Abstract
Stroma tissue surrounding cancer cells plays an important role in tumor development and behavior. In colorectal cancer, it has been found that the amount of stroma within the primary tumor is of prognostic value. We therefore have evaluated the prognostic value of this tumor–stroma ratio for breast cancer. A cohort of 574 early breast cancer patients, primarily treated with surgery between 1985 and 1994 was analyzed for the tumor–stroma ratio. The percentage of stroma was visually estimated on Haematoxylin-Eosin (H&E) stained histological sections. Patients with more than 50% intra-tumor stroma were quantified as stroma rich and patients with less than 50% as stroma poor. For the total group of patients, stroma-rich tumors had a shorter relapse-free period (RFP) (P = 0.001) and overall survival (OS) (P = 0.025) compared to stroma-poor tumors. Tumor–stroma ratio was an independent prognostic parameter for the total group of patients (P < 0.001) and also in stratified analysis based on systemic treatment. Importantly, in the triple-negative cancer subpopulation, patients with stroma-rich tumors had a 2.92 times higher risk of relapse (P = 0.006) compared to those with stroma-poor tumors, independently of other clinico-pathological parameters. Five-year RFP-rates for triple-negative cancer patients with stroma-rich compared to stroma-poor tumors were 56 and 81%, respectively. Tumor–stroma ratio has proven to be an independent prognostic factor for RFP in breast cancer patients and especially in the triple-negative cancer subpopulation. Tumor–stroma ratio could be easily implemented in routine daily pathology diagnostics, as it is simple to determine, reproducible, and performed in quick time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since current prognostic and predictive factors for breast cancer do still not provide optimal risk stratification, additional information is necessary for improved tailored treatment for the individual patient.
In a recent review, four main molecular classes of breast cancer were reported, which have been identified by gene expression profiling [1]. According to the classification of Perou et al., these four classes are proposed as basal-like type tumors, luminal cancers (A and B), and Her-2 positive cancers. These subgroups correspond reasonably well to the clinical characterization on the basis of hormone receptor status, HER2 expression, and differentiation level [2]. At least 15% of the breast cancers are basal-like, of which the majority comprises the so-called triple-negative cancers [3]. Triple-negative breast cancer is a subtype of invasive breast cancer defined as estrogen receptor (ER)-negative, progesterone receptor (PgR)-negative, and HER-2-negative phenotypes. These triple-negative breast tumors are more likely to affect younger women and are associated with a more advanced stage. Regardless of the stage at diagnosis, women with triple-negative breast cancers have a worse outcome compared to other breast cancer subtypes [4]. Currently, there are no specific treatment guidelines for triple-negative breast cancer [5]. At this moment, neither currently used clinico-pathological parameters nor molecular profiling techniques are able to subdivide this set of patients with respect to prognosis [1, 6, 7]. Therefore, there is a strong need to develop additional parameters for this subgroup.
Metastasis is the leading cause of morbidity and mortality in early breast cancer patients [8]. Tumor invasion and metastasis are considered to be a multifactor process involving complex interactions of biological pathways [9]. Tumor-associated stroma and cancer-associated fibroblasts may play an important role in these tumor progression phases [10]. It is hypothesized that processes similar to wound-healing response are activated, which results in cell motility, angiogenesis, and matrix remodeling. In fact, for breast cancer, a wound-response gene expression signature, a fibromatosis signature, and a stromal signature have been found to be predictors of patient outcome and tumor progression [10–13]. These data suggest that stroma in the vicinity of tumors undergoes, or may be affected by, changes during tumor progression. A recent study of our group further supports this hypothesis. In this study, tumors with distinct patterns of intra-tumor stromal percentage were identified in a population of colon cancer stage II patients who had not received chemotherapy or radiotherapy. We defined two groups, stroma-low (<50%) and stroma-high (≥50%) patients, showing statistically significant differences in survival [14, 15]. In this study, we hypothesize that tumors with a high stroma production (stroma-rich tumors) may reflect poor tumor biology and thus a worse outcome. We have evaluated the prognostic value of this stroma parameter in a set of 574 breast cancer patients. Analyses were stratified for adjuvant systemic therapy to correct for possible interactions and furthermore for the triple-negative cancer patients to determine the prognostic value of the tumor–stroma ratio within this subset of patients.
