Abstract
Tumor necrosis factor α (TNFα) is a pleiotropic cytokine which can regulate a wide variety of cellular responses. Low concentrations of TNFα seem to increase tumor growth and progression. The −308 G/A polymorphism in TNFα has been implicated in breast cancer risk but the published data remain inconclusive. In order to derive a more precise estimation of the relationship, a meta-analysis was performed by searching PubMed, Web of Science, ScienceDirect, EBSCO, CNKI, and Chinese Biomedicine Database. 11 studies including 10,184 cases and 12,911 controls were collected for TNFα −308 G/A polymorphism. Crude ORs with 95% CIs were used to assess the strength of association between the TNFα −308 G/A polymorphism and breast cancer risk. The pooled ORs were performed for codominant model (GG versus AA; GA versus AA), dominant model (GG + GA versus AA), recessive model (GG versus GA + AA), and G allele versus A allele, respectively. Overall, significantly elevated breast cancer risk was found for recessive model (OR = 1.10, 95% CI = 1.04–1.17) and for G allele versus A allele (OR = 1.08, 95% CI = 1.02–1.14). In the subgroup analysis by ethnicity, significantly increased risks were also found among Caucasians for recessive model and for G allele versus A allele (for recessive model: OR = 1.10, 95% CI = 1.04–1.17; for G allele versus A allele: OR = 1.09, 95% CI = 1.03–1.14). However, no significant associations were found among Asians for all genetic models. In conclusion, this meta-analysis suggests that the TNFα −308 G allele is a risk factor for developing breast cancer, especially for Caucasians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is by far the most frequent cancer of women [1]. It is expected to be the second leading cause of USA cancer deaths in 2008, preceded only by lung cancer [2]. The mechanism of breast carcinogenesis is still not fully understood. Low-penetrance susceptibility genes combining with environmental factors have been suggested to be important in the development of cancer [3]. To date, many reports have been published on common low-penetrant genes associated with an increased breast cancer risk [4]. An important one is tumor necrosis factor α (TNFα), a member of the TNF/TNFR cytokine superfamily which is involved in maintenance and homeostasis of the immune system, inflammation, and host defence [5]. Although TNFα was originally characterized to cause hemorrhagic tumor necrosis at high concentrations in many types of cancer, low concentrations of TNFα seem to increase tumor growth and progression [6]. Studies examining the role of the TNFα gene in breast cancer growth have revealed evidence for TNFα as a breast tumor promoter [7]. An important polymorphism (rs1800629, −308 G/A), located 308 bp upstream from the TNF start site within the promoter region of TNFα contains a G-to-A substitution and has been considered to influence the TNFα transcriptional activity [8, 9]. The association between TNFα levels and breast cancer risk has been observed in previous study [10]. Therefore, it is conceivable that the TNFα −308 G/A polymorphism may have relationship with the breast cancer risk. To date, a number of studies have investigated the role of TNFα −308 G/A polymorphism in breast cancer risk [11–25]. However, the results of those studies remain inconclusive, potentially due to the possible small effect of the polymorphism on breast cancer risk or the relatively small sample size in each of published studies. Hence, a meta-analysis of 11 eligible studies involving 10,184 cases and 12,911 controls was performed to help us derive a more precise estimation of the relationship between TNFα −308 G/A polymorphism and breast cancer risk.
Methods
Publication search
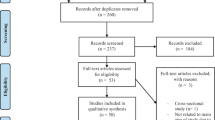
We searched the articles using the search terms “tumor necrosis factor α”, “TNFα”, “polymorphism”, and “breast” in PubMed, Web of Science, ScienceDirect, EBSCO, CNKI, and Chinese Biomedicine Database without a language limitation, and the last search was updated on December 7, 2009. All searched studies’ bibliographies were checked for other relevant publications. Review articles were hand-searched to find additional eligible studies. Only published studies with full text articles were included. When overlapping data of the same patient population were included in more than one publication, only the most recent or complete study was used in this meta-analysis.
