Abstract
We evaluated the efficacy and safety of sequential therapy with trastuzumab monotherapy (H-mono) followed by H plus docetaxel (D) after disease progression (H → H + D) versus combination therapy with H + D as first-line therapy. Patients with human epidermal growth factor receptor type 2 (HER2)-positive metastatic breast cancer (MBC) and left ventricular ejection fraction >50% were randomly assigned to either (a) H → H + D [H, once weekly 2 mg/kg (loading dose, 4 mg/kg); D, once every 3 weeks 60 mg/m2] or (b) H + D. Primary endpoints were progression-free survival (PFS) for the H-mono stage of the H → H + D group and H + D group and overall survival (OS) for both groups. Secondary endpoints were overall response rate, time to treatment failure, second PFS and safety. The planned number of patients was 160 patients in total. Of 112 patients enrolled, 107 were eligible. After 112 patients were enrolled, the Independent Data Monitoring Committee recommended stopping enrollment because PFS and OS were greater in the H + D group than the H → H + D group. Median PFS was 445 days in the H + D group versus 114 days for H-mono in the H → H + D group [hazard ratio (HR), 4.24; P < 0.01]. OS was significantly longer in the H + D group (HR, 2.72; P = 0.04). H + D therapy is significantly superior to H → H + D therapy as first-line therapy in patients with HER2-positive MBC, especially in terms of OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is difficult to establish the standard therapeutic approach for patients with metastatic breast cancer (MBC) because of diverse tumor biology and the need to consider individual patient characteristics and personal preferences. The algorithm for MBC proposed by Hortobagyi in 1998 [1] is based on hormone therapy sensitivity in addition to disease status and risk of progression. In 2001, Piccart proposed a new algorithm [2] by adding HER2 expression status and trastuzumab therapy to Hortobagyi’s algorithm.
Trastuzumab (Herceptin®, Genentech, Inc., Roche, Chugai) monotherapy (H-mono) and trastuzumab combined with chemotherapy (H + CT) are both recommended in the NCCN Guidelines as first-line treatment for MBC [3]. However, to date, the relative superiority of the two strategies has not been directly compared.
Addition of trastuzumab significantly improves the efficacy of first-line chemotherapy in patients with HER2-positive MBC, as demonstrated in a large-scale phase III trial [4], which showed an overall response rate (ORR) of 50% and overall survival (OS) of 25.1 months for trastuzumab in combination with anthracycline and paclitaxel. Furthermore, after this clinical trial started, a similar additive effect was shown for trastuzumab in combination with first-line docetaxel (Taxotere®, Sanofi-Aventis), with the combination achieving an ORR of 61% and OS of 31.2 months [5]. In comparison, an ORR of 26% and OS of 24.4 months were reported in patients with HER2-positive MBC treated with H-mono as first-line therapy [6]. H + CT therefore appears to increase ORR compared with H-mono; however, the two strategies result in similar OS although they have not been directly compared in clinical trials. If OS is equivalent with the two strategies, then it can be assumed that starting treatment with H-mono should provide quality of life benefits compared with first-line H + CT. We conducted this phase III comparative trial to compare sequential and combination trastuzumab-based strategies. Trastuzumab was continued after disease progression in the H-mono group because the benefits of this approach have been reported from multiple retrospective studies [7–10] and continuous use is frequently used in clinical practice. Of note, the benefits of continuous administration of trastuzumab after disease progression have also been reported from recent prospective trials [11, 12]. Data from this trial, designed to compare H-mono and H + CT as used in current clinical practice, suggest a preferred option for trastuzumab-based first-line therapy for HER2-positive MBC.
Patients and methods
Trial design
This was a phase III, open-label, randomized, multi-center, comparative trial in patients with HER2-positive MBC. Patients were randomly assigned to one of two groups: initial treatment with trastuzumab alone, followed by combination therapy with trastuzumab and docetaxel after disease progression (H → H + D), or initial combination therapy with trastuzumab and docetaxel (H + D). In the H → H + D group, trastuzumab was administered weekly with a starting dose of 4 mg/kg followed by 2 mg/kg as the second and subsequent doses, and docetaxel 60 mg/m2 was administered every 3 weeks following disease progression. Disease progression was defined as an increase in the size of the target lesion or the appearance of a new lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) [13]. In the H + D group, trastuzumab and docetaxel were administered from the start of treatment using the same doses and schedules as described for the sequential therapy group. A minimization procedure with the biased coin method, using the status of liver metastases, and previous treatment with paclitaxel and anthracyclines as adjustment valuables, was applied for central randomization.
