Abstract
Inhibition or downregulation of Bcl-2 represents a new therapeutic approach to by-pass chemoresistance in cancer cells. Therefore, we explored the potential of this approach in breast cancer cells. Cisplatin and paclitaxel induced apoptosis in a dose-dependent manner in MCF-7 (drug-sensitive) and MDA-MB-231 (drug-insensitive) cells. Furthermore, when we transiently silenced Bcl-2, both cisplatin and paclitaxel induced apoptosis more than parental cells. Dose dependent induction of apoptosis by drugs was enhanced by the pre-treatment of these cells with HA14-1, a Bcl-2 inhibitor. Although the effect of cisplatin was significant on both cell lines, the effect of paclitaxel was much less potent only in MDA-MB-231 cells. To further understand the distinct role of drugs in MDA-MB-231 cells pretreated with HA14-1, caspases and Bcl-2 family proteins were studied. The apoptotic effect of cisplatin with or without HA14-1 pre-treatment is shown to be caspase-dependent. Among pro-apoptotic Bcl-2 proteins, Bax and Puma were found to be up-regulated whereas Bcl-2 and Bcl-xL were down-regulated when cells were pretreated with HA14-1 followed by paclitaxel or cisplatin. Enforced Bcl-2 expression in MDA-MB-231 cells abrogated the sensitizing effect of HA14-1 in cisplatin induced apoptosis. These results suggest that the potentiating effect of HA14-1 is drug and cell type specific and may not only depend on the inhibition of Bcl-2. Importantly, alteration of other pro-apoptotic or anti-apoptotic Bcl-2 family members may dictate the apoptotic response when HA14-1 is combined with chemotherapeutic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is regulated by the complex interactions between two groups of Bcl-2 family members: pro-apoptotic proteins (Bax/Bak-like proteins and BH3-only proteins) and anti-apoptotic proteins (e.g., Bcl-2, Bcl-xL, Mcl-1). The mechanistic actions of pro-apoptotic and anti-apoptotic Bcl-2 proteins have been proposed to antagonize each other through heterodimerization in mitochondrial apoptotic regulation [1]. Bcl-2 inhibits mitochondrial permeabilization, which is induced by pro-apoptotic Bax, and Bak, and subsequent cell death. The functional inhibition of Bax or Bak by Bcl-2 relies, in part, on the ability of Bcl-2 to bind to the conserved BH3-domain of pro-apoptotic Bcl-2 family proteins through its hydrophobic cleft. Bcl-2 also sequesters activator BH3-domain proteins (Bid and Bim) and blocks the activation of Bax and Bak. Following death stimuli, sensitizer BH3-only proteins (Bad, Bmf, Puma, Noxa, Bik) displace Bax or Bak or activator BH3-only proteins from their anti-apoptotic partner to promote cell death [2, 3].
Apoptosis is the primary mechanism that causes many malign cells to die when subjected to chemotherapy or radiotherapy. However, resistant clones exist by the overexpression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL. It is implicated in chemoresistance mechanism, as overexpression of Bcl-2 may inhibit apoptosis induced by many of the currently available anticancer drugs [4, 5]. The targeting of anti-apoptotic function of Bcl-2 in tumor cells is an attractive strategy for either restoring the normal apoptotic process or making these cells more susceptible to chemotherapy or radiotherapy [3].
Widely used anticancer drugs including cisplatin and paclitaxel exert their toxicity generally through induction of apoptosis. Both drugs have distinct mechanism through by which the activating apoptotic response occurs through their chemical properties. Paclitaxel is a mitotic blocker by stabilizing microtubules whereas cisplatin is a platinum derived chemotherapeutic agent that triggers DNA damage. Although they induce apoptosis, chemoresistance is a major obstacle in the cancer therapy.
