Abstract
According to current treatment standards, patients with metastatic breast cancer at diagnosis receive palliative therapy. Local treatment of the breast is only recommended if the primary tumor is symptomatic. Recent studies suggest that surgical removal of the primary tumor has a favorable impact on the prognosis of patients with primary metastatic breast cancer. We performed a systematic review of the literature to weigh the evidence for and against breast surgery in this patient group. Ten retrospective studies were found in which the use of breast surgery in primary metastatic breast cancer and its impact on survival was examined. The hazard ratios of the studies were pooled to provide an estimate of the overall effect of surgery, and the results and conclusions of the studies were analyzed. A crude analysis, without adjustment for potential confounders, showed that surgical removal of the breast lesion in stage-IV disease was associated with a significantly higher overall survival rate in seven of the ten studies, and a trend toward a better survival in the three remaining studies. Surgery of the primary tumor appeared to be an independent factor for an improved survival in the multivariate analyses from the individual studies, with hazard ratios ranging from 0.47 to 0.71. The pooled hazard ratio for overall mortality was 0.65 (95% CI 0.59–0.72) in favor of the patients undergoing surgery. This systematic review of the literature suggests that surgery of the primary breast tumor in patients with stage-IV disease at initial presentation does have a positive impact on survival. In order to provide a definite answer on whether local tumor control in patients with primary metastatic disease improves survival, a randomized controlled trial comparing systemic therapy with and without breast surgery is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 11% of all women in the western world will develop breast cancer. Of all breast cancer patients 3–10% has distant metastases at initial presentation [1]. Median survival of these patients is in the range of 16–24 months and is determined by several factors, including number and site of metastatic lesions, and tumor characteristics such as hormone receptor and HER2neu status [2, 3]. During the last decades, the treatment of metastatic breast cancer has undergone considerable changes, with taxanes and third-generation aromatase-inhibitors being introduced in the nineties of the previous century and, among others, trastuzumab and bevacizumab in the current decade [4–6].
Until now, we continue to adhere to the concept that metastatic breast cancer is an incurable disease. In line with this concept, aggressive local therapy is thought to provide no survival advantage, and the primary goals of local treatment are the prevention or palliation of symptoms. Therefore, local treatment of the primary tumor is only recommended if the primary tumor is symptomatic. However, recent studies suggest that breast surgery has a favorable impact on the prognosis of patients with primary metastatic breast cancer, and that it may be time to reconsider the treatment paradigm “no surgery of the primary tumor” [7–13]. On the other hand, not all studies addressing this subject have reported a better prognosis for the patients undergoing surgery [14–16], and the investigators of these studies suggest that the beneficial effect seen in other studies may be the result of selection bias.
In this review of the literature we try to weigh the evidence for and against breast surgery in patients with primary metastatic breast cancer. We also explored the literature to look for possible biological mechanisms explaining the findings from clinical studies. We conclude with an overall summary of our review and with a final recommendation on further research on this topic.
Methods
A search was performed in PubMed in May 2009. The following search strategy was used: breast cancer AND (stage-IV OR metastatic) AND surgery AND (“primary tumor” OR “primary tumor”). English journals were taken into account, and only full papers were included. This resulted in 784 hits. After reviewing the abstracts, eleven retrospective studies were found in which the use of local therapy in primary metastatic breast cancer and its impact on survival was examined. One study was excluded as it did not contain a control group without surgery [17]. References of selected studies were checked, but no additional studies were found. The literature search was done by two authors (J. Ruiterkamp and A. Voogd).