The major inclusion criteria were (1) female patients with breast cancer confirmed by tissue diagnosis and cytologic diagnosis, (2) HER2-positive breast cancer (3+ on immunohistochemical [IHC] analysis or gene amplification by fluorescence in situ hybridization [FISH]-positive as determined by the local institution) confirmed in the primary lesion(s) (in the case of bilateral breast cancer, both right and left lesions) or in the target metastatic lesion(s), (3) measurable lesion(s) fulfilling RECIST criteria, (4) Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of zero or one, and (5) left ventricular ejection fraction (LVEF) >50% on echocardiography.
The joint primary endpoints were (1) progression-free survival (PFS; the time from randomization to disease progression or death due to any cause) in the H-mono stage of the H → H + D group and during combination therapy with trastuzumab and docetaxel in the H + D group and (2) overall survival (OS). In the H → H + D group, events and deaths reported during the H-mono stage were included in the analysis. Patients who did not show disease progression at the time of analysis were censored on the day of last observation. Tumor size was evaluated based on RECIST guidelines. The secondary endpoints were (1) time to treatment failure (TTF) in the H-mono stage of the H → H + D group and during combination therapy with trastuzumab and docetaxel in the H + D group, (2) overall response rate (ORR) in the H-mono stage of the H → H + D group and during combination therapy with trastuzumab and docetaxel in the H + D group, (3) ORR in the combination stage with trastuzumab and docetaxel in the H → H + D group, (4) PFS during combination therapy with trastuzumab and docetaxel in the H + D group (2nd PFS), and (5) safety as measured by National Cancer Institute-Common Toxicity Criteria (NCI-CTC Version 2.0). The second PFS was defined as the period from the day of randomization until disease progression or death from any cause after initiating trastuzumab and docetaxel (2nd PD), where the patients in the H → H + D group, who did not proceed to the combination therapy after their first progressive disease (PD), were counted as events and patients who discontinued treatment without confirming first PD were censored. Adverse drug reactions (ADR) were defined as any adverse event for which a causal relationship with at least one of trastuzumab and docetaxel could not be ruled out.
Sample size setting and statistical analysis
The target number of patients was 80 per group and 160 in total. The trial was designed to select the most appropriate therapy by investigating whether PFS with trastuzumab monotherapy is remarkably shorter than with trastuzumab in combination with chemotherapy. The H → H + D group was considered to be clinically inferior to the H + D group if the point estimate of hazard ratio (HR) of the H-mono stage of the H → H + D group versus the H + D group was ≥1.3 in this trial. The necessary number of events was set to 120 by an assessment of study feasibility and the following power consideration: when assuming that the true HR is 1.6, the H → H + D group is judged as inferior to the H + D group with a probability of 87%, whereas, when assuming that the HR is 1, the H → H + D group is judged as not inferior to the H + D group with a probability of 93%. A total of 160 patients were required to obtain data for 120 events from the two groups during the accrual period of 3 years and follow-up of 1 year.
For time to event endpoints (PFS, OS, and TTF), Kaplan–Meier curves were calculated, and differences in hazard rates between the H-mono stage in the H → H + D group (or the H → H + D group) and the H + D group were tested at the 5% significance level by the two-sided log-rank test. In addition, the HR and 95% confidence interval (CI) of each group were calculated using the Cox regression. For ORR, response rates and 95% CI were calculated, with differences between the H-mono stage in the H → H + D group and the H + D group tested at the 5% significance level by the two-sided chi-square test (continuously correction).
Interim analysis
An interim analysis was prospectively planned for 1 year after stating treatment in the 80th patient (corresponding to a half of the target number of patients). If a significant difference in survival was detected between the two groups, with a two-sided significance level of 1% by the log-rank test, a protocol change would be performed, including discontinuation of patient registration to the group with inferior survival or early termination of the trial. Furthermore, it was prospectively planned to review efficacy data during the study period and to make protocol changes or terminate the trial if the efficacy was markedly low in the combination therapy stage of the H → H + D group.