Ethyl-2-amino-6-cyclopentyl-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromone-3-carboxylate (HA14-1), the first reported small molecule antagonist for Bcl-2 protein, was identified by Wang et al. [5]. This simple chemical structure is a putative Bcl-2 inhibitor, which was identified from in silico screens. HA14-1 disrupts the binding interaction of the Bak BH3-domain peptide with Bcl-2 and Bcl-xL proteins [4], strongly inhibits the Bcl-2/Bax interaction [6], and also inhibits the interaction between Bcl-2 and BH3-only protein Bim [7]. Since its discovery, HA14-1 has been shown to enhance the cytotoxic effects of variety of anticancer agents.
In the present study, we demonstrated that transiently silencing of Bcl-2 increased the apoptotic effect of cisplatin and paclitaxel in both cell lines. Chemical inhibition of Bcl-2 by HA14-1 enhanced the apoptosis induced by cisplatin or paclitaxel in MCF-7 cells. However, pre-treatment of HA14-1 significantly enhanced the apoptosis induced by cisplatin in MDA-MB-231 cells and paclitaxel was found to be much less potent. This selective sensitizing effect of HA14-1 was shown to be via modulation of other pro or anti-apoptotic Bcl-2 family members. This is the first study that reveals the selective action of HA14-1 on the apoptotic response of cancer cells when combined with chemotherapeutic drugs.
Materials and methods
Chemicals, antibodies and primers
Bcl-2 inhibitor HA14-1 [ethyl-2-amino-6-bromo-4-(lcyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate], was purchased from Calbiochem. Solutions of HA14-1 were freshly prepared before each experiment (dissolved in DMSO as 5 mM stock). Paclitaxel was dissolved in ethanol to make a 7.2 mM stock solution and stored at 4°C. Cisplatin was provided by Bristol Myers Squibb as 3.3 mM solution. G418 (Sigma) was prepared as a 50 mg/ml stock solution and kept at 4°C in aseptic conditions.
The pan-caspase inhibitor (Z-VAD-FMK), caspase-3 inhibitor (Z-DEVD-FMK) and caspase-9 inhibitor (Z-LEHD-FMK) were purchased from BD Biosciences.
Anti-Bcl-2 (1:1,000), Bcl-xL (1:2,000), Bax (1:2,000), Puma (1:1,000), β-actin (1:2,000) were purchased from cell signaling technology (CST). HRP-conjugated secondary anti-rabbit antibody (1:5,000) was from CST. Anti PARP and cleaved PARP, cleaved caspase-3 and pro-caspase-3, cleaved caspase-9 and pro-caspase-9 (each 1:1,000) were from CST. The appropriate primers for quantitative RT-PCR experiments were purchased from Geneglobe (Qiagen).
Cell culture and stable cell line
MCF-7 (HTB-22, ATCC) and MDA-MB-231 cells (HTB-26, ATCC) were maintained in RPMI1640 (Biological Industries) with 2 mM l-glutamine, 10% fetal calf serum (Pan Biotech), 1% nonessential amino acids (Biological Industries), and 100 U/100 mg ml−1 penicillin/streptomycin (Biological Industries) and grown in the presence of 5% CO2 in humidified air at 37°C.
For establishing stable cell lines, MDA-MB-231 cells were transfected using FugeneHD (Roche) with 3 μg plasmid pCI-neo alone (Promega) and pCI-neo-Bcl-2 according to the manufacturer’s instructions. The G418-resistant (700 μg/ml) clones were analyzed for Bcl-2 expression by qRT-PCR.
Cell viability assay
Cells were seeded in 96-well plates and incubated for 24 h with various concentrations of cisplatin (0–250 μM), paclitaxel (0–100 nM) and Bcl-2 specific inhibitor HA14-1 (0–10 μM). Cell viability was determined by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (Sigma) assay, which is based on the conversion of MTT to MTT-formazan by mitochondrial enzymes. Absorbance was determined at 570 nm with a Biorad microplate reader.
