A/A +A/G genotype was compared with the G/G genotype. In addition, meta-analysis results indicated that the A/A genotype of TNFA −308 was a risk factor for BC in African (A/A vs. G/G OR = 4.085 95%CI = 1.460–11.425; A/A vs. G/A OR = 4.861 95%CI = 1.746–13.527; A/A vs. G/A + G/G OR = 4.246 95%CI = 1.551–11.625), but not in Caucasian or Asian individuals. In conclusion, the results of this meta-analysis indicate that the TNFA −308 A allele may be an important protective factor for BC in European individuals, but it is not likely to confer susceptibility to BC in worldwide populations. In addition, the AA genotype of TNFA −308 may be a risk factor for BC in African individuals. Besides, other polymorphisms were not associated with BC susceptibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common cancers worldwide and a major cause of mortality in women in developed countries. Over the past several years, breast cancer incidence rates have increased by approximately 30% in Westernized counties owing to changes in reproductive patterns and, more recently, to increased screening [1]. BC pathogenesis is a multistep and multifactorial process owing to a complex series of interactions among genetic, environmental, and endocrine factors [2]. Among the former, genetic polymorphisms, such as variants of pro- and anti-inflammatory cytokines such as -interleukin (IL) and tumor necrosis factors (TNF), have been most extensively investigated.
TNF-alpha is a potent pro-inflammatory cytokine initially identified as a serum factor that induces necrosis of transplanted tumors in mice [3]. It is one of the earliest cytokines to be produced in inflammatory response [4]. This production initiates a cytokine cascade involving the production of IL-1, IL-6, and other mediators, as well as TNF itself. High levels of TNF-alpha may cause organ dysfunction [5]. It has been shown that the blood level of T TNF-alpha is increased in solid tumors [6]. Therefore, it seems likely that the expression level of TNF-alpha may be involved in cancer pathogenesis and progression.
Several single-nucleotide polymorphisms in the TNFA promoter can greatly influence the expression level of TNF-alpha [7]. Among these, a common polymorphism in the promoter, a G to A substitution at position −308, has been studied intensively as a putative determinant of susceptibility to various disease, including rheumatoid arthritis, psoriasis, and BC; although the effect is small, the A allele of the TNFA −308 polymorphism is significantly associated with increased TNF-alpha production [8]. Similarly, the T allele of TNF-A 857 polymorphism located in the 5′-flanking region also shows higher transcriptional activity [9].
In fact, numerous studies have investigated genetic polymorphisms and BC susceptibility or progression. However, the association between BC risk and polymorphisms found in TNFA is still controversial. Many studies have found that pro-inflammatory genotypes of TNFA were associated with BC risk [10, 11]. However, other studies have suggested that polymorphisms of TNFA may not be significantly associated with BC risk. These mixed results were likely due to small sample sizes and low statistical power. The low statistical power of individual studies to detect small differences between cases and controls is one factor to explain the lack of conclusive results.
To better address the association between TNFA polymorphisms and BC risk, we performed a meta-analysis of all eligible studies, including a subgroup analysis based on ethnicity.
Materials and methods
Identification of eligible studies
We performed an extensive search of studies that examined the association of the TNFA polymorphisms with BC. All eligible studies were identified by searching the PubMed database. The following terms were used: (“lacteal gland” OR “mammary gland” OR “breast”) AND (“cancer” OR “carcinoma”) AND (“polymorphism” OR “polymorphisms”) AND (“tumor necrosis factor-alpha” OR “TNF-alpha” OR “TNFA”OR “TNF-A”). References of cited articles were reviewed to identify additional studies not indexed by Medline. No language or country restrictions were applied. Included studies were required to meet the following criteria: (a) based on unrelated individuals, pedigree data were excluded; (b) genotype distributions of both cases and controls were available; and (3) genotype distribution of the control population must be in Hardy–Weinberg equilibrium (HWE).
