Abstract
The aim of this study is to test the hypothesis that antiangiogenic treatment with sunitinib consolidation can prolong remissions induced by taxane-based chemotherapy in women with metastatic breast cancer. The method involves a two-arm open-label (2:1 randomization) multicenter, randomized phase II trial evaluating the efficacy of sunitinib (arm A) versus no therapy (arm B) in patients with HER-2-negative metastatic breast cancer who achieved an objective response to taxane-based chemotherapy. The results of this study indicates that the primary endpoint of progression-free survival (PFS) ≥5 months was achieved in 10 of 36 patients (28%) in arm A and 4 of 19 patients (21%) in arm B. The median PFS was 2.8 and 3.1 months, respectively. A protocol amendment to the sunitinib dosing schedule was made because 53% (17/32) of patients treated at a starting dose of 50 mg (4 weeks on/2 weeks off) required dose reduction. Changing the starting dose to sunitinib 37.5 mg continuously resulted in dose reductions in 44% (7/16) of patients. Grades III–IV toxicity occurred in 69% of patients in arm A (fatigue 31%, musculoskeletal pain 11%, neutropenia and thrombopenia 8%) and 11% in arm B. The proof-of-principle study does not confirm the hypothesis that sunitinib consolidation therapy can lead to a predefined clinically relevant proportion of patients with PFS of ≥5 months after an objective response to taxanes. Furthermore, toxicity was significant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although early breast can often be cured, there remains a significant risk of developing metastases, a clinical situation that is generally incurable. Several antitumor therapies, such as hormone therapy for hormone-sensitive disease, or chemotherapy, are capable of providing significant benefit, including increased survival and quality of life. An interesting category of drugs has emerged in cancer treatment, targeting the blood supply. This strategy, called antiangiogenic therapy, is rapidly evolving, and the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab has recently been approved by the FDA and EMEA for use in combination with chemotherapy in patients with metastatic breast cancer.
New drugs are generally tested in patients with metastatic disease, at a stage associated with a bulky tumor mass. However, antiangiogenic compounds may be more beneficial in the prevention of malignant progression when the tumor load has been reduced. Metastatic breast tumors are relatively chemosensitive and a significant proportion of patients achieve an objective clinical response. Taxanes are considered the most active chemotherapeutic drugs in breast cancer. Tumor shrinkage following induction chemotherapy provides an excellent model to test the hypothesis that antiangiogenic drugs can prevent revascularization and malignant regrowth.
Sunitinib is an orally administered small molecule inhibitor of multiple tyrosine kinase receptors including VEGF, platelet-derived growth factor (PDGF), c-KIT, and RET. Sunitinib is established as a first-line therapy in metastatic renal cancer, in which the angiogenic pathway is constitutively activated, and as second-line therapy after imatinib for inoperable gastrointestinal stromal tumors (GIST), by targeting the mutant c-KIT receptor. In other cancers including breast cancer, modest single-agent antitumor activity has been observed [1]. This study was designed to test the hypothesis that sunitinib, a compound with a clinically proven antiangiogenic effect, can delay tumor progression after a taxane-induced objective partial or complete response (PR or CR).
Materials and methods
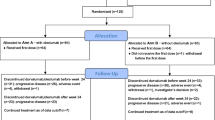
Patients with metastatic breast cancer achieving an objective response with taxane chemotherapy were included in this two-arm, open-label, randomized (randomization 2:1), multicenter, phase II clinical trial. The purpose of the study was to evaluate the ability of sunitinib consolidation therapy (arm A) to delay tumor progression in the setting of reduced tumor burden following induction chemotherapy. Arm B (no therapy) was used as control.
Patient population
Eligible patients had histologically proven, HER2-negative, measurable metastatic breast cancer at the start of taxane therapy. Patients achieving a PR or CR according to RECIST criteria after completing taxane-based therapy were included. No more than two lines of chemotherapy in the metastatic setting (with taxanes included as last line) were allowed. Patients received four to six cycles of taxane therapy on a three-weekly schedule or 8–18 doses on a weekly schedule. The recommended taxane schedules were six cycles every 3 weeks (6 doses over 18 weeks) or 12–16 weekly doses. Hormonal therapy (tamoxifen, aromatase inhibitors, or other hormone suppressing therapies) was not allowed during the study period.
All patients gave written informed consent, and the study was approved by the ethics committees of the participating institutions.