M30 apoptosense ELISA assay
Cytokeratin-18 cleavage indicates the activation of caspases and was measured using reagents from Peviva AB. MCF-7 and MDA-MB-231 breast cancer cells were seeded on 96-well plates. A solution of 10% NP-40 was added and mixed for 5 min at room temperature. Two replicates of 25 μl cell lysate/medium were transferred into a specific M30 Apoptosense ELISA assay. This assay was performed according to the manufacturer’s instructions. After the washing step, stop solution was added to each well, and samples were incubated in the dark for 5 min. The spectrophotometric measurement was made at 450 nm.
RNA extraction and reverse transcriptase assay
Total RNA was isolated using a trizol reagent (Invitrogen) following the procedure described by the manufacturer. Total RNA was digested with RNase-free DNase (Boehringer Mannheim) for 15 min at 37°C.
Reverse transcription (RT) was performed using a specific RT kit (Sensiscript, Qiagen). The RT reaction mixture contained 1 μl of total RNA, 500 ng of oligo(dT) primer, 5× RT reaction buffer, 10 mM dNTPs, and 200 U of a reverse transcriptase (Qiagen) in a total volume of 20 μl. All samples were incubated at 37°C for 1 h. The quantity of cDNA was calculated using spectrophotometry by determination of optical density at 260 nm (OD260). Purity was calculated using the OD260/280 ratio.
Transfection of siRNA
MCF-7 and MDA-MB-231 cells were placed in a six well plate 24 h prior to transfection. Cells were transfected with 20 nM Hs_Bcl-2_9_siRNA (Qiagen-Gene Globe) using 1:6 ratio siRNA specific transfection reagent (RNAifect, Qiagen) following the manufacturer’s protocol. After 48 h incubation, the silencing effect was checked with qRT-PCR and western blot analysis.
Real time RT-PCR
Real-time PCR was performed in 96-well 0.2 ml thin wall PCR plates using the iCycler Thermal Cycler (Bio-Rad) and carried out using the QuantiTect SYBR Green PCR Master Mix (Qiagen), which contained HotStarTaq DNA Polymerase, QuantiTect SYBR Green PCR Buffer, and SYBR Green I. The real-time PCR reaction mixture contained 1× QuantiTect SYBR Green PCR Master Mix, 0.3 mM primer pairs, and 500 ng cDNA in a total volume of 25 μl. The mixture was heated initially at 95°C for 15 min to activate the HotStarTaq DNA Polymerase and then this was followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 50–54°C for 1 min, and extension at 72°C for 1 min. Furthermore, the number of amplified products was identified by melt curve analysis. The melt curve protocols designed for increment temperatures of 0.5°C with a starting temperature of 45°C and ending at 90°C were repeated to ensure that primer dimers and other nonspecific products had been minimized or eliminated.
Immunoblot analysis
MCF-7 and MDA-MB-231 cells were treated with the appropriate concentrations of drugs. First, all samples were washed with ice-cold PBS and lysed on ice in a solution containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, Nonidet P-40 0.5%, (v/v), 1 mM EDTA, 0.5 mM PMSF, 1 mM DTT, protease inhibitor cocktail (Complete, Roche). After cell lysis, cell debris was removed by centrifugation for 15 min at 13,200g , and protein concentrations were determined with a Bradford protein assay. Total protein lysates (30 μg) were separated on a 12% SDS–PAGE and transferred onto PVDF membranes. The membranes were then blocked with 5% milk blocking solution in Tris buffer saline (TBS)-Tween20 and incubated with appropriate primary and horseradish peroxidase (HRP)-conjugated secondary antibodies (CST) in antibody buffer containing 5% (v/v) milk blocking solution. After washes with TBS-Tween 20, proteins were analyzed using an enhanced chemiluminescence detection system (ECL or ECL-Advance, Amersham Pharmacia Biotech) and exposed to Hyperfilm-ECL (Amersham Pharmacia Biotech).