Materials and methods
Study population
In a retrospective cohort study, patients were included with non-metastatic invasive breast cancer who were primarily treated with surgery in the Leiden University Medical Center (LUMC) between 1985 and 1994 (n = 677). Patients with a history of cancer (other than basal cell carcinoma of the skin or in situ carcinoma) or bilateral tumors were excluded. The following data were available: age, tumor grade, histological type, TNM stage, local and systemic therapy, locoregional/distant recurrence, second primaries, and overall survival. Expression of ER, PgR, Ki67, and HER2 were a priori centrally determined according to standard diagnostic procedure, using standard histological staining protocols and automated microscopy determining the histoscore for quantification. The historscore considers both intensity and percentage of stained cells and, therefore, is considered to be a very complete method for quantification. In addition, all tumors were histologically graded according to current pathological standards by a pathologist (VS).(van Nes JGH, de Kruijf EM, Faratian D, van de Velde CJH, Putter H, Falconer C, Smit VTHBM, Kay C, van de Vijver MJ, Kuppen PJK, Bartlett JMS COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Accepted for publication) Systemic treatment criteria of breast cancer patients from 1985 to 1994 were as follows: endocrine tamoxifen treatment was administered to patients with positive lymph node metastasis; chemotherapy was given to younger patients with larger and higher grade tumors and more positive lymph node metastasis. Approval was obtained from the Leiden University Medical Center Medical Ethics Committee. All the samples were handled in a coded fashion, according to National ethical guidelines (“Code for Proper Secondary Use of Human Tissue”, Dutch Federation of Medical Scientific Societies). The REMARK criteria were respected for analyses of the tumor–stroma ratio and writing of this article [16].
Staining and evaluation
Formalin-fixed paraffin-embedded (FFPE) tumor blocks of the primary tumor were collected from pathology archives. Sections were cut and stained with Haematoxylin and Eosin (H&E) according to standard histological protocols. Tumor–stroma ratio was quantified as described before [14]. Using a 5× objective, the most invasive tumor area of the whole tissue slide was selected. Subsequently, using a 10× objective, only fields were scored where both stroma and tumor were present and, most importantly, tumor cells were seen on all sides of the microscopical image field (north–east–south–west). The tumor–stroma ratio was visually estimated in a blinded manner by two investigators (EDK, JVN) and scored per tenfold percentage (10, 20, 30% etc.). In case of heterogeneity, the highest stromal percentage was considered decisive.
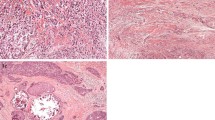
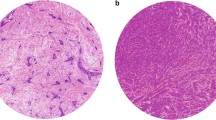
In some cases of necrosis, mucus-forming tumors or tumors with compartments of ductal carcinoma in situ (DCIS), quantification of the tumor–stroma ratio was more difficult, but still possible. In these cases, quantification was performed by ignoring these compartments (by visual eye balling) when determining the stroma percentages. Representative examples of microscopical fields selected for tumor–stroma ratio quantification from stroma-rich and stroma-poor tumors are shown in Fig. 1.