Randomization
Eligible patients were randomly allocated to arm A (sunitinib) or arm B (control) between 3 and 5 weeks after the last three-weekly taxane administration or between 2 and 4 weeks after completing weekly taxane therapy. Patients were stratified according to the institution, disease-free interval (0 = primary metastatic disease; 0–2 years; 2–5 years; >5 years), dominant site of disease (non-visceral, single, or multiple visceral sites), and response to taxanes (CR or PR). Chemotherapy and hormone therapy were not allowed in arm B during the study period. At the time of disease progression, patients in arm B were allowed to cross over to sunitinib.
Dosing
In arm A, sunitinib was initially administered orally at a daily dose of 50 mg for 4 weeks followed by a drug-free interval of 2 weeks (1 cycle = 6 weeks). However, the protocol was amended on January 25, 2008 in light of new data showing tumor regrowth during the 2-week interval in sunitinib dosing [1], and the occurrence of toxicity in the first cohort of 32 patients treated with 50 mg sunitinib in arm A or after progression in arm B. Following the protocol amendment, sunitinib was given orally at a dose of 37.5 mg/d continuously in 6-week cycles. Continuous once-daily administration of sunitinib 37.5 mg has demonstrated very similar activity to sunitinib 50 mg/d on the 4/2 week intermittent schedule [2]. In addition, the manageable safety profile of continuous dosing provides additional flexibility in dose scheduling. The reduced, continuous-dose regimen has been adopted as the regimen of choice in subsequent phase III studies.
Sunitinib was continued until disease progression or for as long as either the patient or the treating oncologist considered continuation of therapy to be in the patient’s best interest. The dosing schedule in arm A was also used for patients from arm B starting sunitinib after progression.
Sunitinib was interrupted temporarily in patients experiencing severe treatment-related toxicity and restarted at a reduced dose (no lower than 25 mg) if appropriate.
Study procedures
Radiological assessments of tumors were performed at baseline and in the week before starting new cycles of sunitinib. After four cycles, imaging studies were repeated every two cycles until disease progression or withdrawal from the study. Imaging studies were also performed to confirm suspected disease progression.
Endpoints
The primary endpoint was the proportion of patients alive and progression-free 5 months after starting sunitinib. The trial was not powered to allow formal comparison between the investigational arm and the control arm (which was intended for descriptive purposes only). Secondary endpoints included other measures of antitumor efficacy (overall survival, OS) in both treatment arms and the safety and tolerability of sunitinib.
Progression-free survival (PFS) was defined as the time from randomization to first documentation of objective tumor progression, or to death from any cause. PFS data were censored on the day following the date of the last tumor assessment documenting the absence of progressive disease. Toxicity and tolerability were graded according to the National Cancer Institute, Common Terminology Criteria for Adverse Events (version 3.0). All patients who received at least one dose of study treatment were evaluated for efficacy, toxicity, and safety.
The total duration of taxane chemotherapy allowed was approximately 10–20 weeks (4–6 cycles of three-weekly therapy or 8–18 doses of weekly therapy). A longer duration of taxane therapy was not allowed because of inadequate data to support an improvement in outcome with prolonged chemotherapy in a palliative setting in patients with metastatic breast cancer [3]. Moreover, prolongation of chemotherapy is associated with increased toxicity. Based on data from historic studies, the duration of taxane response was estimated to be about 7 months, depending on the type of taxane (paclitaxel or docetaxel) and the clinical setting (first- or second-line treatment of metastatic disease) [4–12]. In practice, taxanes are generally continued for a few months after an objective response is achieved, and therefore, we estimated the median PFS after discontinuation of taxane therapy would be 5 months in these patients.
Statistics
Evaluable patients were classified as having met the primary endpoint if they were alive and free of tumor progression 5 months after starting study therapy. The study was designed as a one-stage, three-outcome phase II trial [13]. The cutoff values for ineffectiveness and effectiveness were 45 and 65%, respectively. The type I (α) and type II (β) error rates were set at 0.05. Therefore, 36 patients treated with sunitinib were required for the study. If 18 patients or fewer were progression-free and alive at 5 months, sunitinib would be declared insufficiently active, whereas, if at least 22 patients were progression-free and alive at 5 months, sunitinib would be declared active and continuation of the trial using a phase III design would be recommended. If, however, between 19 and 21 patients were progression-free and alive at 5 months, no formal statistical conclusion would be possible. The control group of 18 patients receiving no treatment was intended for descriptive purposes (no formal comparison was planned).
