Abstract
An immunohistochemical study was performed using tissue arrays and specific antibodies against MMPs -1, -2, -7, -9, -11, -13, -14, and TIMPs -1, -2 and -3. More than 5,000 determinations on cancer specimens from 124 patients with invasive breast cancer were performed at the center of the tumor and the invasive front. Immunostaining for MMPs/TIMPs by fibroblasts was evaluated. To identify specific groups of tumors with distinct expression profiles, the data obtained from both fibroblast populations were analyzed by unsupervised hierarchical cluster analysis. Intratumor stromal fibroblasts more frequently showed expression of MMP-2, -7, and -14, and TIMP-3, but less frequently of MMP-9 than fibroblasts at the invasive front. Multivariate analysis showed that a high profile of MMPs and TIMPs staining in both fibroblast populations was the most potent predictor factor of distant metastases, whereas a low staining profile in fibroblasts was associated with a low risk of metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classically, the evaluation of prognostic factors has been performed only on the malignant epithelial cells. However, the influence of stromal gene and protein expression patterns on the biological and clinical heterogeneity of the disease is poorly understood. Under normal physiological conditions, stroma acts as an important barrier to the transformation of epithelial cells [1] Nevertheless, the stromal compartment undergoes changes in response to emerging epithelial lesions and has a key role in cancer initiation and progression, including the recruitment of new stromal cells that provide factor involved in cell growth and matrix remodeling [1–3]Recent data indicate that whereas the gene expression signatures derived from whole tumors generate clusters associated with estrogen receptors (ER) and HER2 status [4, 5] differential gene expression from the tumor stroma generates clusters linked to clinical outcome in breast cancer [6] In addition, there is recent evidence pointing to the contribution of inter-patient stromal variability to the biological and clinical heterogeneity of breast cancer [7–15]. Accordingly, we have recently reported that the expression of several metalloproteases (MMPs) and their inhibitors (TIMPs) by intratumor stromal fibroblasts was significantly and independently associated with a high rate of distant metastases [16].
MMPs play an essential role in the degradation of the stromal connective tissue and basement membrane components, which are key elements in tumor invasion and metastasis. In addition, MMPs are able to impact in vivo on tumor cell behaviour as a consequence of their capacity to cleave growth factors, cell surface receptors, cell adhesion molecules, and chemokines/cytoquines [17–19]. Furthermore, by cleaving proapoptotic factors, MMPs produce a more aggressive phenotype via generation of apoptotic resistant cells [20]. MMPs also regulate cancer-related angiogenesis, positively through their ability to mobilize or activate proangiogenic factors [21], and negatively via generation of angiogenesis inhibitors, such as angiostatin and endostatin, cleaved from large protein precursors [22] On the other hand, it is now accepted that TIMPs are multifactorial proteins also involved in the induction of proliferation and the inhibition of apoptosis [23, 24].
The fact that the expression of MMPs and TIMPs by intratumor stromal fibroblasts could be of clinical relevance led us to consider the biological and clinical potential significance of the expression of this enzymatic system by fibroblasts located at the invasive front of breast carcinomas, since the invasive front is the area where some of the most important interactions between cancer cells and the tumor supporting stroma take place [25]. Therefore, the pattern of MMPs/TIMPs expression by fibroblasts at the invasive front could reflect prognosis better than at other tumor areas or, even provide useful complementary information of clinical interest. Consequently, the aims of the present work were to compare the expression of MMPs and TIMPs by intratumor stromal fibroblasts and by those located at the invasive front of breast carcinomas, their relationship with clinicopathological characteristics and their prognostic significance. Our results demonstrate different patterns of expression of MMPs and TIMPs between fibroblasts belonging to those two tumor areas, and their combined evaluation may provide a highly predictive tool of distant metastases.