Statistical analysis
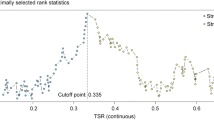
Data were analyzed using the statistical package SPSS for Windows 15.0 (SPSS Inc, Chicago, IL, USA). Cut-off of the tumor–stroma ratio was taken as 50% as previously determined in colon cancer by maximum discriminative power, which was also confirmed in this breast cancer population (supplementary Table 1) [14]. Intra-observer variability was analyzed using the Cohen’s kappa coefficient. Relationship between tumor–stroma ratio and well-established factors was investigated with the chi-squared test. The Kaplan–Meier method and log-rank test were used for analysis and comparison of survival curves. Relapse-free period rates were reported as cumulative incidence functions, after accounting for death as competing risk [17]. Cox regression was used for univariate and multivariate analyses of relapse-free period (RFP) and overall survival (OS). For all the models, we performed a link test for possible interaction. In addition, goodness of fit and proportional hazard assumptions were checked with COX–Snell residuals and Schoenfeld residuals, respectively. Variables included in multivariate analysis were all those variables which were of influence on outcome of patients in univariate analysis (P < 0.1). RFP was the time of date of surgery until a relapse (locoregional or distant relapse); OS was the time of date of surgery until death. Stratifications based on adjuvant treatment, to account for adjuvant therapy effects on prognosis of patients, and on triple-negative breast carcinoma patients were performed in survival analyses.
Results
Clinico-pathological features
The FFPE blocks were available from pathology archives for 86% (574/677) of the patients. No statistically significant differences in clinico-pathological parameters were seen between patients with and without available FFPE blocks. Median age of patients was 57 years (23–96) and the median follow-up time was 19 years (14–24 years) of patients alive. Clinico-pathological, local/systemic treatment, and outcome characteristics of these patients are shown in Table 1.
Tumor–stroma ratio in breast cancer
Routine H&E-stained slides from the most invasive part of the tumor were microscopically analyzed for the presence of stromal involvement using a 5× and a 10× objective. We observed areas with abundant stroma (stroma-rich) with a size as large as one microscopic field (10× objective, 100× total magnification), but also larger areas matching 2–4 fields were seen or even more, independent of the size of the tumor. Estimation of the tumor–stroma ratio was performed successfully in all the tumors (100%). Cohen’s kappa coefficient revealed an almost perfect agreement in classification (kappa = 0.85; 94% concordance in classification) for a set of tumors (32% of total set) scored by both observers.
Tumor–stroma ratio and prognostic associations with outcome
Out of the whole cohort (n = 574), 264 (46%) patients had a relapse of disease, and 370 (54%) patients deceased. A total of 388 (68%) patients were classified as stroma-rich and 186 (32%) patients as stroma-poor. A worse RFP (Hazard ratio (HR) 1.62; 95% CI 1.23–2.13; P = 0.001) (Table 2) and OS (HR1.29; 95% CI 1.03–1.60; P = 0.025) (supplementary Table 2) were found for patients with stroma-rich tumors as compared to patients with stroma-poor tumors, with 5 year RFP and OS of, respectively, 64 vs. 79%, and 71 vs. 83% (Fig. 2a, b). In multivariate analysis, the tumor–stroma ratio was an independent prognostic variable for RFP (HR 1.97; 95% CI 1.47–2.64; P < 0.001) (Table 2) and OS (HR: 1.50; 95% CI 1.18–1.91; P = 0.001) (supplementary Table 2) independent of other clinico-pathological parameters.
Next, in order to account for systemic therapy effects, subanalyses were performed based on systemic therapy administration. In the group of patients that received only local therapy, 66% (244/369) had a stroma-rich tumor, and 34% (125/369) a stroma-poor tumor. Patients with a stroma-rich tumor showed a worse clinical outcome for RFP (HR 1.55; 95% CI 1.10–2.18; P = 0.012) and OS (HR 1.25; 95% CI 0.97–1.62; P = 0.098), with 5 year RFP of, respectively, 66 and 81%, and OS of 76 and 82%. In multivariate analysis, tumor–stroma ratio was an independent parameter for RFP (HR 2.06; 95% CI 1.42–2.97; P < 0.001).
The prognostic influence of tumor–stroma ratio in patients treated with chemotherapy (130/574) and endocrine therapy (93/574) was determined. Within the patients treated with chemotherapy, 68% (88/130) had stroma-rich tumors and 32% (42/130) stroma-poor tumors. Patients with a stroma-rich tumor showed a trend toward a worse outcome for RFP (HR 1.66; 95% CI 0.94–2.93; P = 0.082) and OS (HR 1.72; 95% CI 0.99–2.98; P = 0.054), with 5 year RFP of respectively 63 and 79%, and OS 65 and 83%. In multivariate analysis, tumor–stroma ratio showed to be of independent influence on RFP (HR 1.83; 95% CI 1.04–3.25; P = 0.038).