Statistical pooling of study results
Overall survival was the outcome of interest. Breast-cancer-specific survival was used for studies that did not report overall survival. The decision to combine these outcome measures was justified by the fact that the difference between overall survival and breast-cancer-specific survival is usually very small because of the poor prognosis of patients with stage-IV breast cancer. The hazard ratios (HRs) of the studies were pooled using the Review Manager software (RevMan 5.0.21) [18]. The HR is the preferred statistic for pooling time-to-event outcomes because it incorporates data from the entire Kaplan–Meier curve and allows for censoring. When available, the HR was extracted directly from the papers. The standard errors of the HR estimates were calculated from the reported 95% confidence intervals (95% CI) or P values using the methods described by Parmar et al. [19]. These values were analyzed using the generic inverse variance method. A random effects model was used to calculate the overall effect. Pooled results are expressed as HRs with 95% CI. HRs less than one favor surgery and HRs greater than one favor no surgery.
Results
An overview of the study characteristics is shown in Table 1. All 10 studies selected for the review were retrospective in design. Seven studies were based on more than 200 patients. For four studies data from population-based registries were used, including up to 16,023 patients in one study. The remaining studies were based on hospital registries, including 147–622 patients. Most studies were based on registries from the nineties and onward; four studies were initiated in the seventies or eighties. In all studies, one-third to half of all patients was treated with surgery of the primary tumor.
Univariate results from the individual studies
A crude analysis, without adjustment for potential confounders, showed that surgical removal of the breast lesion in stage-IV disease was associated with a significantly higher overall survival rate (P < 0.05) in seven of the ten studies, and a trend toward a better survival in the three remaining studies.
Of the two studies that took into account the surgical margins, one showed that the better survival was only observed in the patients whose primary breast tumor had been removed with free surgical margins, whereas the other study showed that the survival benefit was more pronounced in patients with free surgical margins [7, 9].
The few studies addressing the impact of axillary lymph node dissection did not find a significant contribution of nodal dissection to the prognosis [9, 13].
Separate discussion on ‘negative’ studies
Three studies did not find a positive effect of surgery on overall survival in patients with primary metastatic breast cancer [14–16].
Bafford et al. [14] found that, although on multivariate analysis survival was significantly superior in the surgery group (median survival 4.13 years versus 2.36 years; HR 0.47; P = 0.003), this benefit was confined to the patients operated upon before diagnosis of metastatic disease (median survival 4.05 years). The authors argued that the effect of surgery may be caused by “stage migration bias”, meaning that patients who benefited from surgery probably are the ones with smaller breast tumors and asymptomatic metastases, who already had a better overall survival from the start. However, in this study the authors adjusted for age, number of sites of metastases, use of systemic therapy and estrogen receptor (ER), and HER2neu status, thus taking into account possible confounders. A univariate comparison of two subgroups (stage-IV diagnosis before or after surgery) with the no-surgery group provides a lower level of evidence when compared with the overall multivariate analysis. Therefore, we are not convinced by these data that breast surgery only benefits patients who had a diagnosis of metastatic disease after primary breast surgery.
Leung et al. [15] compared survival between different patient groups. Median survival for patients who underwent surgery was 25 months, compared with 13 months for patients who did not receive surgery (P = 0.004). Of 157 patients, 84 were treated with chemotherapy whereas 73 not. Median survival was 25 months for the patients treated with chemotherapy when compared with only 8 months for those not treated with chemotherapy. When taking the effects of chemotherapy into account, surgery by itself no longer appeared to have a significant impact on survival. An obvious limitation of the study, also stated by the authors, is the limited number of patients included.
In the largest ‘negative’ study, it was suggested that case selection bias and coding errors may explain an important part of the survival advantage observed in stage-IV breast cancer patients undergoing surgery [16]. The investigators used case-matching, according to age, year of diagnosis, site of metastatic disease, hormone receptor status, and use of systemic therapy, to make patients with and without surgery more comparable and to remove bias by these factors. Overall, there was a statistically significant survival benefit for patients treated with surgery. Moreover, the evidence pointed at the same direction in the various subgroup analyses. In nearly all case-matched subgroups there was still a significant survival benefit from breast surgery. In some subgroups there was no statistical significance difference, however, the survival curves yet were more favorable for the surgery-treated groups, and lack of significance may have resulted from the small numbers of patients included per subgroup; for example one subgroup contained only eight patients. In conclusion, we consider this study as a positive study with respect to impact of breast surgery in stage-IV disease.