Data extraction
Information was carefully extracted from all eligible publications independently by two of the authors, according to the inclusion criteria. Disagreement was resolved by discussion between the authors. If these two authors could not reach a consensus, a third author was consulted to resolve the dispute and a final majority decision was made. For each study, the following information was collected: the first author’s last name, year of publication, country in which the study was performed, ethnicity of the study population, numbers of genotyped cases and controls, source of control groups (population-or hospital-based), source of DNA, and other variables that could be sources of bias. Patient ethnicity was categorized as Caucasian, Asian, and African.
Statistical analysis
Meta-analysis
We calculated summary odd ratios (ORs) corresponding to a 95% confidence interval (CI) to assess the strength of association between TNFA polymorphisms and breast cancer. We examined the association between TNFA −308 allele A and BC risk compared with that for allele G (A vs. G); homozygote AA was contrasted with GG. Recessive (AA vs. GA + GG) and dominant (AA + GA vs. GG) models for allele A were also used. The same contrasts were performed for allele T of the TNFA −857 polymorphism, allele C of the TNFA −1031 polymorphism, and allele C of the TNFA −1210 polymorphism, respectively. As the AA genotypes of TNFA −238 were much less frequent than GA and GG genotypes, so we just examined the contrast of the allelic effect of A (minor allele) versus G (common allele), and also examined the contrast of A/A + G/A versus G/G genotypes. The same contrasts were performed for allele A of the TNFA −863 polymorphism.
We used the Cochrane Q-test to assess the heterogeneity among studies. If the Q-test revealed a P value of more than 0.05, the fixed-effects model (the Mantel–Haenszel method) was selected to pool the data [12]. Otherwise, the random-effects model (the DerSimonian and Laird method) was used [13]. For sensitivity analysis, we excluded smaller studies and recalculated the summary ORs (95% CIs) using only larger studies to reflect the influence of the individual data. The potential publication bias was estimated by visual inspection of funnel plots [14], in which the standard error of log (OR) of each study was plotted against its log (OR); an asymmetric plot indicates a possible publication bias. We also used the method of Begg and Mazumdar [15] to calculate P values for rank correlation and Egger’s weighted regression method [16] to calculate P values for bias (P < 0.05 was considered representative of statistical significance). All statistical analyses were performed using STATA (v.10.1; Stata Corporation, College Station, TX).
Results
Included studies
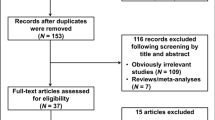
Thirteen relevant studies with a total number of 10,236 cases and 13,143 controls were included in this analysis [10, 11, 17–27]. Characteristics of the included studies are shown in Table 1. The most commonly investigated genotypes were TNFA −308, −238, −863, −1031, −1210, and −857, which were reported in 13, 3, 2, 3, 2, and 2 studies, respectively. All studies used healthy volunteers or blood donors as control subjects. Populations were categorized into Caucasian, Asian, and African.
Effect of allele and subgroup analysis
TNF-A −308
Summary results of this meta-analysis for the association between the TNFA −308 polymorphism and BC are shown in Table 2. The meta-analysis did not reveal an association between BC and the TNFA −308 A allele in the overall population (OR = 1.050, 95%CI = 0.889–1.241; Fig. 1). However, stratification by ethnicity indicated that the TNFA −308 A allele was associated with a decreased risk of BC compared with the G allele in Caucasian individuals (OR = 0.927, 95%CI = 0.879–0.978; Fig. 2). The overall OR for the A/A versus G/G genotype of the TNFA promoter −308 was 0.976(95%CI 0.824–1.155), and an association was not found. However, stratification by ethnicity indicated that the AA genotype was a risk factor for BC in African, but not in Caucasian or Asian populations. The same results were seen when we compared the A/A and G/A genotypes or the A/A and G/A + G/G genotypes. For the A/A + G/A versus GG genotype, the OR was 1.203 (95%CI 0.851–1.229), 0.915 (95%CI 0.861–0.972), 1.774 (95%CI 0.447–7.037), and 1.746 (95%CI 0.476–6.048) in the overall, Caucasian, Asian, and African populations, respectively.
