Abstract
Uncontrolled proliferation is a defining feature of the malignant phenotype. Ki67 is a marker for proliferating cells and is overexpressed in many breast cancers. Atypical hyperplasia is a premalignant lesion of the breast (relative risk ~ 4.0). Here, we asked if Ki67 expression could stratify risk in women with atypia. Ki67 expression was assessed immunohistochemically by digital image analysis in archival sections from 192 women with atypia diagnosed at the Mayo Clinic 1/1/67–12/31/91. Risk factor and follow-up data were obtained via study questionnaire and medical records. Observed breast cancer events were compared to population expected rates (Iowa SEER) using standardized incidence ratios (SIRs). We examined two endpoints: risk of breast cancer within 10 years and after 10 years of atypia biopsy. Thirty-two (16.7%) of the 192 women developed breast cancer over a median of 14.6 years. Thirty percent (58) of the atypias had ≥2% cells staining for Ki67. In these women, the risk of breast cancer within 10 years after atypia was increased (SIR 4.42 [2.21–8.84]) but not in those with <2% staining. Specifically, the cumulative incidence for breast cancer at 10 years was 14% in the high Ki67 vs. 3% in the low Ki67 group. Conversely, after 10 years, risk in the low Ki67 group rose significantly (SIR 5.69 [3.63–8.92]) vs. no further increased risk in the high Ki67 group (SIR 0.78 [0.11–5.55]). Ki67 appears to be a time-varying biomarker of risk of breast cancer in women with atypical hyperplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women with atypical hyperplasia have a fourfold increased risk of breast cancer compared to women in the general population [1–4] with a cumulative incidence approaching 30% at 25 years [2]. Because of their high-risk status, women with atypia are frequently counseled to follow heightened surveillance and to take tamoxifen for chemoprevention [5]; some even consider prophylactic mastectomy. Thus, these women need accurate, individualized risk assessment.
The most frequently used model for risk prediction in women with atypia is the Gail model, but our group has recently shown that this model lacks sufficient accuracy in predicting risk of individual women with atypia [6]. From a biologic standpoint, atypical hyperplasia is recognized as a precursor lesion to both noninvasive and invasive breast cancer. Thus, atypia represents a unique platform from which to evaluate possible mediators and processes in breast carcinogenesis and to determine whether biomarkers that reflect such processes could better stratify risk for these women.
Imbalance in the normal regulation of cell proliferation is a defining feature of the cancer phenotype [7]. We evaluated proliferation status in tissue samples from women with atypical hyperplasia using Ki67, a monoclonal antibody that recognizes a nuclear antigen present in proliferating cells [8]. Ki67 expression increases progressively across the continuum from benign breast disease, to ductal carcinoma in situ (DCIS), to invasive breast cancer [9–11]. In invasive breast cancer, higher Ki67 levels have been shown to correlate with worse clinical outcome in numerous studies [12, 13]. In DCIS, higher Ki67 is associated with higher grade lesions [14].
To our knowledge, Ki67 has not been examined in a cohort of women with atypia who were followed long term for subsequent breast cancer events. Here, we examine the expression of Ki67 in 192 women with atypia and assess its association with patient age, type of atypia, family history, risk of progression to breast cancer, and time to breast cancer.
Methods
Study population
The Mayo Benign Breast Disease cohort includes 9,376 women aged 18–85 who had excisional breast biopsies with benign findings at the Mayo Clinic in Rochester, MN between January 1, 1967, and December 31, 1991 [1, 15]. In this cohort, 331 women had atypical hyperplasia [2]. Benign biopsies were reviewed by the study pathologists (DWV and CR) using current classification systems [4, 16]. Paraffin-embedded, formalin-fixed tissue for Ki67 staining was available for 192 of the women with atypia.
Follow-up and risk factor data
Follow-up for breast cancer events and risk factor information were obtained through Mayo medical records and a study questionnaire. Family history was classified as negative, strong, or weak. Criteria for a strong family history were: at least one first-degree relative with breast cancer before the age of 50 years or two or more relatives with breast cancer, with at least one being a first degree relative. Any lesser degree of family history was considered to be weak. All protocol procedures and patient contact materials were reviewed and approved by the Institutional Review Board of the Mayo Clinic. Completion and return of the patient contact materials were considered to be implied consent.