In patients treated with endocrine therapy, stroma-poor and stroma-rich tumors were found in 71% (66/93) and 29% (27/93) of the tumors, respectively. Associations with outcome were similar to those found for the chemotherapy-treated patients. Patients with stroma-rich tumors showed a trend toward a significantly worse RFP (HR 1.98; 95% CI 0.95–4.11; P = 0.068) and OS (HR 1.72; 95% CI 0.99–2.98; P = 0.054), with 5 year RFP of, respectively, 58 and 70%, and OS of 61 and 89%. In a multivariate analysis for RFP, both tumor stage and tumor–stroma ratio (HR 2.59; 95% CI 1.13–5.91; P = 0.024) resulted as independent prognostic factors.
For all subanalyses based on systemic treatment, interaction models were introduced in a Cox regression analysis. These analyses revealed that there was no statistically significant interaction between tumor–stroma ratio and systemic therapy (P = 0.684), chemotherapy (P = 0.916), and endocrine therapy (P = 0.506). These results further indicated that the effect on clinical outcome of tumor–stroma ratio was unrelated to systemic therapy.
Tumor–stroma ratio and prognostic associations with outcome in triple-negative breast carcinomas
Of the set of patients with a triple-negative breast carcinoma (n = 82) 35 (43%) patients had a relapse of disease, and 55 (67%) patients deceased. Of these patients, 56% (46/82) had a stroma-rich tumour, and 44% (36/82) stroma-poor. Patients with stroma-rich tumors had a statistically significant worse outcome compared to patients with stroma-poor tumors for RFP (HR 3.91; 95% CI 1.49–6.83; P = 0.003) (Table 3) and showed a trend toward a worse survival for OS (HR 1.60; 95% CI 0.93–2.74; P = 0.088) (supplementary Table 3), with 5 year RFP and OS of respectively 56 vs. 81% and 44 vs. 83% (Fig. 3a, b). In a multivariate analysis, grade, nodal status, and tumor–stroma ratio were of independent influence on RFP (tumor–stroma ratio: HR 2.92; 95% CI 1.36–6.32; P = 0.006) (Table 3). When corrected for other clinico-pathological parameters, patients with stroma-rich tumors had a statistically significant worse OS compared to stroma-poor tumors (HR 1.87; 95% CI 1.07–3.26; P = 0.028) (supplementary Table 3).
Kaplan–Meier graphs for tumor–stroma ratio for triple-negative carcinoma patients. Patients with stroma-rich tumors show a significant worse relapse-free period (a) compared to patients with stroma-poor tumors. A trend was seen toward a worse survival for patients with stroma-rich tumors compared to patients with stroma poor tumors (b)
Triple-negative carcinoma patients treated with local therapy only showed similar results for influence of tumor–stroma ratio on RFP (HR 4.12; 95% CI 1.49–11.39; P = 0.006). Five-year RFP for patients with stroma-rich compared to stroma-poor tumors was, respectively, 58 and 88%. The tumor–stroma ratio was independent of all other variables, since it was the only parameter which met multivariate inclusion criteria.
Tumor–stroma ratio remained of independent influence on outcome in patients in the non-triple-negative group, when corrected for other clinico-pathological parameters (RFP: HR 1.50 95% CI 1.09–2.07 P = 0.013).
An interaction model for tumor–stroma ratio with triple-negative cancer patients was introduced in Cox regression analysis. This revealed a significant interaction between the two variables on clinical outcome (P = 0.018), further supporting our hypothesis that a relationship between tumor–stroma ratio and triple-negative cancer exists.