Inclusion criteria
The following inclusion criteria were used for the literature selection: (a) articles about TNFα −308 G/A polymorphism and breast cancer risk, (b) case–control studies, and (c) sufficient published data for estimating an odds ratio (OR) with 95% confidence interval (CI).
Data extraction
Two investigators (Fang and Yao) extracted information from all eligible publications independently according to the inclusion criteria listed above. Disagreement was resolved by discussion between the two investigators. The following characteristics were collected from each study: first author’s surname, publication date, country of origin, ethnicity, source of control groups, total number of cases and controls, and numbers of cases and controls with the GG, GA, and AA genotypes, respectively. For those studies of different ethnic groups, data were extracted separately for each of the ethnic groups, categorized as Asian, Caucasian, and unknown ethnicity. We did not define any minimum number of patients to include a study in our meta-analysis.
Statistical methods
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the strength of the association between TNFα −308 G/A polymorphism and breast cancer risk. We explored the association for codominant model (GG versus AA; GA versus AA), dominant model (GG + GA versus AA), recessive model (GG versus GA + AA) and G allele versus A allele, respectively. In order to evaluate the ethnicity-specific effect, subgroup analyses were performed by ethnicities as well. A χ2-based Q-test was performed to check the heterogeneity [26]. If the Q-test revealed a P value of more than 0.10, it indicates a lack of heterogeneity among the studies and the pooled ORs were calculated according to the fixed effects model (the Mantel–Haenszel method) [27]. Otherwise, the random effects model (the DerSimonian–Laird method) [28] was used. The one-way sensitivity analyses were performed to assess the stability of the meta-analysis’ results. An estimate of potential publication bias was also carried out using the funnel plot and the Egger’s linear regression test. An asymmetric funnel plot suggests a possible publication bias, and a P less than 0.05 in the Egger’s test was considered representative of statistically significant publication bias [29]. All statistical analyses listed above were performed with the software Stata version 10.0 (Stata Corporation, College Station, TX).
Results
Study characteristics
In total, 11 studies with 10,184 cases and 12,911 controls met the inclusion criteria and were used in the pooled analyses [11–21]. Table 1 lists the studies identified and their main characteristics. Of the 11 studies, sample sizes ranged from 190 to 10,145. There were 9 studies of Caucasians, 1 study of Asians, and 1 study of unknown ethnicity. Among the 11 studies, 9 articles used controls derived from healthy populations and 2 articles used hospital-based controls. Genotypes’ distributions in the controls of 10 studies were in agreement with Hardy–Weinberg equilibrium.
Main results
The main results of this meta-analysis were listed in Table 2. Overall, when all the eligible studies were pooled into the meta-analysis, significantly increased breast cancer risk was found for recessive model (OR = 1.10, 95% CI = 1.04–1.17) (Fig. 1) and for G allele versus A allele (OR = 1.08, 95% CI = 1.02–1.14). In the stratified analysis by ethnicity, significantly increased risks were also found among Caucasians for recessive model and for G allele versus A allele (for recessive model: OR = 1.10, 95% CI = 1.04–1.17; for G allele versus A allele: OR = 1.09, 95% CI = 1.03–1.14) (Table 2); however, no significantly increased risk was found among Asians for all genetic models (Table 2).
Sensitivity analyses and publication bias
Our results suggested the influences of the individual data set to the pooled ORs were not significant. In addition, neither Begg’s funnel plot nor Egger’s test suggest any obvious evidence of publication bias (data not presented).