Statistical analysis
All samples were evaluated statistically using an excel calculation file. Relative expression of mRNA was shown as mean ± standard deviation, and the student’s t-test was applied to understand the probability efficiency. Differences were regarded as statistically significant at values of P < 0.05.
Results
Cisplatin and paclitaxel induces apoptosis
We used MCF-7 (p53 wt) and MDA-MB-231 (p53 mt) breast cancer cells to investigate whether cisplatin or paclitaxel decreases cell viability through the induction of apoptosis.
To assess the cytotoxic effect of cisplatin or paclitaxel, we treated MCF-7 and MDA-MB-231 cells at various concentrations of these drugs for 24 h and we assessed the cell viability using MTT assay. Since moderate cytotoxic effects were observed at 30 μM cisplatin and 20 nM paclitaxel (30 and 15% decrease in cell viability in MCF-7 cells and 29 and 23% decrease in cell viability in MDA-MB-231 cells, respectively; Fig. 1a–b), these concentrations were used in further experiments.
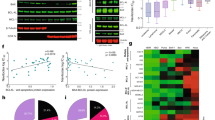
Determination of drug induced cytotoxic response and apoptosis. a and b The cytotoxic effect of cisplatin or paclitaxel was determined by MTT assay following exposure of MCF-7 and MDA-MB-231 cells to drugs for 24 h, respectively. These results represent the mean (±SEM) values obtained from at least two different assays (with three different cell cultures). c Determination of drug induced apoptosis by M30 Apoptosense ELISA assay. MCF-7 and MDA-MB-231 cells were grown up on 96 well plates, respectively. Cells were treated with 30 μM cisplatin or 20 nM paclitaxel for 24 h. The concentration of M30 antigen is presented as Units per Liter (U/l). Each experiment was repeated three times from two different cell culture experiments
To confirm that the decrease in cell viability was indeed due to apoptosis, we performed the M30 Apoptosense ELISA assay (Fig. 1c). A 30 μM cisplatin increased M30 antigen levels by 2.5- and 1.9-fold in MCF-7 and MDA-MB-231 cells, respectively. We determined that 20 nM paclitaxel increased 2.1- and 1.6-fold M30 antigen levels compared to the control samples in both cell lines, respectively (drug vs. control **P < 0.01).
Bcl-2 siRNA enhances drug induced apoptosis in MCF-7 and MDA-MB-231 cells
The efficiency of Bcl-2 siRNA on Bcl-2 protein levels was assessed at 24 and 48 h following transfection. Transfection of Bcl-2 siRNA markedly down-regulated Bcl-2 within 48 h. We did not detect any effect of scramble siRNA on Bcl-2 protein levels (Fig. 2a). Transfection ratio was determined as 1:6 for siRNA: transfection reagent complex.
Silencing of Bcl-2 by siRNA enhanced drug induced apoptosis. a Bcl-2 siRNA silencing effect and efficient transfection reagent complex ratio was determined by western blotting. b Cell viability was determined by MTT assay. After silencing, MCF-7 and MDA-MB-231 breast cancer cells were treated with 30 μM cisplatin or 20 nM paclitaxel for 24 h. c Apoptotic cell death was determined by M30 assay in drug exposed MCF-7 and MDA-MB-231 cells following silencing of Bcl-2. NS is scramble siRNA
To investigate if treatment with Bcl-2 siRNA itself had any effect on cell viability, we performed the MTT assay after 48 h transfection. The results here (Fig. 2b) showed that Bcl-2 siRNA did not decrease cell viability. When transfection of Bcl-2 siRNA was followed by treatment with cisplatin, cell viability further reduced by 52 and 65% in MCF-7 and MDA-MB-231 cells, compared to treatment with scramble siRNA and cisplatin, respectively. Similarly, transfection of Bcl-2 siRNA followed by treatment with paclitaxel, reduced cell viability by 43 and 57% in both cell lines, compared to treatment with scramble siRNA and paclitaxel, respectively. These findings were consistent with a possible increase in apoptosis and we performed M30 Apoptosense ELISA assay. Down-regulation of Bcl-2 by siRNA resulted in increased apoptotic response by chemotherapeutic drugs. As demonstrated in Fig. 2c, each chemotherapeutic drug induced M30 antigen levels due to apoptotic cell death more than parental cells in the transiently Bcl-2 down-regulated cells (P < 0.05). This indicates that siRNAs for Bcl-2 sensitized cells to both cisplatin and paclitaxel.