For all Cox regression models, we performed a link test. There seems to be no interaction in the models: For OS, the whole patient cohort and triple-negative group link test P-values were, respectively, P = 0.6 and P = 0.9; For RFP, the whole patient cohort, patients only treated with local therapy, chemotherapy-treated patients, endocrine-treated patients, all triple-negative cancer patients, and triple-negative cancer patients who received only local therapy, link test P-values were, respectively: 0.05, 0.2, 0.2, 0.5, 0.9, and 0.9. Besides this, we checked for goodness of fit visually with COX–Snell residuals; all the models showed to have a good model fit. Finally, we checked for the proportional hazards assumption based on the Schoenfeld residuals for the models with patients who received systemic therapy (chemotherapy treated: P = 0.2; endocrine treated: P = 0.3) and triple-negative patients (P = 0.6) for RFP, and found no evidence that the models violated the proportional hazards assumption.
Discussion
Our study shows that the tumor–stroma ratio is an independent prognostic factor for breast cancer patients. Stroma-rich tumors were associated with an increased risk of relapse. Importantly, for the triple-negative breast carcinoma patient group, a group for which no definite prognostic biomarkers are available, the tumor–stroma ratio appeared to be a prognostic parameter which significantly correlates with a better 5 year relapse-free period rate of 81% (stoma-poor) compared to 56% (stroma-rich) [7]. In addition, tumor–stroma ratio showed the same prognostic power for patients treated with endocrine and chemotherapy, indicating that the prognostic effect was independent of systemic therapy. Importantly, high intra-observer agreement Kappa values for tumor–stroma ratio, as found in this study and prior studies on colorectal cancer and esophageal cancer, convincingly demonstrate that tumor–stroma ratio is a highly reproducible method. For our breast cancer population, Cohen’s kappa coefficient was 0.85. For colorectal cancer, kappa values of three different pathologists’ intra-observer agreements varied between 0.596 and 0.702 [14, 15]. For esophageal cancer, Cohen’s kappa coefficient was 0.859 [18]. These kappa values can be translated as almost to be in perfect agreements. In addition, the tumor–stroma ratio can be easily assessed and provides no additional costs above standard diagnostics [15]. Therefore, we believe that the tumor–stroma ratio may be a candidate parameter to be implemented as a standard procedure within pathology laboratories.
Many prognostic and predictive factors have been found for breast cancer. The ASCO guidelines advice the use of different prognostic and predictive factors in clinical practice: ER, PgR, HER2, urokinases plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1), and gene profiles detected with multiparameter gene expression assays [19]. ER, PgR, and HER2 are well known parameters and are determined by the use of immunohistochemistry. For ER and PgR, different antibodies and quantification methods (i.e., percentage of positive stained cells, Allred score, and histoscore) are used, which are reported with variable results [20]. For the determination of HER2 expression, many different antibodies are utilized in clinical practice, which may result in a broad aspect of results [21]. However, because of the great prognostic and predictive effect of HER2 and the many side-effects and high cost of trastuzumab therapy, accurate determination of HER2 overexpression in tumors is of crucial importance. UPA and PAI-1 markers are still subject of debate and currently evaluated in the prospective Node-Negative Breast Cancer III (NNBC 3) Europe Trial [22]. Microarray-based prognostic tools, like the MammaPrint, a 70-gene expression profile, and Oncotype DX, a 21-gene expression profile, can be used for prognostication of breast cancer patients according to the ASCO guidelines [23]. However, the clinical value of gene expression profiling is currently being debated and under investigation [24, 25]. Recent evidence shows that gene profiles do not outperform prognostication with current clinico-pathological parameters [26, 27]. Evidence showing that complex gene signatures quantify evident tumor characteristics, such as tumor grade, ER, HER2, cell cycle, and cell proliferation, is accumulating [26, 28–30]. Moreover, it is noteworthy that gene expression arrays are not suitable for all tumors. Frozen material is often needed, and when applying gene expression array analyses for tumor tissue, it is common practice to select those parts of the tissue in which tumor cells form the major component, as admixtures of abundant stroma and inflammatory cells will lead to masking of amplifications and deletions. This may lead to exclusion of tumors with a high percentage of stroma (>50%) for gene expressionarray analysis, as these samples do not meet the criteria for a reliable array. Our study shows that a high percentage of intra-tumor stroma results in a worse outcome for patients, and obviously this set of patients is not eligible for gene expression analysis. Therefore, we suspect that arraying techniques may form a selection bias for patients with a better prognosis and that the tumor–stroma ratio provides additional prognostic information to gene expression profiles. Adjuvant! Online (http://www.adjuvantonline.com) is another tool to quantitatively estimate the prognosis and response to systemic treatment of patients with early breast cancer. It is based on age, ER status, histological grade, tumor size, and lymph node status. Although Adjuvant! Online is a useful method to predict the prognosis of disease and benefit of treatment, it needs more validation and modification because it is too optimistic for subgroups enriched with adverse prognostic factors such as HER2 overexpression and negative PgR [31]. Our tumor–stroma ratio parameter was of prognostic value independently of all parameters on which Adjuvant! Online is based and is, therefore, a candidate parameter to prognostication in addition to this digital tool.