Results
Patient population
A total of 56 patients were enrolled between February 7, 2006 and March 17, 2009. Patient and treatment characteristics are shown in Table 1. One patient randomized to receive sunitinib progressed before starting treatment and died a few weeks later. The remaining 55 patients were evaluated for efficacy and toxicity. To four patients in arm A (11%) and two in arm B (11%), the taxane regimen leading to objective response was administered for a duration longer than the protocol-specified 20 weeks (range 22–27 weeks); however, the analysis was intent to treat, the differences were balanced, and median number of taxane administrations was identical in both arms. Two patients in arm A who had received two lines of chemotherapy before taxane-induced objective response were also included in the analysis.
Efficacy
The primary endpoint (progression-free at 5 months) was achieved by 10/36 patients (28%) in arm A and 4/19 (21%) in arm B. Median PFS was 2.8 months in arm A and 3.1 months in arm B. Kaplan–Meier curves for PFS and OS are shown in Fig. 1.
In arm B, 12/19 patients received crossover treatment with sunitinib after progression. The response rate was 25% in this group.
Toxicity
The main side effects in both arms of the study are described in Table 2.
During the study, new data emerged about a dosing schedule of sunitinib 37.5 mg continuously instead of sunitinib 50 mg 4 weeks on, 2 weeks off. Since we observed significant grade III toxicity and dose reductions in the first 32 patients treated with the intermittent 50 mg dosing schedule, a protocol amendment was made in January, 2008 to change the starting dose of sunitinib to 37.5 mg continuously. Sunitinib dose reductions were required in 53% (17/32) of patients treated at a starting dose of 50 mg and in 44% (7/16) treated with the continuous dosing schedule.
Dose reductions and delays
Before the dosing amendment, 26 patients in arm A and six from arm B after crossover received sunitinib at 50 mg. Of the patients treated with the 50 mg dosing schedule, 47% (15/32) received one cycle of sunitinib, 25% two cycles, 13% three cycles, and 15% four or more. In addition, 53% (17/32) required at least one dose reduction and 63% required at least one dose interruption.
After the protocol amendment, ten patients in arm A and six from arm B after crossover received sunitinib 37.5 mg continuously. Of patients treated with the continuous schedule, 44% (7/16) received 1 cycle of sunitinib, 25% two cycles, 0% three cycles, and 31% four or more. At least one dose reduction was required in 47% (7/16) and at least one dose interruption in 56%.
Discussion
In this study, we tested the hypothesis that an antiangiogenic agent such as sunitinib can significantly prolong PFS following a meaningful reduction in tumor mass (PR or CR) induced by chemotherapy (taxanes). It was hypothesized that antiangiogenic agents are most effective in the setting where the tumor has been reduced and regrowth occurs after stopping chemotherapy. It is thought that neoangiogenesis is required for tumor regrowth after cytoreduction and, therefore, blocking angiogenesis could lead to prolonged tumor control. Unfortunately, the results of this proof-of-principle study do not support this hypothesis. This was not a phase III trial comparing sunitinib consolidation therapy with placebo and no formal comparison can be made between study arms. However, the median PFS with sunitinib in a consolidation setting was only 2.8 months, and only 28% of patients achieved the 5-month PFS endpoint, which was far below the initially defined and presumed clinically relevant cutoff of at least 45% and preferably more than 65%. It should be acknowledged that median PFS in the control arm (3.1 months) was also lower than hypothesized based on previous studies (5 months). The Kaplan–Meier curve for PFS seems to indicate that the curves slightly diverge after 4 months, but there were too few patients with prolonged disease control in the sunitinib arm to allow any definitive conclusions to be made. One of the major challenges with angiogenesis inhibitors is the lack of reliable predictive biomarkers to select patients most likely to benefit.
Another important consideration is the significant toxicity of sunitinib, which resulted in dose reduction in more than 50% of patients. Following the protocol-specified dose amendment, 47% of patients still required dose reduction. This degree of dose reduction appears to be relatively higher than rates reported for single-agent sunitinib in other settings, such as renal cancer [14]. It can be hypothesized that cumulative toxicity associated with 3–4 months of taxane therapy only partially resolved and that side effects were exacerbated when sunitinib was started. Fatigue is a known side effect of taxanes and the main toxicity associated with sunitinib. It is possible, therefore, that sequential administration of taxanes and sunitinib leads to exacerbation or accumulation of fatigue.