Materials and methods
Patient selection, characteristics and tissue specimen handling
This study is comprised of 124 women with a histologically confirmed diagnosis of early breast cancer and treated between 1990 and 2003. We selected women with the following inclusion criteria: invasive ductal carcinoma, at least six histopathologically assessed axillary lymph nodes and a minimum of 5 years of follow-up for those women without tumor recurrence. The exclusion criteria were the following: metastatic disease at presentation, prior history of any kind of malignant tumor, bilateral breast cancer at presentation, having received any type of neoadjuvant therapy, development of loco-regional recurrence during the follow-up period, development of a second primary cancer, and absence of sufficient tissue in the paraffin blocks used for manufacturing the TMAs. From a total of 1,264 patients fulfilling these criteria, we randomly selected a sample size of 124 patients in accordance to four different groups with similar size and stratified with regard to nodal status and to the development of metastatic disease, which were the key measure variables of the study. Thus, we included an important number of events in both node-positive and -negative patient subgroups (half of the cases that developed distant metastases during the follow-up period are included in each subgroup) in order to guarantee the statistical power of the survival analysis. Patient characteristics included in the two main groups, with or without distant metastases, are listed in Table 1.
Women were treated according to the guidelines used in our institution. The study adhered to national regulations and was approved by our institution Ethics and Investigation Committee. The end-point was distant metastatic relapse. The median follow-up period in patients without metastases was 85 months, and 46 months in patients with metastases. In addition, in the present study we analyzed the expression of the factors in normal mammary tissues obtained from 14 women that underwent cytoreductive surgery of the breast (age range: 35–60 years).
Tissue microarrays and immunohistochemistry
Breast carcinoma tissue samples were obtained at the time of surgery. Routinely fixed (overnight in 10% buffered formalin), paraffin-embedded tumor samples stored in our pathology laboratories were used. Histopathologically representative tumor areas were defined on haematoxylin and eosin-stained sections and marked on the slide. Tumor tissue array (TMA) blocks were obtained by punching a tissue cylinder (core) with a diameter of 1.5 mm through a histologically representative area of each ‘donor’ tumor block, which was then inserted into an empty ‘recipient’ tissue array paraffin block using a manual tissue arrayer (Beecker Instruments, Sun Praerie, WI, USA) as described elsewhere [26]. Collection of tissue cores was carried out under highly controlled conditions. Areas of non-necrotic cancerous tissue were selected for arraying by two experienced pathologists (L. O. González and A. M. Merino). A total of four cores were used for each case. Two of these cores in each case corresponded to the tumor central area, and the other two cores corresponded to the invasive front. This method, evaluating two cores (double redundancy) of each tumor area has been shown to correlate well with conventional immunohistochemical staining [27]. The invasive front was defined as the tumoral advancing edge. This corresponds to a 2 mm margin surrounding the tumor and containing cancerous cells. From the 124 tumor samples available, four TMA blocks were prepared, each one containing 31 primary tumor samples, as well as internal controls including four normal breast tissue samples from two healthy women who underwent reductive mammary surgery. These latter samples contained epithelial components on which immunohistochemistry was not seen with any of the antibodies used.
Serial 5-μm sections of the high-density TMA blocks were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slide. One section from each tissue array block was stained with H&E, and these slides were then reviewed to confirm that the sample was representative of the original tumor. Immunohistochemical staining was done on these sections of TMA fixed in 10% buffered formalin and embedded in paraffin using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Antibodies for MMPs and TIMPs were obtained from Neomarker (Lab Vision Corporation, Fremont, CA, USA). The dilution for each antibody was established based on negative and positive controls (1/50 for MMP-2, -7, -14, and TIMP-2; 1/100 for MMP- 9, -13, TIMP-1, and -3; and 1/200 for MMP-1, -11). The negative control was DakoCytomation mouse serum diluted to the same mouse IgG concentration as the primary antibody. All the dilutions were made in Antibody Diluent, (Dako) and incubated for 30 min at room temperature. In a prior report, we confirmed the presence of the evaluated proteins by Western blot analysis of breast tumor cytosol samples. A single band of the expected molecular mass was observed for each protein [28]. We also used other antibodies for several factors, such as MMP-2 (policlonal, 1/50, Abcam Cambridge UK.), -13 (clone 181-15A12, 1/100, Calbiochem MERCK KgaA Darmstadt Germany), -11(clone SC3-05, 1/100, Calbiochem MERCK KgaA Darmstadt Germany). On the other hand, we also used antibodies against cytokeratins (AE1–AE3, DAKO 1/1) and vimentin (DAKO 1/100) to distinguish fibroblasts from tumoral cells.