Multivariate analyses from the individual studies
Interpretation of the results of the univariate analysis is limited by the fact that the decision to remove the primary tumor may have been guided by factors that are themselves related to the outcome of the disease. In most studies, the patients who had surgery were significantly younger when compared to the patients who did not had surgery [7–10, 12, 13]. These patients had also smaller primary tumors and more often just one metastatic localization [8, 9, 12, 13]. Some studies reported that patients who had surgery also had less extensive regional disease [8, 15]. The patients who received surgery were also less likely to have concomitant diseases [13]. They were more likely to be Caucasian and to have tumors with positive estrogen and progesterone receptors [10, 12]. They also received more often local radiotherapy [9, 13, 14]. Some studies reported that the use of chemotherapy was lower among women who underwent surgery [9]. In contrast, other studies found that these women were more likely to receive chemotherapy, hormonal therapy, or both than patients who did not have surgery [8, 13]. In one study patients, who received surgery were significant older than patients who were not treated with surgery [12]. Thus, a multivariable analysis is required to adjust for these potential confounders.
In Table 2, the results of the studies in which a multivariable analysis has been performed are summarized. In general, results of the univariate analysis were confirmed. Surgery of the primary tumor appeared to be an independent factor for an improved survival in patients with stage-IV breast cancer. HRs range from 0.47 to 0.71.
Most studies adjusted for age, tumor size, hormone receptor status, and site of metastatic disease. Some of the studies also adjusted for the use of systemic therapy (mostly for chemotherapy), sometimes also for hormonal treatment and radiotherapy, and axillary lymph node status. Only two studies corrected for comorbidity. The following variables with significant impact on survival have been identified: age, ER, and progesterone receptor (PR) status, (number of) sites of metastases, use of systemic therapy (chemotherapy and sometimes hormonal treatment), and surgical margins.
Statistical pooling of study results
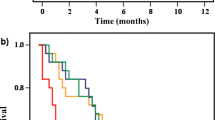
HRs for overall mortality and standard errors for the estimated HRs were reported or could be calculated for all studies, except for the study by Cady et al., which was a matched pair analysis, and the study by Leung et al. (Fig. 1). The papers by Rapiti et al. and Khan et al. did not report the HR for the total group of patients undergoing surgery versus no surgery, but only for patients with free and positive surgical margins (vs. no surgery) separately. The HRs for these subgroups were both included in the pooled analysis, together with the HRs of the other studies (Fig. 1). For the study by Khan et al., the HR for the patients with positive surgical margins was significantly different from the HR for patients with negative margins. Significant heterogeneity was observed by visual inspection of the forest plot and by calculating the Chi-square test for heterogeneity (P < 0.0001) and the I 2 percentage (81%). For that reason a random effects model was chosen to pool the HRs. The pooled HR for overall mortality was 0.65 (95% CI 0.59–0.72) in favor of the patients undergoing surgery (Fig. 1).
Discussion
In this systematic review, we weighted the evidence for and against breast surgery in patients with primary metastatic breast cancer. Surgery of the breast tumor was associated with a statistically significant better overall survival in seven of the ten studies, and a trend for significant better survival in the three remaining studies. All studies were non-randomized and conducted retrospectively. Surgery with free surgical margins even caused a larger difference in overall survival. There were several other factors contributing to a better overall survival, like younger age, smaller primary tumor, and having just one metastatic site. In the multivariate analyses, there has been a correction for these factors. In retrospective analyses, however, there is still a risk of residual confounding, even if multivariate analyses on known prognostic and therapeutic factors have been performed. Evident limitations of the existing literature are that patients who underwent surgery also receive effective systemic therapy at the same time, whereas patients with a better prognosis are also more likely to get their tumor resected.