Discussion
It has been suggested that single nucleotide polymorphisms (SNPs) are the most common sources of human genetic variation, and they may contribute to an individual’s susceptibility to cancer [30]. So far, growing number of studies have investigated the role of TNFα −308 G/A in the development of breast cancer, but the results are inconclusive. Hence we performed this meta-analysis of 11 studies, involving 10,184 cases and 12,911 controls, to estimate the association specifically. Our results indicated that the TNFα −308 G allele is a risk factor for developing breast cancer. This result may be biologically plausible. Some studies have reported that the TNFα −308 G/A polymorphism can alter TNFα’s expression levels [8, 9]. Since TNFα is known to have both pro- and anti-carcinogenic properties [10] and chronically produced TNFα can play the role of a tumor promoter [18], the −308 G/A polymorphism of TNFα may act as an indirect breast cancer risk factor through changing the expression level of TNFα. In the subgroup analysis, significant association was found in Caucasians but not in Asians for recessive model and G allele versus A allele, suggesting a possible role of ethnic differences in genetic backgrounds and the environment they lived in.
The results of our meta-analysis strongly support the conclusion that the TNFα −308 G allele is a risk factor for developing breast cancer according to the large sample size and the significant associations for the recessive model and for G allele versus A allele in total and in Caucasians. However, there still may be some limitations in this meta-analysis. First, the controls included in our analysis were not uniformly defined. Although most of the controls were selected mainly from healthy populations, some were hospital-based. Therefore, these studies may have included the control groups who have different risks of developing breast cancer so that non-differential misclassification bias was possible. Second, in the subgroup analyses, there is only one study of Asians and the sample of Asians was relatively small, not having enough statistical power to explore the real association. Third, our data lack the information of age, sex, environmental factors, and lifestyle, so that our results were based on unadjusted estimates and a more precise analysis should be conducted in the future.
In conclusion, our meta-analysis with a large sample size strongly suggests that the TNFα −308 G allele is a risk factor for developing breast cancer, especially for Caucasians. However, further prospective researches with more convincing experimental proofs are necessary and expected. Such researches may eventually lead to our better, comprehensive understanding of the association mechanism between the TNFα −308 G/A polymorphism and breast cancer risk.
References
Kellen E, Vansant G, Christiaens MR, Neven P, Van Limbergen E (2009) Lifestyle changes and breast cancer prognosis: a review. Breast Cancer Res Treat 114:13–22
Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin. doi:10.3322/CA.2007.0010
Lichtenstein P, Holm NV, Verkasalo PK (2000) Environmental and heritable factors in the causation of cancer. N Engl J Med 343:78–85
Dunning AM, Healey CS, Pharoah PDP, Teare MD, Ponder BAJ, Easton DF (1999) A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:843–854
Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 25:409–416
Wu S, Boyer CM, Whitaker RS, Berchuck A, Wiener JR, Weinberg JB, Bast RC Jr (1993) Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res 53:1939–1944
Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Bequelin W, Salatino M, Charreau EH, Frahm I, Sapia S, Brouckaert P, Elizalde PV, Schillaci R (2008) TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res 314:509–529
Kroeger KM, Carville KS, Abraham LJ (1997) The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol 34:391–399
Wilson AG, di Giovine FS, Blakemore AI, Duff GW (1992) Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet 1:353
Balkwill F (2002) Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13:135–141