The use of systemic therapy for triple-negative breast cancer patients remains controversial. Triple-negative carcinomas, in general, have a relatively poor prognosis, but are a heterogeneous group [5]. In the triple-negative carcinomas, a subgroup of patients has been associated with improved prognosis which could not be identified by gene expression-based prognostic tools [1, 6, 7]. According to a meta-analysis of publicly available gene-expression and clinical data, all gene-expression signatures showed to have the best performance on survival prediction for ER-positive tumors, whereas they were not significantly of influence on outcome for tumors with HER2 overexpression and triple-negative carcinomas [30]. In this article we show that the tumor–stroma ratio is a strong independent prognosticator for triple-negative carcinoma patients.
The majority of studies of neoplastic transformation have focused on events that occur within cancer cells. Other studies have addressed the microenvironment of tumor cells supporting tumor progression [12, 13, 32]. Recent study now provides more insight into possible initiation and progression of malignant cells. The phenomenon of tumor–stroma ratio might be explained by biological processes involved with breast cancer invasion and metastasis. Various mechanisms underlie these processes, including loss of cell adhesion, proteolysis, matrix remodeling, and cytoskeletal rearrangements. Increasing evidence supports the role of the tumor microenvironment (i.e., fibroblasts, myoepithelial cells, macrophages, proteases etc.) in stimulating tumor progression, invasion, and metastasis [32]. Increase in abundance of fibroblasts in a tumor causes deposition of fibrotic extra-cellular matrix (ECM). Changes in ECM structure can be further stimulated by proteases, which degrade stroma. Together, this results in disruption of epithelial tissue and remodeling of the ECM, facilitating invasion of tumors cells. In addition, fibroblasts secrete growth factors, angiogenic factors, and inflammatory factors, which all contribute to tumor progression and expansion [33, 34]. In vivo studies on cell lines and in mice support this theory of cancer-associated fibroblasts. In prostatic tumors and breast tumors, it was shown that fibroblast from tumor environment, compared to fibroblasts derived from areas that were not intimately associated with invasive carcinoma, significantly increased growth of epithelium and provided better support for cancer growth [35, 36]. These findings all support the hypothesis that tumor-associated stroma is of influence on the tumor’s invasive behavior.
Future plans are necessary to validate our findings, especially for the triple-negative patients on a larger patient set, to analyze the underlying mechanisms of the stroma formation using molecular techniques and model systems, and ultimately come to the development of new agents.
In conclusion, tumor–stroma ratio has shown to be a prognostic parameter in breast cancer patients, independently of other clinico-pathological parameters and systemic therapy. Furthermore, we were able to stratify the triple-negative cancer subgroup according to their risk of relapse, with a three times higher risk of relapse for patients with stoma-rich tumors. The tumor–stroma ratio is easy to determine, reproducible, and quickly performed for all types of breast cancers. In our opinion, it is a candidate parameter that could easily be implemented in routine pathology diagnostics, to optimize risk stratification for breast cancer patients, especially for the triple-negative carcinoma subgroup.