Tissue sections were deparaffinized in xylene and then rehydrated in graded concentrations of ethyl alcohol (100, 96, 80, 70%, then water). To enhance antigen retrieval only for some antibodies, TMA sections were microwave treated in a H2800 Microwave Processor (EBSciences, East Granby, CT, USA) in citrate buffer (Target Retrieval Solution; Dako) at 99°C for 16 min. Endogenous peroxidase activity was blocked by incubating the slides in peroxidase-blocking solution (Dako) for 5 min. The EnVision Detection Kit (Dako) was used as the reactivity detection system. Sections were counterstained with haematoxylin, dehydrated with ethanol and permanently coverslipped.
For each antibody preparation studied, the location of immunoreactivity, percentage of reactive area and intensity were determined. All the cases were semiquantified for each protein-stained area. An image analysis system with the Olympus BX51 microscope and soft analysis (analySIS®, Soft imaging system, Münster, Germany) were used as follows: tumor sections were stained with antibodies according to the method explained above and counterstained with haematoxylin. There were different optical thresholds for both stains. Each core was scanned with a 400× power objective in two fields per core. Fields were selected searching for the protein-reactive areas. The computer program selected and traced a line around antibody-reactive areas (higher optical threshold: red spots), with the remaining, non-stained areas (haematoxylin-stained tissue with lower optical threshold) standing out as a blue background. Any field had an area ratio of stained (red) versus non-stained (blue). A final area ratio was obtained after averaging two fields. To evaluate immunostaining intensity we used a numeric score ranging from 0 to 3, reflecting the intensity as follows: 0, no reactivity; 1, weak reactivity; 2, moderate reactivity; and 3, intense reactivity. Using an Excel spreadsheet, the mean score was obtained by multiplying the intensity score (I) by the percentage of reactivity area (PA) and the results were added together (total score: I × PA). This overall score was then averaged with the number of cores that were done for each patient. If there was no tumor in a particular core, then no score was given. In addition, for each tumor the mean score of two core biopsy samples was calculated. This scoring evaluation was based on a global evaluation of staining areas corresponding to tumoral cells as well as to stromal cells. Nevertheless, in the present work we also evaluate the immunohistochemical staining by exclusively stromal fibroblastic-like cells. We distinguished stromal cells from cancer cells because these latter cells are larger in size. In addition, fibroblasts are spindle cells whereas mononuclear inflammatory cells are round cells. On the other hand, while cancer cells are arranged forming either acinar or trabecullar pattern, stromal cells are isolated. Moreover, we used two markers to distinguish fibroblasts from tumoral cells: cytokeratins and vimentin, as it was described above.
Statistical analysis
Differences in percentages were calculated with the chi-square test. Immunostaining score values for each protein were expressed as a median (range). Correlation between score values was calculated by using the Spearman correlation test. Comparison of immunostaining values between groups was made with the Mann–Whitney or Kruskall–Wallis tests. Statistical results were corrected applying Bonferroni’s correction. P < 0.05 was considered significant. For metastasis-free survival analysis we used the Cox’s univariate method. Cox’s regression model was used to examine interactions of different prognostic factors in a multivariate analysis. Expression profiles were analyzed by a unsupervised hierarchical clustering method that organizes proteins in a tree structures, based on their similarity. Data was reformatted as follows: “−3” designated negative staining, “3” positive staining, and missing data was left blank. We used the Cluster 3.0 program (average linkage, uncentered correlation). Results were displayed with the Treeview program [29]. The SPSS 11.5 program (SPSS Inc., Chicago, IL, USA) was used for all calculations.
Results
More than 5,000 determinations in cancer specimens from 124 patients with primary invasive ductal carcinoma of the breast and controls were performed on TMAs. Minimal internal variance of score data between duplicate tissue cores from the same patients and the same tumor areas was detected in the tissue arrays, showing a high agreement for each protein (r > 0.95 and P < 0.0001, for each protein). Thus, we have previously described a validation study for MMPs and TIMPs in invasive breast carcinomas [16].