Histopathologic review
A diagnosis of atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH) was based on criteria of Page et al. [16]. For each example of atypical hyperplasia, the number of separate foci was defined [2]. Multifocal atypia required the identification of atypia in more than one terminal duct lobular unit, as defined by clear separation from another terminal duct lobular unit by nonspecialized interlobular mammary stroma [2].
Immunostaining
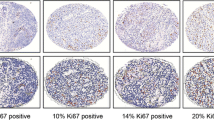
Ki67/MIB-1 assessment: tumor proliferation was determined by digital image analysis with an Automated Cellular Imaging System (ACIS III®, DakoCytomation, San Juan Capistrano, CA) using 5 μm-thick formalin-fixed, paraffin-embedded sections. All analyses were performed in the Molecular Imaging Laboratory within the Department of Pathology at Mayo Clinic. Detection of the Ki-67/MIB-1 was determined by immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded (FFPE) samples, which were deparaffinized with three changes of xylene and rehydrated in a series of alcohols (100, 95, then 70% EtOH) and rinsed well in running distilled water. Slides were then placed in a preheated 0.1 mM EDTA, pH 8.0 retrieval buffer for 30 min and then cooled in the buffer for 5 min followed by a 5 min rinse in running distilled water. After the HIER (heat-inactivated epitope retrieval) step, slides were placed on the DAKO Autostainer for the following procedure (at room temperature). Sections were incubated with 3% H2O2 in ethanol for 5 min to inactivate the endogenous peroxides. Sections were incubated in 1:100 Ki67/MIB-1 (M7240, DAKO, Carpenteria, CA) for 30 min. Sections were rinsed with TBST wash buffer. Secondary incubation was in the DUAL+/HRP-labeled polymer (K4061, DAKO, Carpenteria, CA) for 15 min. The slides were rinsed with TBST wash buffer. Sections were then incubated in 3,3′-diaminobenzidine (DAB+) (K3467, DAKO) for 5 min, counterstained with modified Schmidt’s hematoxylin for 5 min, followed by a 3-min tap water rinse to blue sections, dehydrated through graded alcohols and cleared in three changes of xylene and mounted with a permanent mounting media. Ki67 expression was assessed with the nuclear antibody MIB-1 (Immunotech, Marseille, France; 1:400 dilution) using a standard avidin–biotin complex method. Slides were processed with an automatic stainer (BioTek®, Ventana Medical System, Tucson, AZ) and diluted hematoxylin was used as a nuclear counterstain. Nuclei expressing the antigen that reacts with Ki67/MIB-1 were identified by the brown chromogen diaminobenzidine. Representative Ki67 staining is illustrated in Fig. 1. Areas of atypia were marked as ADH or ALH on each slide by a pathologist. Each slide was scanned on the ACIS III to generate an image for quantitative analysis. On each image, all areas of atypical epithelium (stroma was excluded) as marked by the pathologist were outlined using the free-form tool for analysis by the software. The instrument provided a Ki67/MIB-1 percentage (representing the proportion of the nuclear area positive for MIB-1) and intensity (representing the average stain concentration over the region collected on a scale of 0 to 255) for each specimen. For women with more than one area of atypia, per-woman summary measures of percent staining were calculated using a weighted average of the multiple values, with weights proportional to the size of each given area. Summary measures of intensity for each woman were calculated in a similar fashion, but used both area-size and area-specific percentage staining as weighting variables to account for potential differences in these factors across the multiple areas per women.
Statistical analysis
Data were summarized descriptively using frequencies and percentages for categorical variables and medians and interquartile ranges (IQRs) for continuous variables. Among the 331 women with atypical hyperplasia, we compared distributions of demographic and clinical variables by tissue availability for Ki67 staining using chi-square tests of significance.