Although no patient discontinued sunitinib permanently before progression, a large proportion of patients required dose interruptions, sometimes prolonged, before a lower dose could be restarted. In non-clinical models, dose interruption of antiangiogenic agents can lead to reactivation [15] and even acceleration of angiogenesis. It is conceivable that such a scenario may have happened in this study population. On the other hand, a recent preliminary analysis of the AVADO trial did not show any accelerated growth after bevacizumab was discontinued, suggesting that metastatic spread does not accelerate in breast cancer after stopping antiangiogenic agents [16]. The hypothesis that continuous and sustained inhibition of angiogenesis inhibition can prolong PFS without causing excessive toxicity could be tested with sunitinib administered at a lower starting dose (e.g., 25 mg daily continuously) or with other angiogenesis inhibitors. It is important to answer this question definitively because continuous inhibition of angiogenesis after induction chemotherapy combined with antiangiogenic therapy is considered to be standard practice in all previous and ongoing studies. While it has not been shown that this approach is effective, it is evident that toxicity is clinically relevant with some classes of agent, and the cost of therapy is high. Furthermore, this study does not support the concept of consolidation antiangiogenic therapy in patients with metastatic breast cancer responding to taxanes.
In addition to insufficient dose intensity and possible regrowth of tumor during interruption of sunitinib treatment, there are several other reasons for the lack of efficacy observed. For instance, alternative angiogenic pathways can be activated, or tumors can obtain oxygen and nutrients through co-option of blood vessels. Liver metastases were present in the large majority of patients in this study, and it has been clearly described that such tumors can grow in a liver replacement pattern by exploiting the existing liver blood supply [17].
Another plausible explanation for the lack of important single-agent activity of sunitinib in breast cancer, in contrast to renal cell cancer, is that there is no documented constitutive activation of the VEGF pathway. Moreover, there is no proof that other sunitinib targets (PDGF, c-KIT) are activated in breast cancer. It seems that in breast cancer (in contrast to renal cancer and GIST), antiangiogenic drugs work essentially in combination with chemotherapy, as shown by the E2100 [18], AVADO, and RIBBON trials. One of the possible mechanisms for this phenomenon is normalization of the tumor vasculature, thereby increasing chemotherapy delivery to the tumor site [19]. Response rates with bevacizumab [20] and sunitinib [1] monotherapy have been around 10% in pretreated patients. A recent randomized phase III study [20] showed that the PFS for sunitinib (2.8 months) was lower than for capecitabine (4.2 months) in patients with metastatic breast cancer failing anthracyclines and taxanes. The latter study suggests that antiangiogenic drugs should be used and further developed in combination with chemotherapy. Furthermore, such findings bring into question the use of single-agent antiangiogenic drugs in the adjuvant setting, which is being assessed in ongoing phase III studies. Two further phase III trials of sunitinib in patients with metastatic breast cancer were presented at ASCO 2010. In a first-line study, the addition of sunitinib to docetaxel significantly improved response rate (51% compared with 39%), but did not improve PFS, the primary endpoint of the study (hazard ratio 0.92; median 8.6 vs. 8.3 months) [21]. In the setting of second-line therapy or beyond, the addition of sunitinib to capecitabine did not improve PFS or response rate [22]. It is remarkable to observe in our study that 3/12 patients had an objective response to sunitinib after progression in the control arm (crossover). Although numbers are very small, this finding is consistent with the study of Burstein and colleagues [1] that showed an objective response rate of 11% for single-agent sunitinib. It appears that sunitinib has antitumor activity in a minority of patients, but this does not lead to improved PFS for the majority of patients. There is a great need to find biomarkers to predict the efficacy of antiangiogenic therapy. It is possible that antiangiogenic drugs like sunitinib are active within a small, specific subgroup of patients. Nevertheless, accumulated data from this study and other recent clinical trials do not support the use of sunitinib in the treatment of breast cancer.