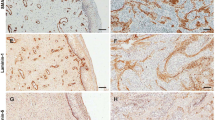
Figure 1 shows representative examples of MMPs and TIMPs expression by fibroblasts in the center of the tumor or at the invasive front of breast carcinomas. Immunostaining for these proteins has a cytoplasmic location in all positive cases. With regard to MMP-14 expression, it is of note that the immunostaining showed cytoplasmic and membrane location. As Table 2 shows, the expression of MMPs and TIMPs by fibroblasts varied among tumors. Fibroblasts at center of the tumor showed more frequently positive expression of MMP-2 (P < 0.01), -7 (P < 0.0001) and -14 (P < 0.0001), and TIMP-3 (P < 0.0001) than fibroblasts at the invasive front; while fibroblasts at the invasive front showed more frequently positive expression of MMP-9 (P < 0.0001). However, there were no significant differences between fibroblasts of these paired sets of tissue samples for MMP-1, -11 and -13, and TIMP-1 and -2. We analyzed the expression of these factors in normal mammary tissues which were obtained from 14 women that underwent cytoreductive surgery of the breast. Fibroblasts from these normal tissue samples were negative for MMP-2, -7, -9, -11, -13, and -14; TIMP-1, -2, and -3. Only one of the 14 cases showed a weak staining for MMP-1 in some fibroblasts.
400×, examples of MMPs and TIMPs expression by fibroblasts (arrows) in the center (left) of the tumor and at the invasive front (right) of breast carcinoma. a–b, expression of MMP-7; c–d, expression of TIMP-3; e–f, expression of MMP-11; g–h, expression of MMP-9; i–j, expression of MMP-2; k–l, expression of MMP-1
The concordances in expression of factors between intratumor stromal fibroblasts and those at the invasive front were of 74.7% for MMP-1, 69.1% for MMP-2, 34.8% for MMP-7, 60% for MMP-9, 65.8% for MMP-11, 54.1% for MMP-13, 50.8% for MMP-14, 56.6% for TIMP-1, 60.1% for TIMP-2, 60.1%, and of 42.9% for TIMP-3.
We also analyzed the concordances in the expression of factors between fibroblasts at tumoral center and those ones at the invasive front, and these same proteins expressions by tumoral cells in the respective localizations. According to fibroblasts and tumoral cells at tumoral center, our results show the following concordances: 92.4% for MMP-1, 75.4% for MMP-2, 85.3% for MMP-7, 40.6% for MMP-9, 80.4% for MMP-11, 75.8% for MMP-13, 91.2% for MMP-14, 52.1% for TIMP-1, 55.3% for TIMP-2, and 76.6% for TIMP-3. For fibroblast and tumoral cells at invasive front, our results show the following concordances: 91.9% for MMP-1, 72.2% for MMP-2, 40.5% for MMP-7, 42.2% for MMP-9, 30.1% for MMP-11, 69.2% for MMP-13, 56.8% for MMP-14, 49.6% for TIMP-1, 46.2% for TIMP-2, and 53.8% for TIMP-3.
We also compared the expression of MMPs and TIMPs by these two fibroblastic populations in the center of the tumor with the global immunohistochemical staining. In this tumor area, and in addition to fibroblasts, the expression of these factors was predominantly located in tumor cells but also in a significant percentage of mononuclear cells, such as previously was reported by our team [16, 28]. Table 3 shows the relationship between the expression of MMPs and TIMPs by intratumor stromal fibroblasts and by those at the invasive front. Our results demonstrated that the expression of each protein by intratumor stromal fibroblasts was significantly and positively associated with their corresponding score value (P < 0.05, for TIMP-1; and P < 0.0001, for another MMPs/TIMPs). However, expression of MMPs and TIMPs by fibroblasts at the invasive front only showed association with their corresponding score vales for MMP-2 and -13 (P < 0.05, for both), and TIMP-1, -3 (P < 0.05, for both) and -2 (P < 0.0001; Table 3).
In the present work we also examined the possible relationship between the expression of MMPs or TIMPs by fibroblasts located at the invasive front or in the intratumor stroma and the clinicopathological characteristics of patients and tumors. Among all of these clinicopathological factors considered in the study, we found no significant relationships between the expression of MMPs or TIMPs by fibroblasts at the invasive front and patient’s age or menopausal status, PR, peritumor inflammation (Fig. 2), advancing tumor edge, perineural invasion, vascular invasion and necrosis (data not shown). However, our results did demonstrate a significant relationship between expression of MMPs or TIMPs by fibroblasts at the invasive front and tumor size, nodal status, histological grade and ER. As it is shown in Table 4, MMP-7 expression by fibroblasts at the invasive front was positively and significantly associated with a larger tumor size (P = 0.004), positive nodal status (P = 0.0038), and tumors with a higher degree of undifferentiation (P = 0.038); whereas MMP-1 expression was associated with positive nodal status (P = 0.019), and MMP-14 was associated with desmoplastic reaction (P = 0.03).