References
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J 360:790–800
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer 109:1721–1728
Reis-Filho JS, Tutt AN (2008) Triple negative tumours: a critical review. Histopathology 52:108–118
Fan C, Oh DS, Wessels L et al (2006) Concordance among gene-expression-based predictors for breast cancer. N Engl J Med 355:560–569
Fulford LG, Reis-Filho JS, Ryder K et al (2007) Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res 9:R4
Kryj M, Maciejewski B, Withers HR, Taylor JM (1997) Incidence and kinetics of distant metastases in patients with operable breast cancer. Neoplasma 44:3–11
Yokota J (2000) Tumor progression and metastasis. Carcinogenesis 21:497–503
Chang HY, Nuyten DS, Sneddon JB et al (2005) Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci 102:3738–3743
Dvorak HF (1986) Tumors: wounds that do not heal, similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Beck AH, Espinosa I, Gilks CB, van de Rijn M, West RB (2008) The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab Invest 88:591–601
Finak G, Bertos N, Pepin F et al (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14:518–527
Mesker WE, Junggeburt JM, Szuhai K et al (2007) The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 29:387–398
Mesker WE, Liefers G, Junggeburt JMC et al (2009) Presence of a high amount of stroma and downregulation of smad-4 predict a worse survival for stage I-II colon cancer patients. Cell Oncol 31:169–178
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430
Courrech Staal EF, Wouters MW, van Sandick JW, et al (2010) The stromal part of adenocarcinomas of the oesophagus: does it conceal targets for therapy? Eur J Cancer 46:720–728
Harris L, Fritsche H, Mennel R et al (2007) American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312
Thomas J, Hanby A, Pinder S et al (2008) Implications of inconsistent measurement of ER status in non-invasive breast cancer: a study of 1, 684 cases from the Sloane project. Breast J 14:33–38
Nunes CB, Rocha RM, Reis-Filho JS et al (2008) Comparative analysis of six different antibodies against Her2 including the novel rabbit monoclonal antibody (SP3) and chromogenic in situ hybridisation in breast carcinomas. J Clin Pathol 61:934–938
Annecke K, Schmitt M, Euler U et al (2008) uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Adv Clin Chem 45:31–45
van de Vijver MJ, He YD, van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Ein-Dor L, Kela I, Getz G, Givol D, Domany E (2005) Outcome signature genes in breast cancer: is there a unique set? Bioinformatics 21:171–178
Michiels S, Koscielny S, Hill C (2005) Prediction of cancer outcome with microarrays: a multiple random validation strategy. Lancet 365:488–492
Haibe-Kains B, Desmedt C, Sotiriou C, Bontempi G (2008) A comparative study of survival models for breast cancer prognostication based on microarray data: does a single gene beat them all? Bioinformatics 24:2200–2208
Sotiriou C, Piccart MJ (2007) Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer 7:545–553
Haibe-Kains B, Desmedt C, Piette F et al (2008) Comparison of prognostic gene expression signatures for breast cancer. BMC Genomics 9:394
Thomassen M, Tan Q, Eiriksdottir F, Bak M, Cold S, Kruse TA (2007) Comparison of gene sets for expression profiling: prediction of metastasis from low-malignant breast cancer. Clin Cancer Res 13:5355–5360
Wirapati P, Sotiriou C, Kunkel S et al (2008) Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 10:R65
Buyse M, Loi S, Van’t Veer L et al (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98:1183–1192
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011
Orimo A, Gupta PB, Sgroi DC et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Acknowledgments
We would like to thank the Dutch Cancer Society (UL 2007-3968) for financial support. In addition, we thank Dr. E. Bastiaannet and Dr. J.M.C. Junggeburt for additional statistical help, Mr. J. Molenaar for his help with the database, and Mr. K. van de Ham for pictures of representative tumor fields.
Author information
Authors and Affiliations
Corresponding author
Additional information
An invited commentary to this article can be found at doi:10.1007/s10549-010-0930-z
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Kruijf, E.M., van Nes, J.G.H., van de Velde, C.J.H. et al. Tumor–stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 125, 687–696 (2011). https://doi.org/10.1007/s10549-010-0855-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0855-6