HA14-1 selectively sensitizes MCF-7 and MDA-MB-231 cells to apoptosis induced by cisplatin and paclitaxel
Since anti-apoptotic Bcl-2 family members likely contribute to the resistance in cancer chemotherapy by raising the threshold for apoptosis, we investigated whether the addition of HA14-1 would potentiate the apoptotic induction by chemotherapeutics. Initially we determined dose dependent effect of HA14-1 on MCF-7 and MDA-MB-231 cells. Cells were exposed to various concentrations of HA14-1 for 24 h (Fig. 3a–d) and cell viability was determined by MTT assay.
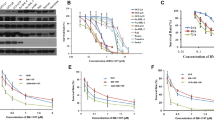
Sensitizing effect of HA14-1 is drug and cell type specific. a and c MCF-7 and MDA-MB-231 cells were treated with cisplatin (30 μM), various concentrations of HA14-1 (0–10 μM) and their combinations. Cell viability was evaluated by MTT assay. Columns represent the mean (±SEM) values obtained from at least two different assays (with three different cell cultures), each comprising six replicates. b and d MCF-7 and MDA-MB-231 cells were exposed to paclitaxel (20 nM), various concentrations of HA14-1 (0–10 μM) and their combinations. Cell viability was checked by MTT assay. Columns represent the mean (±SEM) values obtained from at least two different assays (with three different cell cultures), each comprising six replicates. e MCF-7 and MDA-MB-231 cells were treated with HA14-1 alone or pre-treated with HA14-1 followed by cisplatin or paclitaxel. Apoptotic responses were determined by M30 Apoptosense ELISA assay. The concentration of M30 antigen is presented as Units per Liter (U/L). Each experiment was replicated three times from two different cell culture experiments. f The modulation of Bcl-2 expression within 24 h time interval in 10 μM HA14-1 exposed breast cancer cells. β-actin was used as loading control
HA14-1, between 1 and 5 μM concentrations for MCF-7 cells and between 1 and 8 μM concentrations for MDA-MB-231 cells did not significantly decrease cell viability in both cell lines as compared to control (P > 0.05). Whereas HA14-1 (10 μM) reduced cell viability by 22 and 15% in MCF-7 and MDA-MB-231 cells, respectively (P < 0.05; Fig. 3a–d). We found out that pre-treatment of HA14-1 markedly enhanced the cytotoxic effect of cisplatin or paclitaxel as compared to drug alone treatment in MCF-7 cells (Fig. 3a–b). However, HA14-1 (10 μM) pre-treatment followed by cisplatin significantly increased the cytotoxic effect of cisplatin by 41% (Fig. 3c; P < 0.05; cisplatin vs. HA14-1 plus cisplatin), 10 μM HA14-1 pre-treatment did not enhance the cytotoxic effect of paclitaxel in MDA-MB-231 cells (paclitaxel vs. HA14-1 plus paclitaxel; Fig. 3d, P > 0.05).
These findings were also confirmed with M30 Apoptosense ELISA assay which determines apoptotic cell populations. Pre-treatment of 10 μM HA14-1 followed by 30 μM cisplatin increased M30 antigen concentration by 3.7- and 2.9-fold in MCF-7 and MDA-MB-231 cells as compare to cisplatin alone, respectively (P < 0.05). However, pre-treatment of 10 μM HA14-1 followed by 20 nM paclitaxel treatment significantly increased apoptosis by 3.1-fold in MCF-7 cells (P < 0.05), by contrast pre-treatment of 10 μM HA14-1 followed by 20 nM paclitaxel treatment did not significantly increased M30 antigen levels as compared to paclitaxel alone in MDA-MB-231 cells (1.9- vs. 2.0-fold; P > 0.05; Fig. 3e).