On the other hand, as Table 4 shows, MMP-7 expression by fibroblasts in the center of the tumor was also positevely correlated to node involvement (P = 0.0001). Nevertheless, we found several other different associations between expression of MMPs or TIMPs by fibroblasts in the center of the tumor and some clinicopathological characteristics. So, expression of MMP-1 and -14 by these fibroblasts was associated with peritumor inflammation (P = 0.024 and P = 0.006, respectively), MMP-7 and -13 with desmoplastic reaction (P = 0.02 and P = 0.0001, respectively), MMP-9 with ER negative status (P = 0.005), TIMP-1 with node-positive status (P < 0.0001) and ER-negative status (P = 0.04), and TIMP-3 with the expansive type of tumor advancing edge (P = 0.002).
In addition, we studied the possible influence of single MMPs and TIMPs expression by fibroblasts at the invasive front or those in the center of the tumor on distant relapse-free survival. As it is shown in Table 5, multivariate analysis showed that expression by fibroblasts in the center of the tumor of MMP-9, -11, -13, and TIMP-2, was significantly associated with a high rate of distant metastases. With regard to the prognostic significance of the expressions of MMPs and TIMPs by fibroblasts at the invasive front, our results demonstrated that MMP-11 and -14, and TIMP-1 and -2, were also significantly and independently associated with a higher probability of shortened relapse-free survival (Table 4).
To identify specific groups of tumors with distinct MMP/TIMP immunohistochemical expression profiles as well as their possible prognostic importance, the obtained data were evaluated by unsupervised hierarchical cluster analysis for each cellular type. This algorithm placed proteins on the horizontal axis and samples on the vertical axis based on similarity of their expression profiles. This way, it produced a dendogram with well-defined cluster of cases for fibroblasts in the center of the tumor as well as for fibroblasts at the invasive front. Therefore, the dendogram showed a first-order division of the tumors into two distinct MMP/TIMP molecular profiles, one for fibroblasts in the center of the tumor (designated as group 1A, n = 58 and group 2A, n = 66; Fig. 3a) and another one for fibroblasts at the invasive front (designated as group 1B, n = 47 and group 2B, n = 77; Fig. 3b). MMP-1, -9, -11, -13 and -14, and TIMP-2, were identified by showing a significantly higher expression in groups 1A and 1B, compared with groups 2A and 2B (in both fibroblast populations). TIMP-3 was also identified by showing a significantly higher expression in group 1A than in group 2A (in fibroblasts in the center of the tumor); whereas MMP-7 and TIMP-1 were identified by showing significant high expression in group 1B compared with group 2B (fibroblasts at the invasive front). Likewise, it was also remarkable our findings indicating that patients with fibroblasts located either in the center of the tumor or at the invasive front (groups 1A and 1B) and belonging to a high molecular MMP/TIMP profile expression group, had the highest probability of distant metastases; whereas patients with both fibroblast populations belonging to a low molecular MMP/TIMP profile group (groups 2A and 2B) had the lowest probability of distant metastases (P < 0.0001; Table 4; Fig. 4). Multivariate analysis according to Cox model demonstrated that tumor stage [II: relative risk (RR) confidence interval (CI) = 1.9 (0.9–3.9); III: 3.1 (1.3–6.1); P < 0.001] and ER status [positive: 0.5 (0.3–0.9), P < 0.001] was significantly and independently associated with relapse-free survival. However, this same analysis also demonstrated that clustering for fibroblast populations was the most potent independent factor associated with relapse-free survival [groups 1A and 1B: 9.4 (4.2–20.9), P < 0.0001; Table 4].