The length of follow-up for each woman in the study was defined as the number of days from her benign biopsy to the date of her breast cancer diagnosis, prophylactic mastectomy, LCIS, death, or last contact. We estimated relative risks, overall and by strata of Ki67 staining levels, with standardized incidence ratios (SIRs) by dividing the observed numbers of breast cancer incident by population-based expected values. The approach allowed us to compare rates of breast cancer in our cohort with that of the general population rather than an internal referent group, recognizing that all women in our cohort were at some increased risk of breast cancer from their diagnosis of atypical hyperplasia. Expected values were calculated by apportioning each woman’s person-years of follow-up into 5-year age and calendar-period categories and multiplying these by the corresponding breast cancer incidence rates from the Iowa Surveillance, Epidemiology, and End Results (SEER) registry. This reference population was chosen because of its demographic similarities to the Mayo Clinic population (80% of cohort members reside in the upper Midwest). Risk analyses were performed within two time-dependent strata, one that focused on follow-up and outcomes that occurred in the first 10 years post biopsy and one that focused exclusively on follow-up and outcomes that occurred after the first 10 years post biopsy. Differences between the standardized incidence ratios across Ki67 staining levels were compared using a Poisson regression analysis that accounted for the population-based expected event rate for each individual using an offset term. Separate analyses were carried out for percent staining and intensity of staining.
We visually displayed observed and expected event rates using cumulative incidence curves, while accounting for the effects of death as a competing risk [17]. Expected events were calculated for each 1-year follow-up interval in a manner similar to that used for determining SIRS. A modified Kaplan–Meier approach was used to accumulate expected incidence over these intervals. The expected curve was smoothed using linear interpolation. All statistical tests were two-sided, and all analyses were conducted using the SAS (SAS Institute, Inc., Cary, NC) software system.
Results
Patient characteristics
Within our atypia cohort of 331 women, archived tissue was available for Ki67 staining for 192 women. We compared these 192 subjects to the remaining 139 and observed no significant differences in terms of distributions of case status, age at atypia, family history of breast cancer, and for breast cancer patients, time to diagnosis (P > 0.05 for each attribute). Clinical features for the subjects included in this study are presented in Table 1, along with histopathologic features of the atypias. Median post biopsy follow-up was 14.6 years for the 192 women, 32 of whom (16.7%) have developed breast cancer.
Ki67 immunostaining of atypia samples
In the 192 samples, we analyzed both percent cells positive for Ki67 and staining intensity. The correlation between the two measures was 0.98; thus, we report the values by percent cells positive, as is customary when reporting Ki67 values. The median value for percent positive cells for Ki67 was 1.0% and the 75th percentile value was 2.3%. Based on the empirical distribution of staining values in our cohort, we selected a cutoff of 2% cells positive to define groups of individuals with higher or lower degrees of staining. Examples of low (<2%) and high (≥2%) staining of Ki67 are shown in Fig. 1. We looked for patterns in Ki67 levels by age at biopsy, type of atypia (ADH, ALH, or both), number of foci of atypia, and family history, and no differences were observed, as shown in Table 1. For 174 of these 192 women, we also have COX-2 staining data [18]. There was an association between higher Ki67 expression and higher COX-2 expression (P = 0.03).
Association of Ki67 expression with breast cancer risk
Among cases, we examined the time from atypia biopsy to breast cancer diagnosis by Ki67 immunostaining. Among the 32 patients who developed breast cancer, those with ≥2% Ki67 expression had a shorter time to breast cancer (median = 5.5 years, IQR = 3.2–7.2) than those with <2% cells staining positive for Ki67 (median = 13.8 years, IQR = 11.6–20.3). This difference is reflected in the results displayed in Table 2 and Fig. 2. We, therefore, examined the risk of breast cancer by Ki67 levels within two time periods: over the first 10 years of follow-up and in the follow-up after 10 years. As shown in Table 2, we found a positive association between Ki67 overexpression (≥2% of cells positive) and the risk of breast cancer in the first 10 years of follow-up (SIR = 4.42 [95% CI 2.21–8.84]). This excess risk resulted in a 10-year cumulative incidence of 14.1%, significantly higher than what is expected in the population at large (Fig. 2). In contrast, in the women with low Ki67 expression, we found no increased risk of breast cancer in the first 10 years, with SIR 1.01 (95% CI 0.38–2.70), which was significantly lower than the women with high expression (P = 0.01). The 10-year cumulative incidence of breast cancer was 3% for those women with low Ki-67 staining values, in line with population averages. After 10 years, risk increased significantly in the low Ki67 group [SIR 5.69 (3.63–8.92)] vs. no increased risk in the high Ki67 group [SIR 0.78 (0.11–5.55)]. We formally examined this apparent time-dependent difference in incidence patterns by Ki67 staining levels via Poisson regression analysis, and found a statistically significant interaction between pre- vs. post-10 years incidence and high vs. low staining levels (P < 0.001). Considering all years of follow-up (median 14.6), Ki67 level was not associated with risk of breast cancer (P = 0.84).