Two recent retrospective studies evaluated whether locoregional treatment, either surgery or radiotherapy, would impact survival apart from protection against uncontrolled chest wall disease and irrespective of treatment of the primary breast tumor. Local control was maintained in 82% of all patients in the surgical group versus 34% in the group without surgery (P = 0.001). Moreover, chest wall control was associated with improved overall survival regardless of whether surgical resection of the tumor was performed, with a hazard ratio of 0.42 (P < 0.0002) [20]. Another study also analyzed the effect of locoregional treatment, locoregional radiotherapy and/or surgery, and its effect on overall survival. Patients who received locoregional treatment had a 3-year survival rate of 43.3%, whereas patients who did not receive locoregional therapy had a 3-year survival rate of 26.7% (P = 0.00002). In multivariable analysis, locoregional treatment was associated with a 30% reduction in the risk of death (HR: 0.70, 95% CI: 0.58–0.85) [21].
Axillary dissection has been regarded as a staging rather than a therapeutic procedure, but a meta-analysis including nearly 3,000 patients showed an average survival benefit of more than 5% from axillary lymph node dissection in the non-metastasized setting [22]. Several studies now support the idea that uncontrolled regional disease in the axilla can also act as a source for systemic tumor (re)seeding in the metastasized setting [23, 24]. The study of Kahn et al. [7] provides some evidence of an additional beneficial effect of axillary clearance when performed in the same session as the breast tumor removal. This was confirmed by other study that shows that patients who underwent an axillary lymph node dissection tended to have a better overall survival than those without axillary dissection, though the difference was restricted to the first year after treatment [13].
If the total tumor burden really plays a role in survival and the primary tumor can be considered as a metastatic site, then the removal of the breast lesion is part of a multimodality strategy in preventing further growth and dissemination of the disease [6]. This hypothesis was confirmed by several studies which found a strong correlation between the level of circulating tumor cells (CTCs) and the prognosis of metastatic breast cancer by showing that the number of circulating tumor cells before treatment is an independent predictor of overall survival in patients with metastatic breast cancer [25–27]. Also patients who after treatment converted from elevated CTCs to nonelevated levels show a clinical response [28]. Thereby, an association is found between the median CTC level, determined in the course of the treatment, and the time to progression in metastatic breast cancer [29]. This may indicate that the clinical response is correlated with a decrease in CTCs and thus with a reduction of tumor burden.
Improvement in survival can also be caused by the fact that surgical resection restores the immune system, even in patients with metastatic disease [30]. Tumor-induced immunosuppression is a mechanism allowing tumors to escape immune destruction. It is thought that immunosuppression intensifies with increasing tumor burden. Surgery reduces the quantity of immunosuppressive factors and allowing the immune response to recover. Opposite to the proposed biological mechanisms in favor of surgical removal of the primary tumor, there have been observations indicating that surgical resection of the breast lesion in metastatic disease may accelerate relapse by two mechanisms: 1. due to removal of inhibitors of angiogenesis there will be an angiogenic surge; 2. surgical wounding will lead to the release of growth and immunosuppressive factors [10, 31]. Some other studies also suggest that the growth of distant metastases even may be stimulated after the primary breast tumor has been removed; this hypothesis is partly sustained by experimental studies and partly by doctor’s personal experience [32]. Our analysis of available retrospective studies does not support this view.
In conclusion, our literature review suggests that surgery of the primary breast tumor in patients with stage-IV disease at initial presentation does have a positive impact on survival. In order to provide a definite answer on whether local tumor control in patients with primary metastatic disease improves survival, a randomized controlled trial comparing systemic therapy with or without breast surgery is needed. Such a trial may run for many years to include enough patients, but we feel that this next step is logical in the light of the retrospective evidence. In the Netherlands as well as in the United States plans for such a trial are made. In such prospective trials, it may also be feasible to add quality of life questionnaires, to determine psychological effects of locoregional treatment. Hopefully these efforts will result in an evidence-based conclusion on this very important subject of the treatment of patients with primary metastatic breast cancer.