Hierarchical clustering analysis of global MMPs/TIMPs expression in the different cells types of breast cancer as measured by immunohistochemistry on TMA. Graphical representation of hierarchical clustering results in fibroblasts in the center of the tumor (a), and fibroblasts at the invasive front (b). Rows, tumor samples; columns, MMPs/TIMPs. Protein expressions are depicted according to a color scale: red, positive staining; green, negative staining; gray, missing data. Two major clusters of tumors (1 and 2) are shown in both fibroblast populations
Kaplan–Meier survival curves as function of the immunostaining expression by fibroblasts in the center of the tumor of: MMP-9 (a), MMP-11 (b), MMP-13 (c), TIMP-2 (d), and TIMP-3 (e); expression by fibroblasts at the invasive front of: MMP-11 (f), MMP-14 (g), TIMP-1 (h), and TIMP-2 (i); as function of two major clusters of tumors (Group1 and Group 2) shown in fibroblasts in the center of the tumor (j), in fibroblasts at the invasive front (k), and in combination of the different cluster groups (l)
Discussion
This is, to the best of our knowledge, the first study comparing the expression of MMPs and TIMPs by intratumor stromal fibroblasts and by those located at the invasive front in breast carcinomas. Our results demonstrate differences in the expression of these biological factors implicated in invasion and metastasis between fibroblasts corresponding to those two different tumor areas in a significant percentage of cases. In addition, we have found that those different fibroblast populations might reflect different biological tumor behaviours and, thus, support complementary clinical information in breast cancer patients.
We found significant levels of discordance in the expression of MMPs and TIMPs by intratumor stromal fibroblasts and those located at the invasive front, ranging from 24.3% for MMP-1 to 65.2% for MMP-2. Likewise, clustering analysis showed two different groups, with low and high MMP/TIMP molecular profiles in both fibroblast populations, in the center of the tumor and in the invasive front, but each of them with different MMP/TIMP patterns. These findings led us to consider the existence of functional differences in host fibroblasts in these two tumor areas in a significant percentage of cases, which could signify a new contribution to the knowledge of the tumor heterogeneity of breast carcinomas. It is remarkable that intratumor stromal fibroblasts showed a positive expression of MMP-2, -7 and -14, and TIMP-3 more frequently than fibroblasts at the invasive front, which showed a more frequently expression of MMP-9. This different pattern of expression of MMPs and TIMPs may correspond to differences in cellular density, which is higher in the center of the tumors, and/or to different biological mechanisms of interaction between tumor cells and the fibroblast population of those two different tumor areas. In fact, it has been shown that cell–cell contact between cancer cells and fibroblasts enhanced the production and activation of MMPs by cancer cells, promoting pericellular proteolysis, angiogenesis, and tumor cell invasion [30, 31]. Nevertheless, it is of note the different expression of MMP-2 and -9 by fibroblasts from those two different areas. MMP-2 (Gelatinase A) and MMP-9 (Gelatinase B) are related to tumor invasion and metastasis by their special capacity to degrade the type IV collagen found in basement membranes [32] and to induce angiogenesis [17]. It has also been described that as breast cancer progresses, MMP-2 production increases during the early phases, while activation of MMP-9 occurs during the late cancerous stage [33] Now, our results contribute to show the evidence of a tendency to an inverse expression of these gelatinases by intratumor stromal fibroblasts and the ones located at the invasive front. It is also remarkable the prior finding that high MMP-2 expression in carcinoma cells is positively associated with a high stromal MMP-2 expression, whereas MMP-9 expression in cancer cells and MMP-9 expression in stromal cells is not associated with each other [34]. In this line, our results also show significant and positive relationships between the expression of each MMP/TIMP by intratumor stromal fibroblasts and their corresponding global expression (score values) in the center of the tumor; whereas expression of MMPs/TIMPs by fibroblasts at the invasive front only showed significant, but lower, relationships with score values in the center of the tumor for MMP-2 and -1, -3, and TIMP-1, -2 and -3.Likewise, it was remarkable our finding indicating higher percentages of concordance in MMPs/TIMPs expressions between both fibroblast and tumoral cells at tumoral center compared to the percentages of concordance between these two cellular types at invasive front Thus, these latter findings also contribute to the accumulating evidence of the presence of different phenotypes of fibroblasts belonging to each one of those two differentiated tumor areas.