Results
The interim analysis was scheduled for June 5, 2008 (1 year after the start of treatment of the 80th patient on June 5, 2007). However, a significant difference in survival between the two groups was identified during a review of the efficacy data in March 2008. Consequently, a meeting of the Independent Monitoring Committee (IDMC) was held in June 2008. The HR for PFS was 4.24 (95% CI, 2.48–7.24, P < 0.01 in log-rank test), which was significantly higher than the HR of 1.3 specified in the protocol. This result suggested that trastuzumab monotherapy was significantly inferior to the initial combination of trastuzumab and docetaxel. Furthermore, the OS hazard was significantly lower in the H + D group than in the H → H + D group (P = 0.03 in log-rank test) although the significance level specified for the interim analysis (1%) was not reached. Based on these results, the IDMC made the following three recommendations: (1) discontinue new enrollment to the trial, (2) allow combination with docetaxel before progression of disease in the H-mono group, and (3) complete data analysis and publish the results of the study early. Based on these recommendations, the interim analysis was performed using May 2, 2008 as the data cut-off date. Furthermore, the IDMC recommended that updated OS data be provided when any new information became available. We report here the updated OS data as of September 1, 2008.
Patients
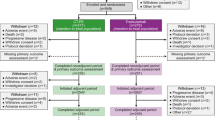
In this trial, 112 patients were enrolled (56 patients per group) between September 2004 and May 2008. The analysis was performed on 111 patients in total, 56 patients assigned to the H → H + D group and 55 patients to the H + D group, because treatment of one patient in the H + D group had not started at the time of data cutoff. Of the 111 patients, 108 patients (H → H + D group, 55; H + D group, 53) were included in the safety population excluding three patients who were not treated (H → H + D group, 1; H + D group, 2). Of the 108 patients included in the safety population, 107 patients (H → H + D group, 54; H + D group, 53) were included in the modified-intention-to-treat (ITT) population (following the intention-to-treat principle) excluding one patient in the H → H + D group who did not meet the eligibility criteria. In the H → H + D group, 44 of 55 patients started on the H-mono regimen showed disease progression. Of the 44 patients, 36 started combination therapy with docetaxel, and 22 of the 36 patients showed further disease progression. Of the 53 patients who started treatment in the H + D group, 24 showed disease progression (Fig. 1). Demographic data, treatment history and tumor-related characteristics were well balanced between the two groups (Table 1).
Study design CONSORT patient enrollment diagram. Open-label randomized, multi-center, comparative trial in patients with HER2-positive MBC. Patients were randomly assigned to one of two groups: initial treatment with trastuzumab alone, followed by combination therapy with trastuzumab and docetaxel after disease progression (H → H + D), or initial combination therapy with trastuzumab and docetaxel (H + D)
Efficacy
The median PFS was 3.7 months (114 days) in the H-mono stage of the H → H + D group and 14.6 months (445 days) in the H + D group. The HR was 4.24 (95% CI, 2.48–7.24, P < 0.01 in log-rank test) corresponding to a longer PFS in the H + D group than in the H → H + D (H-mono stage) (Fig. 2). The Kaplan–Meier curve for OS is shown in Fig. 3 (data cutoff date: September 1, 2008). OS hazard was also significantly lower in the combined group (P = 0.04 in log-rank test) (Fig. 3). The HR was 2.72 (95% CI, 1.03–7.18) although the median of OS was not available because the number of deaths was small in both groups: 13 (24.1%) in the H → H + D group and 6 (11.3%) in the H + D group.
As regards PFS hazards, the H + D regimen was superior to the H-mono regimen in all subgroups except for a subgroup of patients previously treated with paclitaxel (Fig. 4). The likelihood ratio test for interaction based on Cox proportional hazards regression was performed (each factor tested separately). These P values indicated that there were no statistically significant interactions.
The median TTF was 114 days in the H-mono stage of the H → H + D group compared with 332 days in the H + D group. TTF hazard was significantly lower in the H + D group than the H → H + D group (HR 2.81; 95% CI 1.77–4.47, P < 0.01 in log-rank test) although it was shorter than the corresponding median PFS of 445 days. The response rate was significantly higher in the H + D group [67.9% (36/53)] than in the H-mono stage of the H → H + D group [14.8% (8/54)]. PD was reported as the best overall response in 16 patients (29.6%) in the H-mono stage of the H → H + D group compared with none in the H + D group (Table 2). In 36 patients who proceeded to combination therapy in the H → H + D group, response rate was evaluated based on tumor size measured immediately before initiating H + D. The response rate was 47.2% (17/36 patients) and PD was reported as the best overall response in 13.9% (5/36 patients). The median PFS in the combination therapy stage (2nd PFS) of the H → H + D group was 12.4 months (377 days). The HR was 1.35 (95% CI 0.79–2.30, P = 0.27 in log-rank test) compared with the H + D group although it is not appropriate to directly compare second PFS with PFS in the H + D group (Fig. 5).