In order to understand the effect of HA14-1 on Bcl-2 expression, we determined Bcl-2 protein levels following HA14-1 treatment. As shown in Fig. 3f 10 μM HA14-1 did not alter total amount of Bcl-2 at different time points within 24 h of treatment.
HA14-1 pre-treatment potentiates cisplatin-induced apoptosis
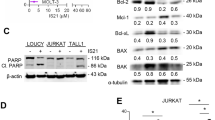
To investigate whether drug-induced apoptosis is mediated by caspases, the proteolytic activation of caspase-3 was examined in drug-insensitive MDA-MB-231 cells. As shown in Fig. 4a. Pre-treatment of cells with HA14-1 followed by paclitaxel induced cleavage of pro-caspase-3 to active form (p19/p17). Whereas paclitaxel alone did not trigger caspase-3 activation. Cisplatin in the presence or absence of HA14-1 was able to induce cleavage of caspase-3. Engagement of apoptosis is further confirmed by the detection of PARP degradation. HA14-1 increased the paclitaxel- and cisplatin-induced PARP cleavage as opposed to drug treatment-only cases. Caspase-9 was also activated by chemotherapeutics. Of note, pre-treatment with HA14-1 followed by cisplatin or paclitaxel treatment induced cleavage of pro-caspase-9 (Fig. 4a).
Cisplatin induced apoptosis is caspase dependent. a The cleavage of caspase-3, caspase-9 and PARP were determined. Thirty micrograms total protein lysate was subjected to 12% SDS–PAGE for western blot analysis with specific antibodies (anti-caspase-3, caspase-9 and PARP). β-actin was used as an internal loading control. b MDA-MB-231 cells were pre-treated with HA14-1 followed by cisplatin or cisplatin alone after 1 h prior treatment pan-caspase general inhibitor Z-VAD-FMK, caspase-3 inhibitor Z-DEVD-FMK and caspase-9 inhibitor Z-LEHD-FMK (each 20 μM) for 24 h. Cell viability was analyzed by MTT assay. Columns represent the mean (±SEM) values obtained from at least two different assays (with two different cell cultures), each comprising four replicates
In our experimental system, we used pan-caspase inhibitor (z-VAD-fmk), caspase-3 inhibitor (z-DEVD-fmk) and caspase-9 inhibitor (z-LEHD-fmk) to address the significance of caspase activation. Cells were exposed to these inhibitors for 1 h. After this, cells were pre-treated with HA14-1 followed by cisplatin or treated with only cisplatin 24 h (Fig. 4b). Cytotoxicity was evaluated by MTT assay.
Treatment with caspase inhibitors, each at 20 μM concentration, prevented cytotoxic effects of cisplatin and HA14-1 plus cisplatin. These findings suggest that the synergistic effect of HA14-1 with cisplatin was caspase-dependent (Fig. 4b).
HA14-1 pre-treatment followed by cisplatin or paclitaxel modulates the expression of Bcl-2 family members
Bcl-2 exerts a significant part of its survival function by physically interacting with pro-apoptotic members of Bcl-2 family. Therefore we next analyzed the mRNA levels of Bcl-2, Bax, Bcl-xL and Puma in MDA-MB-231 cells treated with cisplatin, paclitaxel, HA14-1, HA14-1 plus cisplatin and HA14-1 plus paclitaxel. Pre-treatment of HA14-1 followed by cisplatin downregulated Bcl-2 expression more than threefold whereas this ratio was determined as twofold in cells pretreated HA14-1 followed by paclitaxel as compared to cells treated with paclitaxel alone (Fig. 5a). These findings were verified by immunoblotting results. Pre-treatment of HA14-1 followed by cisplatin led to sharp decrease in Bcl-2 protein levels (Fig. 5b).