Mestiri S, Bouaouina N, Ahmed SB, Khedhaier A, Jrad BB, Remadi S, Chouchane L (2001) Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein hsp70–2: susceptibility and prognostic implications in breast carcinoma. Cancer 91:672–678
Giordani L, Bruzzi P, Lasalandra C, Quaranta M, Schittilli F, Della Ragione F, Iolascon A (2003) Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin Chem 49:1664–1667
Azmy IAF, Balasubramanian SP, Wilson AG, Stephenson TJ, Cox A, Brown NJ, Reed MWR (2004) Role of tumour necrosis factor gene polymorphisms (-308 and -238) in breast cancer susceptibility and severity. Breast Cancer Res 6:R395–R400
Smith KC, Bateman AC, Fussell HM, Howell WM (2004) Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immunogenet 31:167–173
Kamali-Sarvestani E, Merat A, Talei AR (2005) Polymorphism in the genes of alpha and beta tumor necrosis factors (TNF-alpha and TNF-beta) and gamma interferon (IFN-gamma) among Iranian women with breast cancer. Cancer Lett 223:113–119
Scola L, Vaglica M, Crivello A, Palmeri L, Forte GI, Macaluso MC, Giacalone A, Di Noto L, Bongiovanni A, Raimondi C, Accardo A, Verna R, Candore G, Caruso C, Lio D, Palmeri S (2006) Cytokine gene polymorphisms and breast cancer susceptibility. Ann N Y Acad Sci 1089:104–109
Gallicchio L, McSorley MA, Newschaffer CJ, Huang HY, Thuita LW, Hoffman SC, Helzlsouer KJ (2007) Body mass, polymorphisms in obesity-related genes, and the risk of developing breast cancer among women with benign breast disease. Cancer Detect Prev 31:95–101
Gaudet MM, Egan KM, Lissowska J, Newcomb PA, Brinton LA, Titus-Ernstoff L, Yeager M, Chanock S, Welch R, Peplonska B, Trentham-Dietz A, Garcia-Closas M (2007) Genetic variation in tumor necrosis factor and lymphotoxin-alpha (TNF-LTA) and breast cancer risk. Hum Genet 121:483–490
Sirotkovic-Skerlev M, Tamara C, Krizanac S, Kulic A, Pavelic K, Kapitanovic S (2007) TNF alpha promoter polymorphisms analysis in benign and malignant breast lesions. Exp Mol Pathol 83:54–58
Ostashkin AS, Malivanova TF, Yurchenko VA, Mazurenko NN (2008) Tumor necrosis factor gene polymorphisms in breast cancer patients. Russ J Genet 44:1111–1115
The MARIE-GENICA Consortium on Genetic Susceptibility for Menopausal Hormone Therapy Related Breast Cancer RIsk (2009) Polymorphisms in the BRCA1 and ABCB1 genes modulate menopausal hormone therapy associated breast cancer risk in postmenopausal women. Breast Cancer Res Treat. doi:10.1007/s10549-009-0489-8
Skerrett DL, Moore EM, Bernstein DS, Vahdat L (2005) Cytokine genotype polymorphisms in breast carcinoma: Associations of TGF-beta 1 with relapse. Cancer Invest 23:208–214
Chouchane L, Ahmed SB, Baccouche S, Remadi S (1997) Polymorphism in the tumor necrosis factor-alpha promotor region and in the heat shock protein 70 genes associated with malignant tumors. Cancer 80:1489–1496
Gonullu G, Basturk B, Evrensel T, Oral B, Gozkaman A, Manavoglu O (2007) Association of breast cancer and cytokine gene polymorphism in Turkish women. Saudi Med J 28:1728–1733
Kohaar I, Tiwari P, Kumar R, Nasare V, Thakur N, Das BC, Bharadwaj M (2009) Association of single nucleotide polymorphisms (SNPs) in TNF-LTA locus with breast cancer risk in Indian population. Breast Cancer Res Treat 114:347–355
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in metaanalysis detected by a simple, graphical test. BMJ 315:629–634
Wu GY, Hasenberg T, Magdeburg R, Bonninghoff R, Sturm JW, Keese M (2009) Association between EGF, TGF-beta1, VEGF gene polymorphism and colorectal cancer. World J Surg 33:124–129
Acknowledgments
This study was supported by national 973 programs of China Grants 2004CB518605, the national 863 project of China Grants 2006AA020501, and the national key sci-tech special project of China Grants 2008ZX10002-020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, F., Yao, L., Yu, X.J. et al. TNFα −308 G/A polymorphism is associated with breast cancer risk: a meta-analysis involving 10,184 cases and 12,911 controls. Breast Cancer Res Treat 122, 267–271 (2010). https://doi.org/10.1007/s10549-009-0698-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0698-1