Safety
The incidence of ADRs was 96% (53 patients, 746 cases) in the H → H + D group [93% in the H-mono stage (51 patients, 264 cases)] and 100% (53 patients, 908 cases) in the H + D group. The incidence of grade 3/4 ADRs, according to NCI-CTC criteria, was 62% (34 patients, 77 cases) in the H → H + D group [15% in the H-mono stage (eight patients, ten cases)] and 87% (46 patients, 98 cases) in the H + D group. The most frequent grade 3/4 ADRs were leukopenia and neutropenia (Table 3). Grade 3/4 leukopenia and neutropenia were not observed in the H-mono stage of the H → H + D group, but were reported in 20 patients (36%) and 28 patients (51%), respectively, in the overall H → H + D group, and in 32 patients (60%) and 42 patients (79%), respectively, in the H + D group. The markedly higher incidence of grade 3/4 leukopenia and neutropenia in the overall H → H + D group and the H + D group when compared with the H-mono stage of the H → H + D group suggests that these grade 3/4 hematologic toxicities were strongly related to docetaxel. The incidence of grade 3/4 ADRs was lower in the H → H + D group than in the H + D group; however, in the 36 patients switched to combination therapy, the incidence rates for leukopenia (56%) and neutropenia (78%) were similar to rates in the H + D group. Grade 3/4 febrile neutropenia was not observed in the H-mono stage, but reported in two patients (4%) in the overall H → H + D group and in four patients (8%) in the H + D group.
Left ventricular ejection fraction decreased to <50% in two patients (4.1%) in the H → H + D group (none in the H-mono stage) and in one (2.0%) in the H + D group (Table 4). None of these three patients had been previously treated with an anthracycline agent. None of the patients had an LVEF decrease to <45%. A difference between baseline LVEF and most lowered LVEF exceeding ten ejection fraction (EF) points was observed in 12 patients (24.5%) in the overall H → H + D group [7 (18.9%) in the H-mono stage] and in 11 patients (22.0%) in the H + D group. Of the seven patients observed in the H-mono stage, 2 (4.1%) showed a difference exceeding 20 EF points during combination therapy. The decrease in LVEF was similar in H → H + D and H + D groups although a slightly greater effect was observed during the H-mono stage compared with H in combination with docetaxel in both the H → H + D and H + D groups.
Six patients discontinued due to adverse events in the overall H → H + D group (one in the H-mono stage) compared with eight in the H + D group. While adverse event-related discontinuation was more commonly reported in the H + D group than during the H-mono stage, discontinuation due to withdrawal of consent was more common in the H-mono stage than in the H + D group [six in the overall H → H + D group (five in the H-mono stage) and one in the H + D group]. However, a detailed review showed that these five patients in the H-mono stage all withdrew consent after disease progression and refused subsequent administration of docetaxel.
Discussion
This randomized phase III comparative trial in women with HER2-positive MBC was conducted to evaluate sequential treatment with trastuzumab monotherapy followed by the combination of trastuzumab and docetaxel (H → H + D group) compared with initial combination therapy with trastuzumab and docetaxel (H + D group).
Progression-free survival and OS were superior in the H + D group compared with the H → H + D group. The most commonly used treatments in the period following disease progression were trastuzumab, vinorelbine, and capecitabine. Trastuzumab was continued after progression in about 90% of patients. Post-progression therapy was considered to have had no influence on OS because agents used in the follow-up period were well balanced between the two groups.
This trial was designed to test the hypothesis that survival would not be substantially different between initial treatment with trastuzumab monotherapy or the combination of trastuzumab and docetaxel. However, the hypothesis was rejected based on the results and the prospective decision rule that outcome in the H → H + D group was considered to be inferior to that in the H + D group if the point estimate of HR was ≥1.3 (for H → H + D vs. H + D groups).