Modulation Bcl-2 family members in drug exposed MDA-MB-231 cells. a MDA-MB-231 breast cancer cells were pre-treated with HA14-1 followed by cisplatin or paclitaxel, cisplatin alone, paclitaxel alone and HA14-1 alone for 24 h. Bcl-2, Bax, Bcl-xL and Puma copy numbers were calculated using standard curve of β-actin. These values are normalized by dividing with β-actin. The data were represented mean ± SD and representative of independent two experiments, and each experiment was repeated three times. b Thirty micrograms total protein lysate was subjected to western blots with antibodies directed against the Bcl-2 family members. β-actin was used as loading control
Bcl-xL mRNA and protein levels were markedly decreased by cisplatin and paclitaxel treatment as compared to control. Pre-treatment of cells with HA14-1 alone also downregulated Bcl-xL protein levels, even more drastically than cisplatin or paclitaxel treated cells. Of note, treatment with HA14-1 led to a decrease in Bcl-xL mRNA levels similar to treatment with cisplatin and paclitaxel. Treatment of cells with HA14-1 plus cisplatin or HA14-1 plus paclitaxel resulted in decrease in Bcl-xL mRNA and protein levels (Fig. 5a–b).
Treatment of MDA-MB-231 cells with cisplatin or paclitaxel resulted in increased levels of Bax mRNA and protein. In addition, pre-treatment of cells with HA14-1 followed by cisplatin or paclitaxel further increased the expression of Bax mRNA and protein levels.
Treatment with cisplatin induced Puma mRNA and protein levels as compared to control, whereas paclitaxel exert a similar effect. Of note, treatment with HA14-1 alone increased Puma mRNA and protein expression. Further increase in Puma mRNA and protein expression was observed when cells were pre-treated with HA14-1 followed by treatment with cisplatin or paclitaxel.
Bcl-2 over-expression prevents cytotoxic effect of cisplatin
To establish the role of Bcl-2 overexpression in drug induced apoptosis we set up stable Bcl-2 overexpressing MDA-MB-231 cells following G418 selection. Bcl-2 overexpression was determined by qRT-PCR and immunoblotting results (Fig. 6a). Next we investigated the sensitizing effect of HA14-1 on cisplatin induced cell death in MDA-MB-231 Bcl-2 overexpressing cells. As shown in Fig. 6b, cisplatin induced cell viability loss by 21% after 72 h of treatment, whereas pre-treatment of HA14-1 failed to exert additional cytotoxic effect and decreased cell viability by 27% when combined with cisplatin treatment (drug vs. combined treatment P > 0.05).
Bcl-2 overexpression abrogated cisplatin induced apoptosis. a MDA-MB-231 cells were transfected with Bcl-2 plasmid and selected with G418 (700 μg/ml). Bcl-2 mRNA copy number were calculating using standard curve of β-actin. Selected clones were verified by western blotting. b The determination of cell viability in MDA-MB-231Bcl−2+ breast cancer cells. MDA-MB-231Bcl−2+ breast cancer cells were pre-treated with HA14-1 followed by cisplatin for 72 h
Discussion
Resistance to anticancer therapy is a complex problem in both clinical and molecular oncology. Several studies established that over-expression of anti-apoptotic Bcl-2 family proteins confers resistance to apoptosis and decreases efficiency of therapeutics [2, 3]. Since pro-apoptotic Bcl-2 family proteins dock into the BH1-BH2 groove of anti-apoptotic members via their BH3-domain, it has been proposed that BH3 mimetics antagonize the anti-apoptotic members. Currently, a favored strategy for Bcl-2 antagonism is based on small molecules that target Bcl-2 anti-apoptotic proteins by mimicking a BH3 domain [1]. Hence, several compounds have been isolated or chemically synthesized, showing different binding specificity and affinity for these proteins and promoting apoptosis.