Primary analyses were based on a Ki67 staining cutpoint of 2%. However, a defined standard of high staining values for Ki67 in atypia has yet to be established. Thus, we re-examined associations using different cutpoints ranging from 0.5 to 3% and found similar results (data not shown).
Discussion
Atypical hyperplasia is a premalignant lesion associated with a high risk of subsequent breast cancer [1, 2]. Regulation of cell proliferation is a vital physiologic phenomenon that helps to prevent malignant transformation within a cell population. We studied the expression of Ki67, as an accepted marker for proliferating cells, in a sizable cohort of women with atypical hyperplasia who have been followed long term for subsequent breast cancer events. We examined both short-term (within 10 years) and long-term (after 10 years) risk. Women whose atypias had a higher proliferation index had an increased short-term (within 10 years) risk of breast cancer. Importantly, women whose atypia had low Ki67 staining had no increased risk within the first 10 years after atypia but a significantly increased risk after 10 years.
In breast cancer, the presence of a higher proliferative cell population has been shown to correlate with earlier disease progression in studies with sufficient follow-up to look at both short-term and long-term outcomes [19, 20]. The observation is akin to the time-varying recurrence pattern of ER-negative vs. ER-positive breast cancer. With higher grade ER-negative breast cancers, which have higher proliferative indices, recurrences occur earlier [21, 22]. But the disease-free survival curves for ER-positive and ER-negative disease tend to merge with longer follow-up [21–24]. Similarly here, we see a time-varying effect for Ki67 as a biomarker of risk of developing breast cancer. In the high Ki67 group, 89% of the breast cancers occurred in the first 10 years; in the low Ki67 group, 83% of the breast cancers occurred after 10 years. With long follow-up (median 14.6 years), the curves merge.
In the often-proposed continuum of breast carcinogenesis—from normal lobules through hyperplasia to atypia to carcinoma—atypia occupies the highest risk category before cancer develops [25]. Identifying biomarkers that reflect the malignant potential of atypias serves several purposes: risk stratification of individual women at given time periods, clues to etiologic pathways during breast cancer progression, and the identification of relevant targets for risk reducing therapies.
Ki67 has been shown to be a relevant biomarker in several breast cancer settings. Most studies of Ki67 as a prognostic factor in both node-negative and node-positive breast cancer have demonstrated its ability to separate women into better and worse outcomes, at least in univariate analyses [26]. A recent study examined areas of DCIS occurring concomitantly with invasive breast cancer [14]. Areas of low grade DCIS had lower Ki67 expression. In the neoadjuvant setting, changes in Ki67 levels are being used to monitor response to both endocrine therapy and chemotherapy [27–29]. Similarly, there is strong interest in the scientific community to identify surrogate biomarkers for the evaluation of prevention strategies. Our findings raise the possibility that elevated Ki67 in women with atypia could be studied as a surrogate for the impact of various chemoprevention strategies. Our finding of a subgroup of women with atypia and elevated Ki67 levels, who are at higher risk of breast cancer within 10 years, may help explain the efficacy of tamoxifen in the prevention setting for women with atypia [30–32].
Ki67 expression has been examined previously in patients at risk for breast cancer. Shaaban et al. [33] measured Ki67 levels in samples from 38 women with proliferative disease without atypia: later, 16 developed breast cancer and 22 did not. The percentage of proliferating cells was significantly higher in the cases than controls; time to cancer was not reported. Hameed et al. [34] correlated Ki67 levels in 21 atypical ductal hyperplasias diagnosed by core biopsy and the likelihood of finding cancer on the excisional biopsy. Ki67 values were higher in the five women who had concomitant cancer. At least two groups studying women at increased risk of breast cancer by ductal lavage [35] or random periareolar fine needle aspiration [36] have shown that Ki67 expression is higher in samples containing atypical cells. Neither report provided follow-up for later breast cancer events. To our knowledge, ours is the largest cohort of patients with atypical hyperplasia, followed for subsequent breast cancers, in which Ki67 expression has been evaluated.