References
Burstein HJ, Elias AD, Rugo HS et al (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26(11):1810–1816
George S, Blay JY, Casali PG et al (2009) Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45(11):1959–1968
Gennari A, Amadori D, De LM et al (2006) Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol 24(24):3912–3918
Sledge GW, Neuberg D, Bernardo P et al (2003) Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 21(4):588–592
Davidson NG (1996) Single-agent paclitaxel as first-line treatment of metastatic breast cancer: the British experience. Semin Oncol 23(5 Suppl 11):6–10
Gianni L, Munzone E, Capri G et al (1995) Paclitaxel in metastatic breast cancer: a trial of two doses by a 3-hour infusion in patients with disease recurrence after prior therapy with anthracyclines. J Natl Cancer Inst 87(15):1169–1175
Fountzilas G, Athanassiadis A, Kalogera-Fountzila A et al (1997) Paclitaxel by 3-h infusion and carboplatin in anthracycline-resistant advanced breast cancer. A phase II study conducted by the Hellenic Cooperative Oncology Group. Eur J Cancer 33(11):1893–1895
Vici P, Di LL, Conti F et al (1997) Paclitaxel activity in anthracycline refractory breast cancer patients. Tumori 83(3):661–664
Seidman AD, Tiersten A, Hudis C et al (1995) Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol 13(10):2575–2581
Ravdin PM, Burris HA III, Cook G et al (1995) Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol 13(12):2879–2885
Valero V, Jones SE, Von Hoff DD et al (1998) A phase II study of docetaxel in patients with paclitaxel-resistant metastatic breast cancer. J Clin Oncol 16(10):3362–3368
Paridaens R, Biganzoli L, Bruning P et al (2000) Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European organization for research and treatment of cancer randomized study with cross-over. J Clin Oncol 18(4):724–733
Sargent DJ, Chan V, Goldberg RM (2001) A three-outcome design for phase II clinical trials. Control Clin Trials 22(2):117–125
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124
Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15(3):232–239
Harbeck N, Chan A, ten Bokkel Huinink D et al. (2009) No clinical evidence for increase in tumour malignant potential in patients (Pts) with metastatic breast cancer (mBC) treated with bevacizumab (BV) and docetaxel (D) in the phase III AVADO study. San Antonio Breast Cancer Symposium; abstract no. 6089
Stessels F, Van den Eynden G, Van der Auwera I et al (2004) Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90(7):1429–1436
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676
Wildiers H, Guetens G, De BG et al (2003) Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 88(12):1979–1986
Cobleigh MA, Langmuir VK, Sledge GW et al (2003) A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30(5 Suppl 16):117–124
Bergh J, Greil R, Voytko N, Makhson A, Cortes J, Lortholary A, Huang X, Giogettie C, Kern KA, Lichinitser M (2010) Sunitinib (SU) in combination with docetaxel (D) versus D alone for the first-line treatment of advanced breast cancer (ABC). Proc Am Soc Clin Oncol. LBA1010
Crown J, Dieras V, Staroslawska E, Yardley DA, Davidson N, Bachelot TD, Tassell VR, Huang X, Kern KA, Romieu G (2010) Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC). Proc Am Soc Clin Oncol. LBA1011
Acknowledgments
We would like to thank all the participating patients, all the centers who contributed to the study, and Liza Van Eenoo who helped in the coordination of the study and data collection. This study was supported by an unrestricted grant by Pfizer N.V. and used for the performance of this study.
List of recruiting centers and principal investigators: University Hospitals Leuven (Robert Paridaens and Hans Wildiers), Imelda Bonheiden (Wim Wynendaele), AZ St Jan Brugge (Nele Claes), Ziekenhuis Oost Limburg (Jeroen Mebis, Daisy Luyten, Guy Debrock), St-Elisabeth Turnhout (Marc Martens), St-Elisabeth Namen (Peter Vuylsteke and Jean-Charles Goemine), UZ Gent (Hannelore Denys and Veronique Cocquyt), St-Niklaas (Willem Lybaert), Charleroi (Jean-Luc Canon), UZ Brussel (Christel Fontaine and Jacques De Grève), CHC-Liège (Drs C. Focan, MP Graas, F. Kreutz), CHU Sart Tilman Liège (Guy Jerusalem), and Clinique St-pierre Ottignies (Lionel Duck).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed under the auspices of the Belgian Society of Medical Oncology.
Rights and permissions
About this article
Cite this article
Wildiers, H., Fontaine, C., Vuylsteke, P. et al. Multicenter phase II randomized trial evaluating antiangiogenic therapy with sunitinib as consolidation after objective response to taxane chemotherapy in women with HER2-negative metastatic breast cancer. Breast Cancer Res Treat 123, 463–469 (2010). https://doi.org/10.1007/s10549-010-1066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1066-x