Other genotypes
The meta-analysis did not reveal an association between BC and TNFA −238 G>A polymorphism, and the same results were obtained between BC and any other polymorphisms in the overall population (Table 2). As these genotypes were investigated in small number of studies, we did not perform subgroup analysis. And more studies were needed to examine whether BC is associated with these polymorphisms.
Sensitivity analysis
With regard to TNFA −308 polymorphism, the results pattern was not impacted by any single study in all subgroup studies (data not shown).
Publication bias
Funnel plot from comparisons of A versus G of TNFA −308 was generated to assess publication bias (Fig. 3). The Begg’s and Egger’s Test was performed to statistically evaluate funnel plot symmetry; the results indicated no significant publication bias (Table 2).
Discussion
TNFA is located within the HLA class III region in chromosome 6p21.3, and contains several sites of single-nucleotide polymorphisms, which modify gene expression. TNF-alpha has shown an anti-tumor activity in a variety of tumor cell lines, including BC cell lines [28]. TNF-alpha causes arrest of the cell cycle in the transition from G1 to S phase in mammary carcinoma cells [29] and induces apoptosis in tumor cells, similar to the Fas ligand [30]. −238 G/A and −308 G/A promoter polymorphisms of TNF are shown to be associated with the TNF expression both in vivo and in vitro [31–33]. TNFA −857T, −863A, and −1031C are also found to increase TNF promoter activity [9] and lipopolysaccharide-induced TNFA production [34], although contradictory findings have also been reported [35, 36].
This study investigated the relationship between TNFA polymorphisms and BC susceptibility. For TNFA −308 genotypes, the overall results of this meta-analysis showed no significant BC susceptibility with the TNFA −308 promoter A/G polymorphism. However, stratification by ethnicity revealed a significant association between BC risk and the TNFA polymorphism in the European study populations. The available data indicate that the TNFA −308 A allele is an ethnic-specific protective factor for BC, and the A/A genotype is a risk factor for BC in African individuals, although the small number of Caucasian and African studies available to date reduces the confidences of this conclusion. There was no heterogeneity among the Caucasian and African studies, suggesting a strong association of the TNFA −308 A/G polymorphism with BC by ethnicity.
This meta-analysis of the A allele and A/A + A/G genotype of TNFA −308 locus revealed a significant association with BC in European populations, but no such association with the A/A versus G/G, A/A versus G/A genotype, and A/A versus A/G + G/G genotypes. Contrasting results were seen in African individuals. As only eight Caucasian and two African population studies were included, these results should be interpreted with caution. More Caucasian and African studies are needed to confirm this possible association.
The ORs of the A/A versus G/G genotype and AA versus A/G + G/G genotype of TNFA −308 locus were decreased in European populations, versus African and Asian populations, although the difference did not reach statistical significance. This finding may be due to low statistical power owing to the low frequency of the A/A genotype. The meta-analysis consistently showed no heterogeneity among studies in European populations. Taken together, these findings suggest that the TNFA −308 A/G polymorphism may protect against for BC in European individuals. However, because only eight studies in European populations were included, this result should be interpreted with caution. More European studies are needed to determine this possible association.
The finding that the association between the TNFA −308 A/G polymorphism and BC differs according to ethnicity is somewhat surprising; however, many factors may contribute to this difference. First, genetic heterogeneity for BC may exist in different ethnic populations. Second, clinical heterogeneity may be involved, and the contribution of differences in patient populations may cause different results. Third, different linkage disequilibrium (LD) patterns may contribute to the discrepancy. This polymorphism may be in LD with a nearby causal variant in one ethnic group, but not in another. Fourth, the difference might arise from chance, such as type I error, or due to multiple testing that inflates the type I error.