We found significant and positive relationships between MMPs or TIMPs expression either by intratumor stromal fibroblasts, or by those at the invasive front, and clinico-pathological factors indicative of tumor progression. Our results demonstrate that MMP-7 expression by both types of fibroblasts was associated with node-positive status. MMP-7 (matrilysin 1) is a stromelysin that degrades type IV collagen, fibronectin and laminin. It has been shown that MMP-7 is aberrantly expressed in human breast tumors, and that elimination of MMP-7 is associated with low invasiveness and slow tumor growth [35]. Thus, our results are in accordance with experimental studies showing that high intratumor levels of MMP-7 were significantly associated with several parameters indicative of tumor aggressiveness as well as with our recent clinical results indicating that MMP-7 expression in breast cancer is linked to a poorer prognosis [16, 28]. Nevertheless, it is also surprising our finding of several different associations between clinicopathological parameters and MMPs or TIMPs depending on their expression by each of those two populations of fibroblasts. Hence, while expression of MMP-7 by fibroblasts at the invasive front correlated to a larger tumor size or a higher degree of undifferentiation, and expression of MMP-1 correlated to a positive nodal status or the presence of desmoplastic reaction, we found that the expression of MMP-1, -7, -9, -13 and -14, and TIMP-1, by the same cell type correlated to diverse parameters indicative of tumor aggressiveness, such as positive nodes, ER-negative status, desmoplastic reaction or peritumor inflammation. Similarly, we also found the different expression of MMPs and TIMPs by fibroblasts to have a significant value as an independent factor for predicting the occurrence of distant metastases depending on the tumor location of those cells. So, whereas MMP-9, MMP-13 and TIMP-3 expression by fibroblasts in the center of the tumor was associated with distant metastases, MMP-14 and TIMP-1 expression by fibroblasts at the invasive front was associated with that key event of tumor progression. At the present time we do not have a reasonable explanation for the prognostic signification depending on the tumor location of the fibroblasts. Even so, our data suggested that host stromal fibroblasts that appear at sites of active tumor invasion may have a different activation status of biological relevance for tumor growth and progression. Likewise, it was remarkable our finding indicating a high prognostic value of the combination of several molecular profiles of MMP/TIMP expression, based on clustering analysis, of each fibroblast population. Thus, patients with high MMP/TIMP patterns in the corresponding fibroblast populations in the center of the tumor as well as at the invasive front had the highest probability of distant metastases, whereas patients with low MMP/TIMP patterns in both fibroblast populations had the lowest risk of distant metastases.
All of these findings led us to formulate two considerations. On one hand, there is a biological variability in the expression of MMPs and TIMPs by fibroblasts among tumors, depending on their location, in the intratumoral stroma or at the invasive front. On the other hand, we describe new and unexpected findings of different clinical associations between several clinicopathological factors of prognosis and the expression of MMPs and TIMPs by fibroblasts, depending on their location in the tumor scenario. Therefore, our results indicate the importance of evaluating the expression of these factors involved in tumor growth by fibroblasts located in different tumor areas, which provide complementary information on tumor behaviour. Likewise, our data open the possibility of developing further studies on the biological importance of the expression of MMPs and TIMPs by stromal cells in the different areas of breast carcinomas, in order to assess the clinical relevance of tumor heterogeneity as well as to obtain a better knowledge of the role of the stromal cells in breast cancer progression. Presently, the expression profiles of carcinoma-associated stromal cells are only partially known. Our results contribute to show that there are distinct types of fibroblastic reaction patterns that affect breast cancer growth in different ways. Further studies in this line might facilitate the development of therapeutic agents to target and manipulate particular stromal subtypes in the treatment of cancer. Since fibroblast population does not seem to exhibit the extreme genomic changes that are so rampant in malignant epithelial cells, they may be a tractable target for new oncologic therapies.