Results from a randomized controlled trial (HERTAX), using a similar but not identical design to that of the present trial, have been published in abstract form at the ASCO Annual Meeting 2008 [14]. Patients with HER2-positive MBC were randomly allocated to receive first-line treatment with trastuzumab plus docetaxel (H + D) or trastuzumab monotherapy followed at disease progression by docetaxel alone (H → D). In HERTAX, PFS, and ORR were similar in the H + D and H → D groups, but there was a difference of >10 months in median OS between groups (30.5 vs. 20.2 months, respectively). However, the difference in median OS did not achieve statistical significance (HR: 1.45, 95% CI 0.87–2.41; P = 0.15). Although results from our trial and HERTAX showed similar trends in efficacy, the outcomes were slightly different in that no statistically significant difference in OS was demonstrated in HERTAX, PFS was longer in the H + D group in our trial than in HERTAX, and the incidence of adverse events was higher in HERTAX. These differences may be attributable to the different trial designs. After disease progression on H-mono, patients switched to trastuzumab plus docetaxel in the H → H + D group in our trial, whereas they switched to docetaxel monotherapy in HERTAX. The dose of docetaxel was 60 mg/m2 in our trial compared with 100 mg/m2 in HERTAX, and the median number of treatment cycles delivered was eight (range 1–39) versus six, respectively. We believe that the low dose of docetaxel used in our trial allowed delivery of an increased number of cycles resulting in improved efficacy for the combination of trastuzumab and docetaxel and a statistically significant difference in OS between treatment groups. The higher incidence of adverse events in HERTAX compared with our study may also be attributable to the higher dose of docetaxel administered.
In the M77001 study comparing docetaxel monotherapy with docetaxel plus trastuzumab [5], median PFS was 11.7 months and median OS was 31.2 months for the combination. In our study, the median PFS (14.6 months) was higher and the median OS was not reached. Another phase II trial [15–17] investigating combination therapy with docetaxel (100 mg/m2) and trastuzumab did not report superior results to our trial despite using a higher dose of chemotherapy. Febrile neutropenia occurred in 23% of patients treated with trastuzumab and docetaxel in the M77001 study, while the rate was 8% in our trial. This difference may also be attributable to the higher dose of docetaxel in M77001. The results of these studies suggest that superior efficacy and tolerability may be possible with a docetaxel dose of 60 mg/m2 as used in our trial compared with the higher dose of 100 mg/m2 used in HERTAX.
An adverse event of concern was cardiac toxicity. However, congestive heart failure was not reported during the trial in either group, and only two patients in the H → H + D group experienced a decrease in LVEF of over 20 points and none developed LVEF <45%.
Based on the results of our trial, we consider that, for future clinical practice, it is preferable to start first-line treatment of MBC with a combination of trastuzumab and chemotherapy. The docetaxel dose of 60 mg/m2 used in our trial allows combination therapy to be administered in a long-term with manageable adverse events. However, the population in our trial consisted mainly of patients with visceral metastases, and those with only bone metastasis were not included. The most appropriate treatment approach for patients with metastasis limited to the bone should be determined based on individual patient characteristics.
References
Hortobagyi GN (1998) Treatment of breast cancer. N Engl J Med 339:974–984
Piccart MJ (2001) Proposed treatment guidelines for HER2-positive metastatic breast cancer in Europe. Ann Oncol 12(Suppl.1):S89–S94
NCCN Clinical Practice Guidelines in Oncology version 1 (2009) Breast cancer. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726
Fountzilas G, Razis E, Tsavdaridis D et al (2003) Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the Hellenic cooperative oncology group. Clin Breast Cancer 4:120–125
Gelmon KA, Mackey J, Verma S et al (2004) Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 5:52–58
Antoine EC, Extra JM, Vincent-Salomon A et al (2007) Multiple lines of trastuzumab provide a survival benefit for women with metastatic breast cancer: results from the Hermine cohort study [abstract]. Eur J Cancer 5(suppl):213 Abstract 2099