No association was found between BC and TNFA −238, −863, −857, −1031, and −1210 genotypes. As studies investigated these genotypes were not much enough, these results should be interpreted with caution, and more studies are needed.
In conclusion, results of this meta-analysis indicate that the TNFA −308 A allele may protect against BC in European individuals, but it is not likely to confer susceptibility to BC in worldwide populations. In addition, the AA genotype may be a risk factor for BC in African individuals. TNFA −238, −863, −857, −1031, and −1210 genotypes were not association with BC risk. Further detailed investigation with large numbers of worldwide participants is needed to clarify the role of these polymorphisms in BC.
References
Colditz GA, Sellers TA, Trapido E (2006) Epidemiology—identifying the causes and preventability of cancer? Nat Rev Cancer 6(1):75–83
Welch DR, Steeg PS, Rinker-Schaeffer CW (2000) Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res 2(6):408–416
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 72(9):3666–3670
Bazzoni F, Beutler B (1996) The tumor necrosis factor ligand and receptor families. N Engl J Med 334(26):1717–1725
Strieter RM, Kunkel SL, Bone RC (1993) Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med 21(10 Suppl):S447–S463
Ardizzoia A, Lissoni P, Brivio F, Tisi E, Perego MS, Grassi MG, Pittalis S, Crispino S, Barni S, Tancini G (1992) Tumor necrosis factor in solid tumors: increased blood levels in the metastatic disease. J Biol Regul Homeost Agents 6(3):103–107
Raabe T, Bukrinsky M, Currie RA (1998) Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem 273(2):974–980
Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R et al (1998) Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol 113(3):401–406
Higuchi T, Seki N, Kamizono S, Yamada A, Kimura A, Kato H, Itoh K (1998) Polymorphism of the 5’-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens 51(6):605–612
Kohaar I, Tiwari P, Kumar R, Nasare V, Thakur N, Das BC, Bharadwaj M (2009) Association of single nucleotide polymorphisms (SNPs) in TNF-LTA locus with breast cancer risk in Indian population. Breast Cancer Res Treat 114(2):347–355
Mestiri S, Bouaouina N, Ahmed SB, Khedhaier A, Jrad BB, Remadi S, Chouchane L (2001) Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein hsp70-2: susceptibility and prognostic implications in breast carcinoma. Cancer 91(4):672–678
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Munafo MR, Clark TG, Flint J (2004) Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res 129(1):39–44
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
MARIE-GENICA Consortium (2009) Polymorphisms in the BRCA1 and ABCB1 genes modulate menopausal hormone therapy associated breast cancer risk in postmenopausal women. Breast Cancer Res Treat 120(3):727–736
Azmy IA, Balasubramanian SP, Wilson AG, Stephenson TJ, Cox A, Brown NJ, Reed MW (2004) Role of tumour necrosis factor gene polymorphisms (−308 and −238) in breast cancer susceptibility and severity. Breast Cancer Res 6(4):R395–R400
Chouchane L, Ahmed SB, Baccouche S, Remadi S (1997) Polymorphism in the tumor necrosis factor-alpha promotor region and in the heat shock protein 70 genes associated with malignant tumors. Cancer 80(8):1489–1496
Gallicchio L, McSorley MA, Newschaffer CJ, Huang HY, Thuita LW, Hoffman SC, Helzlsouer KJ (2007) Body mass, polymorphisms in obesity-related genes, and the risk of developing breast cancer among women with benign breast disease. Cancer Detect Prev 31(2):95–101
Gaudet MM, Egan KM, Lissowska J, Newcomb PA, Brinton LA, Titus-Ernstoff L, Yeager M, Chanock S, Welch R, Peplonska B et al (2007) Genetic variation in tumor necrosis factor and lymphotoxin-alpha (TNF-LTA) and breast cancer risk. Hum Genet 121(3–4):483–490