References
Bhowmick NA, Moses HL (2005) Tumor–stroma interactions. Curr Opin Genet Dev 15:97–101. doi:10.1016/j.gde.2004.12.003
Kim JB, Stein R, O’Hare MJ (2005) Tumour–stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumour Biol 26:173–185. doi:10.1159/000086950
Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150. doi:10.1146/annurev.pathol.1.110304.100224
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752. doi:10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874. doi:10.1073/pnas.191367098
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A et al (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14:518–527. doi:10.1038/nm1764
West RB, Nuyten DS, Subramanian S, Nielsen TO, Corless CL, Rubin BP, Montgomery K, Zhu S, Patel R, Hernandez-Boussard T et al (2005) Determination of stromal signatures in breast carcinoma. PLoS Biol 3:e187. doi:10.1371/journal.pbio.0030187
Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van’t Veer LJ, Bartelink H et al (2005) Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA 102:3738–3743. doi:10.1073/pnas.0409462102
Bacac M, Provero P, Mayran N, Stehle JC, Fusco C, Stamenkovic I (2006) A mouse stromal response to tumor invasion predicts prostate and breast cancer patient survival. PLoS ONE 1:e32. doi:10.1371/journal.pone.0000032
Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C (2007) Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med 357:2543–2551. doi:10.1056/NEJMoa071825
Fukino K, Shen L, Patocs A, Mutter GL, Eng C (2007) Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA 297:2103–2111. doi:10.1001/jama.297.19.2103
Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, Auvinen P et al (2008) Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol 214:357–367. doi:10.1002/path.2278
Beck AH, Espinosa I, Gilks CB, van de Rijn M, West RB (2008) The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab Invest 88:591–601. doi:10.1038/labinvest.2008.31
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348. doi:10.1016/j.cell.2005.02.034
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563. doi:10.1038/nature06188
Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML, Junquera S, Merino AM, Garcia-Muniz JL (2007) Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer 96:903–911. doi:10.1038/sj.bjc.6603666
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174. doi:10.1038/nrc745
Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118
Manes S, Llorente M, Lacalle RA, Gomez-Mouton C, Kremer L, Mira E, Martinez AC (1999) The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem 274:6935–6945. doi:10.1074/jbc.274.11.6935
Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM (2001) Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3:459–468. doi:10.1038/sj.neo.7900190
Stetler-Stevenson WG (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103:1237–1241. doi:10.1172/JCI6870
Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD (1998) Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 161:6845–6852
Jiang Y, Goldberg ID, Shi YE (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21:2245–2252. doi:10.1038/sj.onc.1205291
Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H, Brunner N (2005) Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 12:215–227. doi:10.1677/erc.1.00719
Giatromanolaki A, Sivridis E, Koukourakis MI (2004) Tumour angiogenesis: vascular growth and survival. APMIS 112:431–440. doi:10.1111/j.1600-0463.2004.apm11207-0804.x
Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB (2002) Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol 117:723–728. doi:10.1309/PEF8-GL6F-YWMC-AG56
Gonzalez L, Corte MD, Vazquez J, Junquera S, Sanchez R, Viña A, Rodriguez J, Lamelas ML, Vizoso F (2008) Study of matrix metalloproteinases and their tissular inhibitors in ductal “in situ” carcinomas of the breast. Histophatology 53:403–415
Gonzalez LO, Pidal I, Junquera S, Corte MD, Vazquez J, Rodriguez JC, Lamelas ML, Merino AM, Garcia-Muniz JL, Vizoso FJ (2007) Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer 97:957–963. doi:10.1038/sj.bjc.6603935
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868. doi:10.1073/pnas.95.25.14863
Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A (2004) Tumor–stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol 92:47–56. doi:10.1016/j.ygyno.2003.09.012
Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N (2001) The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol 194:43–50. doi:10.1002/path.844
Jones JL, Walker RA (1997) Control of matrix metalloproteinase activity in cancer. J Pathol 183:377–379. doi:10.1002/(SICI)1096-9896(199712)183:4<377::AID-PATH951>3.0.CO;2-R
Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411:375–379. doi:10.1038/35077241
Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM (2004) Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res 10:7621–7628. doi:10.1158/1078-0432.CCR-04-1061
Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mason MD, Mokbel K, Mansel RE (2005) Targeting matrilysin and its impact on tumor growth in vivo: the potential implications in breast cancer therapy. Clin Cancer Res 11:6012–6019. doi:10.1158/1078-0432.CCR-05-0275
Acknowledgments
Supported by grants from: FIS-PI040137 and PI070306, Fondo de Inversión Sanitaria del Instituto Carlos III (FIS-Spain), and Obra Social Cajastur.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Casar, J.M., González, L.O., Alvarez, E. et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumor stromal fibroblasts and those at the invasive front of breast carcinomas. Breast Cancer Res Treat 116, 39–52 (2009). https://doi.org/10.1007/s10549-009-0351-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0351-z