Our research group is interested in the development of a risk prediction model for both the short term (e.g. 5 or 10 years) and long term, utilizing tissue-based features. The Gail model’s ability to predict risk both at 5 years and over a woman’s lifetime is one of its strengths. When a woman is being counseled regarding her absolute risk of breast cancer, having data for a 5 or 10-year timeframe is often more helpful than long-term figures, given the pace at which new options for surveillance and risk reduction are being brought into the clinic.
There are strengths, but also limitations, to our study design. To our knowledge, this cohort of women with atypia, where all atypia samples have been confirmed by central pathology review, is the largest such cohort followed long term for breast cancer events. Nonetheless, the sample size is modest. The analysis and quantification of Ki67 staining using digital microscopy, including the assessment of every focus of atypia, should be more accurate and reproducible than manual interpretation [37–39]. Ours is a retrospective cohort study, permitting long follow-up (median 14.6 years) that allows us to examine time trends in breast cancer events.
In summary, we examined Ki67 levels and 10-year and longer-term risk of breast cancer in a cohort of women with atypical hyperplasia. Thirty percent of women with atypia had elevated expression of Ki67; these women had a fourfold increased risk of breast cancer within 10 years after their atypia biopsy. Conversely, those women with low Ki67 expression in their atypia tissue had an increased risk that was concentrated after 10 years of follow-up, compared to the general population. Ki67 appears to be a time-varying biomarker that may help to better stratify risk in women with atypia.
References
Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ 3rd, Visscher DW (2007) Benign breast disease and the risk of breast cancer. N Engl J Med 353(3):229–237
Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, Maloney SD, Pankratz VS, de Groen PC, Lingle WL, Ghosh K, Penheiter L, Tlsty T, Melton LJ 3rd, Reynolds CA, Hartmann LC (2007) Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol 25(19):2671–2677
London SJ, Connolly JL, Schnitt SJ, Colditz GA (1992) A prospective study of benign breast disease and the risk of breast cancer. JAMA 267(7):941–944
Dupont WD, Page DL (1985) Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 312(3):146–151
Chlebowski RT (2000) Reducing the risk of breast cancer. N Engl J Med 343(3):191–198
Pankratz VS, Hartmann LC, Degnim AC, Vierkant RA, Ghosh K, Vachon CM, Frost MH, Maloney SD, Reynolds C, Boughey JC (2008) Assessment of the accuracy of the gail model in women with atypical hyperplasia. J Clin Oncol 26(33):5374–5379
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31(1):13–20
Allred DC, Mohsin SK, Fuqua SA (2001) Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 8(1):47–61
Rudas M, Neumayer R, Gnant MF, Mittelbock M, Jakesz R, Reiner A (1997) p53 protein expression, cell proliferation and steroid hormone receptors in ductal and lobular in situ carcinomas of the breast. Eur J Cancer 33(1):39–44
Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP (2001) Immunodetectable cyclin D(1) is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer 84(8):1064–1069
Imamura H, Haga S, Shimizu T, Watanabe O, Kajiwara T, Aiba M (1999) Prognostic significance of MIB1-determined proliferative activities in intraductal components and invasive foci associated with invasive ductal breast carcinoma. Br J Cancer 79(1):172–178
Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ (2005) Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol 16(11):1723–1739
Balleine RL, Webster LR, Davis S, Salisbury EL, Palazzo JP, Schwartz GF, Cornfield DB, Walker RL, Byth K, Clarke CL, Meltzer PS (2008) Molecular grading of ductal carcinoma in situ of the breast. Clin Cancer Res 14(24):8244–8252
Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton LJ 3rd, Goode EL, Visscher DW (2006) Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst 98(22):1600–1607
Page DL, Dupont WD, Rogers LW, Rados MS (1985) Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55(11):2698–2708
Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18(6):695–706