Giordani L, Bruzzi P, Lasalandra C, Quaranta M, Schittulli F, Della Ragione F, Iolascon A (2003) Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin Chem 49(10):1664–1667
Kamali-Sarvestani E, Merat A, Talei AR (2005) Polymorphism in the genes of alpha and beta tumor necrosis factors (TNF-alpha and TNF-beta) and gamma interferon (IFN-gamma) among Iranian women with breast cancer. Cancer Lett 223(1):113–119
Ostashkin AS, Malivanova TF, Iurchenko VA, Mazurenko NN (2008) Tumor necrosis factor gene polymorphisms in breast cancer patients. Genetika 44(9):1275–1280
Park KS, Mok JW, Ko HE, Tokunaga K, Lee MH (2002) Polymorphisms of tumour necrosis factors A and B in breast cancer. Eur J Immunogenet 29(1):7–10
Scola L, Vaglica M, Crivello A, Palmeri L, Forte GI, Macaluso MC, Giacalone A, Di Noto L, Bongiovanni A, Raimondi C et al (2006) Cytokine gene polymorphisms and breast cancer susceptibility. Ann N Y Acad Sci 1089:104–109
Sirotkovic-Skerlev M, Cacev T, Krizanac S, Kulic A, Pavelic K, Kapitanovic S (2007) TNF alpha promoter polymorphisms analysis in benign and malignant breast lesions. Exp Mol Pathol 83(1):54–58
Kopreski MS, Lipton A, Harvey HA, Kumar R (1996) Growth inhibition of breast cancer cell lines by combinations of anti-P185HER2 monoclonal antibody and cytokines. Anticancer Res 16(1):433–436
Pagliacci MC, Fumi G, Migliorati G, Grignani F, Riccardi C, Nicoletti I (1993) Cytostatic and cytotoxic effects of tumor necrosis factor alpha on MCF-7 human breast tumor cells are differently inhibited by glucocorticoid hormones. Lymphokine Cytokine Res 12(6):439–447
Cai Z, Stancou R, Korner M, Chouaib S (1996) Impairment of Fas-antigen expression in adriamycin-resistant but not TNF-resistant MCF7 tumor cells. Int J Cancer 68(4):535–546
Hagihara M, Shimura T, Sato K, Genga K, Suzuki M, Tsuji K (1995) HLA and tumor necrosis factor beta gene polymorphisms in Okinawa lung cancer patients: comparative study with mainland Japan lung cancer patients. Hum Immunol 43(2):95–100
Messer G, Spengler U, Jung MC, Honold G, Blomer K, Pape GR, Riethmuller G, Weiss EH (1991) Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med 173(1):209–219
Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Hori M, Nakamura Y, Tanaka T (2002) Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet 32(4):650–654
Soga Y, Nishimura F, Ohyama H, Maeda H, Takashiba S, Murayama Y (2003) Tumor necrosis factor-alpha gene (TNF-alpha) −1031/−863, −857 single-nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol 30(6):524–531
Kaijzel EL, Bayley JP, van Krugten MV, Smith L, van de Linde P, Bakker AM, Breedveld FC, Huizinga TW, Verweij CL (2001) Allele-specific quantification of tumor necrosis factor alpha (TNF) transcription and the role of promoter polymorphisms in rheumatoid arthritis patients and healthy individuals. Genes Immun 2(3):135–144
Uglialoro AM, Turbay D, Pesavento PA, Delgado JC, McKenzie FE, Gribben JG, Hartl D, Yunis EJ, Goldfeld AE (1998) Identification of three new single nucleotide polymorphisms in the human tumor necrosis factor-alpha gene promoter. Tissue Antigens 52(4):359–367
Acknowledgments
This project was supported by The National High Technology R&D Program of China [2009AA022701] National 863 Project (2009AA022701) and The National Basic Research Program of China [2010CB534901].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, C., Sun, H., Sun, D. et al. Polymorphisms of tumor necrosis factor-alpha and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 126, 763–770 (2011). https://doi.org/10.1007/s10549-010-1184-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1184-5