Mackey J, Gelmon KA, Verma S et al (2002) Continued use of Herceptin after disease progression in women with HER2-positive (HER2+) metastatic breast cancer (MBC): results from a retrospective analysis of 105 cases [abstract]. J Clin Oncol 21:52a Abstract 207
Von Minckwitz G, Zielinski C, Maarteense E et al (2008) Capecitabine vs. capecitabine + trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: the TBP phase III study (GBG 26/BIG 3–05) [abstract]. J Clin Oncol 26(suppl):47s Abstract 1025
O’Shaughnessy J, Blackwell KL, Burstein H et al (2008) A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [abstract]. J Clin Oncol 26(suppl):44s Abstract 1015
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States, National cancer institute of Canada. J Natl Cancer Inst 92:205–216
Bontenbal M, Seynaeve C, Stouthard J et al (2008) Randomized study comparing efficacy/toxicity of monotherapy trastuzumab followed by monotherapy docetaxel at progression, and combination trastuzumab/docetaxel as first-line chemotherapy in HER2-neu positive, metastatic breast cancer (MBC) (HERTAX study) [abstract]. J Clin Oncol 26(suppl):44s Abstract 1014
Baselga J (2001) Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivotal trial. Oncology 61(suppl 2):14–21
Tripathy D, Slamon D, Leyland-Jones B et al (2000) Treatment beyond progression in the Herceptin pivotal combination chemotherapy trial [abstract]. Breast Cancer Res Treat 64:32 Abstract 25
Bullock K, Blackwell K (2008) Clinical efficacy of taxane–trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist 13:515–525
Acknowledgments
We thank the patients who participated in this trial and their families; the medical, nursing, and research staff at the institutions; the independent data and safety monitoring committee; the monitors, data managers, statisticians, and programmers at Chugai Pharmaceutical and EPS. This study was sponsored and funded by Chugai Pharmaceutical.
Author information
Authors and Affiliations
Corresponding author
Additional information
List of contributions are given in Appendix.
Appendix
Appendix
Contributions
Conception and design: Yasuo Ohashi, Masashi Ando, Toru Watanabe.
Financial support: Chugai Pharmaceutical Co., Ltd.
The following investigators and institutions also participated in the trial:
Junichiro Watanabe, Shizuoka Cancer Center, Shizuoka, Japan; Yoshinori Ito, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan; Keisei Anan, Kitakyushu Municipal Medical Center, Fukuoka, Japan; Yuichi Takatsuka, Kansai Rosai Hospital, Hyogo, Japan; Hitoshi Arioka, Yokohama Rosai Hospital, Kanagawa, Japan; Kenichi Watanabe, Hokkaido Cancer Center, Hokkaido, Japan; Reiki Nishimura, Kumamoto Municipal Hospital, Kumamoto, Japan; Kenjiro Aogi, National Hospital Organization Shikoku Cancer Center, Ehime, Japan; Seigo Nakamura, St. Luke’s International Hospital, Tokyo, Japan; Nobuaki Sato, Niigata Cancer Center Hospital, Niigata, Japan; Masato Koseki, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Hiroshima, Japan; Yutaka Tokuda, Tokai University Hospital, Kanagawa, Japan; Shinji Ohno, National Kyushu Cancer Center, Fukuoka, Japan; Shigeru Murakami, Hiroshima University Hospital, Hiroshima, Japan; Akihiko Chiba, Kanagawa Cancer Center, Kanagawa, Japan; Hideo Inaji, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan; Shintaro Takao, Hyogo Cancer Center, Hyogo, Japan; Hiroko Yamashita, Nagoya City University Hospital, Aichi, Japan; Syouji Oura. Wakayama Medical University Hospital, Wakayama, Japan.
Data analysis and interpretation: Yasuo Ohashi, Shigeto Miura, Kenji Eguchi, Tetsu Shinkai.
Manuscript writing: Kenichi Inoue, Kazuhiko Nakagami, Mitsuhiro Mizutani, Yasuo Hozumi, Yasuhiro Fujiwara, Norikazu Masuda, Fumine Tsukamoto, Mitsue Saito, Shigeto Miura, Kenji Eguchi, Tetsu Shinkai, Masashi Ando, Toru Watanabe, Noriyuki Masuda, Yasuo Ohashi, Muneaki Sano, Shinzaburo Noguchi. Editorial assistance was provided by Medi-Kelsey Limited. All authors contributed materially to drafts and revisions and approved the final manuscript.
Final approval of manuscript: Kenichi Inoue, Yasuo Ohashi, Shinzaburo Noguchi.
Rights and permissions
About this article
Cite this article
Inoue, K., Nakagami, K., Mizutani, M. et al. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res Treat 119, 127–136 (2010). https://doi.org/10.1007/s10549-009-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0498-7