Visscher DW, Pankratz VS, Santisteban M, Reynolds C, Ristimaki A, Vierkant RA, Lingle WL, Frost MH, Hartmann LC (2008) Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J Natl Cancer Inst 100(6):421–427
Liu S, Edgerton SM, Moore DH 2nd, Thor AD (2001) Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res 7(6):1716–1723
Natarajan L, Pu M, Parker BA, Thomson CA, Caan BJ, Flatt SW, Madlensky L, Hajek RA, Al-Delaimy WK, Saquib N, Gold EB, Pierce JP (2009) Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. Am J Epidemiol 169(12):1463–1470
Lundin J, Lehtimaki T, Lundin M, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, Isola J, Joensuu H (2006) Generalisability of survival estimates for patients with breast cancer-a comparison across two population-based series. Eur J Cancer 42(18):3228–3235
Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN (2003) Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat 78(1):105–118
Adami HO, Graffman S, Lindgren A, Sallstrom J (1985) Prognostic implication of estrogen receptor content in breast cancer. Breast Cancer Res Treat 5(3):293–300
Mason BH, Holdaway IM, Mullins PR, Yee LH, Kay RG (1983) Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res 43(6):2985–2990
Santen RJ, Mansel R (2005) Benign breast disorders. N Engl J Med 353(3):275–285
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23(28):7212–7220
Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, Renshaw L, Faratian D, Thomas J, Dowsett M, Krause A, Evans DB, Miller WR, Dixon JM (2006) Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol 24(19):3019–3025
Torrisi R, Bagnardi V, Cardillo A, Bertolini F, Scarano E, Orland L, Mancuso P, Luini A, Calleri A, Viale G, Goldhirsch A, Colleoni M (2008) Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER—and/or PgR-positive breast cancer: clinical and biological activity. Br J Cancer 99(10):1564–1571
Dowsett M, Dunbier AK (2008) Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res 14(24):8019–8026
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 90(18):1371–1388
Riggs BL, Hartmann LC (2003) Selective estrogen-receptor modulators–mechanisms of action and application to clinical practice. N Engl J Med; 348(7):618–629
Dunn BK, Wickerham DL, Ford LG (2005) Prevention of hormone-related cancers: breast cancer. J Clin Oncol 23(2):357–367
Shaaban AM, Sloane JP, West CR, Foster CS (2002) Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol 160(2):597–604
Hameed O, Ghali VS, Tartter PI, Mizrachi H (2005) Immunohistochemical staining for cyclin D1 and Ki-67 aids in the stratification of atypical ductal hyperplasia diagnosed on breast core biopsy. Am J Clin Pathol 124(6):862–872
Cazzaniga M, Severi G, Casadio C, Chiapparini L, Veronesi U, Decensi A (2006) Atypia and Ki-67 expression from ductal lavage in women at different risk for breast cancer. Cancer Epidemiol Biomarkers Prev 15(7):1311–1315
Khan QJ, Kimler BF, Clark J, Metheny T, Zalles CM, Fabian CJ (2005) Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev 14(4):786–789
Bloom K, Harrington D (2004) Enhanced accuracy and reliability of HER-2/neu immunohistochemical scoring using digital microscopy. Am J Clin Pathol 121(5):620–630
Tawfik OW, Kimler BF, Davis M, Donahue JK, Persons DL, Fan F, Hagemeister S, Thomas P, Connor C, Jewell W, Fabian CJ (2006) Comparison of immunohistochemistry by automated cellular imaging system (ACIS) versus fluorescence in situ hybridization in the evaluation of HER-2/neu expression in primary breast carcinoma. Histopathology 48(3):258–267
Zhang K, Prichard JW, Yoder S, De J, Lin F (2007) Utility of SKP2 and MIB-1 in grading follicular lymphoma using quantitative imaging analysis. Hum Pathol 38(6):878–882
Acknowledgments
This work was supported by NCI RO1 grant CA132879, Mayo Clinic Breast Cancer SPORE CA116201, and the Martha and Bruce Atwater Foundation. We thank Linda Murphy for immunostaining; Teresa Allers, Jo Johnson, Mary Campion, Melanie Kasner, and Romayne Thompson for data collection; Joel Worra for database development; Ann Harris and the Survey Research Center for patient follow-up; and Vicki Shea for assistance with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santisteban, M., Reynolds, C., Barr Fritcher, E.G. et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat 121, 431–437 (2010). https://doi.org/10.1007/s10549-009-0534-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0534-7