References
Sant M, Allemani C, Berrino F et al (2004) Breast carcinoma survival in Europe and the United States. Cancer 100:715–722
Ellis MJ, Hayes DF, Lippman ME (2000) Treatment of metastatic breast cancer. In: Harris J et al (eds) Diseases of the breast, 2nd edn. Williams and Wilkins, Philadelphia, pp 749–797
Wood WC, Muss HB, Solin LJ, Olopade OI (2005) Malignant tumors of the breast. In: DeVita VT Jr, Hellman S, Rosenberg SA (eds) Cancer principles and practice of oncology, 7th edn. Lippincott Williams, Philadelphia, pp 1415–1478
Ernst MF, van de Poll-Franse LV, Roukema JA et al (2002) Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002. Breast 16:344–351
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676
Amar S, Roy V, Perez EA (2009) Treatment of metastatic breast cancer: looking towards the future. Breast Cancer Res Treat 114:413–422
Khan SA, Stewart AK, Morrow M (2002) Does aggressive local therapy improve survival in metastatic breast cancer. Surgery 132:620–627
Babiera GV, Rao R, Feng L, Meric-Bernstam F, Kuerer HM, Singletary E et al (2006) Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol 13:776–782
Rapiti E, Verkooijen HM, Vlastos G, Fioretta G, Neyroud-Caspar I, Sappino AP et al (2006) Complete surgical excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol 24:2743–2749
Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA (2007) Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol 14:2187–2194
Fields RC, Donna BJ, Trinkaus K, Zhang Q, Arthur C, Aft R et al (2007) Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol 14:3345–3351
Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM (2007) Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 247:732–738
Ruiterkamp J, Ernst MF, van de Poll-Franse LV, Bosscha K, Tjan-Heijnen VC, Voogd AC (2009) Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol 35:1146–1151
Bafford AC, Burstein HJ, Barkley CR, Smith BL, Lipsitz S, Iglehart JD, Winer EP, Golshan M (2009) Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat 115:7–12
Leung AM, Vu HN, Nguyen KA, Thacker LR, Bear HD (2009) Effects of surgical excision on survival of patients with stage IV breast cancer. J Surg Res. doi:10.1016/j.jss.2008.12.030
Cady B, Nathan NR, Michaelson JS, Golshan M, Smith BL (2008) Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol 15:3384–3395
Carmichael AR, Anderson EDC, Chetty U, Dixon JM (2003) Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol 29:17–19
Review Manager (RevMan) [Computer program]. Version 5.0. (2008) The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
Parmar MKB, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Hazard WH, Gorla SR, Scholtens D, Kiel K, Gradishar WJ, Khan SA (2008) Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer 113:2011–2019
Le Scodan R, Stevens D, Brian E, Floiras JL, Cohen-Solal C, De La Lande B (2009) Breast cancer with synchronous metastases: survival impact of exclusive localregional radiotherapy. J Clin Oncol 27:1375–1381
Orr RK (1998) The impact of prophylactic axillary node dissection on breast cancer survival—a Bayesian meta-analysis. Ann Surg Oncol 6:109–116
Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE et al (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337:956–962
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F et al (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337:949–955
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LWMM, Hayes DF (2006) Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Cancer Res 12:6403–6409
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–791
Cristofanilli M, Hayes DF, Budd GT et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23:1420–1430
Hayes DF, Cristofanilli M, Budd GT, Ellis M, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LWWM (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12:4218–4224
Wong NS, Kahn HJ, Zhang L, Oldfield S, Yang LY, Marks A, Trudeau ME (2006) Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat 99(1):63–69
Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S (2004) Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 64:2205–2211
Retsky M, Bonadonna G, Demicheli R et al (2004) Hypothesis: induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients. Breast Cancer Res 6:372–374
Baum M, Demicheli R, Hruskesky W et al (2005) Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 41:508–515
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiterkamp, J., Voogd, A.C., Bosscha, K. et al. Impact of breast surgery on survival in patients with distant metastases at initial presentation: a systematic review of the literature. Breast Cancer Res Treat 120, 9–16 (2010). https://doi.org/10.1007/s10549-009-0670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0670-0