Among them, HA14-1, is a BH3 mimetic compound, involves changes in Ca2+ homeostasis, inhibition of mitochondrial potential, Bax translocation, reactive oxygen species generation (ROS), cytochrome c release and caspase-9/-3 activation, and subsequently poly(ADP-ribose) polymerase (PARP) cleavage [4, 5, 8–12]. The therapeutic effect of HA14-1 has been described in a variety of tumor cells. HA14-1 cooperates with other drugs such as flavopiridol, bortezomib, dexamethasone, doxorubicin and cytrabine [13–18]. Additionally, HA14-1 could potentiate non-toxic MAPK inhibitors as lethal agents in the cell culture environment [19].
In this study, we determined that cisplatin and paclitaxel exerted distinct modes of action in inducing apoptosis and this effect being cell type specific. Cisplatin and paclitaxel-induced apoptosis is promoted in Bcl-2 silenced MCF-7 and MDA-MB-231 cells more than parental cells (Fig. 2b–c). Therefore we conclude that Bcl-2 is an attractive target for novel treatment strategies of breast carcinoma.
Pre-treatment of HA14-1 significantly potentiated the effect of both drugs in MCF-seven cells. In MDA-MB 231 cells, although pre-treatment of HA14-1 potentiated effect of cisplatin significantly, sensitization was much less when cisplatin was replaced by paclitaxel (Fig. 3c, P < 0.05; Fig. 3d, P > 0.05). These results were further confirmed by M30 Apoptosense ELISA assay which determines caspase-induced cytokeratine 18 cleavage in apoptotic cell populations (Fig. 3e). Additionally, Bcl-2 protein level was checked but not found to be altered by exposure to HA14-1 (Fig. 3f). Neither caspase-9 nor PARP cleavage were found to be drug specific (Fig. 4a) indicating that cisplatin (30 μM) or paclitaxel (20 nM) induced mitochondrial apoptotic pathway in MDA-MB-231 cells. Although cisplatin with or without pre-treatment with HA14-1 increased the cleavage of caspase-3, paclitaxel did not exert same effect in MDA-MB-231 cells. During this process, paclitaxel-induced caspase-3 cleavage is independent from caspase-9 activation. It is also possible that paclitaxel acts via activation of another effector caspase, like caspases-6 or -7, or by an unrelated pathway. Previously, the possible involvement of caspase-7 in paclitaxel-induced apoptosis has been suggested in human esophageal squamous cancer cells and in non-small-cell lung cancer H460 and H520 cell lines [20, 21]. Although pre-treatment of HA14-1 followed by paclitaxel activated caspase-3, HA14-1 did not markedly increase the apoptotic effect of paclitaxel in MDA-MB-231 cells. In order to understand the different sensitizing effects of HA14-1 pre-treatment of paclitaxel, further studies should focus on other effector caspases, or caspase independent cell death mechanisms. We also established that cytotoxic effects of cisplatin or HA14-1 plus cisplatin were prevented by pan-caspase, caspase-9 and caspase-3 inhibitors (Fig. 4b). Thus, we conclude that cisplatin-induced apoptosis occurs by caspase dependent pathways. Consistent with our findings, pan-caspase, caspase-9 and caspase-3 inhibitors prevented HA14-1-induced apoptosis in follicular lymphoma B cells [22].
Bcl-2, Bcl-xL, and Bax have been implicated as major regulators in the control of mitochondrial apoptotic pathway. Bcl-2 and Bcl-xL bind to outer membrane of mitochondria and block cytochrome c efflux. In contrast, upon apoptosis induction, Bax translocates from cytosol to mitochondria where it enhances cytochrome c release through either down-regulation of Bcl-2/Bcl-xL and/or up-regulation of Bax [2, 3]. We presented here, HA14-1 pre-treatment followed by cisplatin or paclitaxel abrogated Bcl-2 and Bcl-xL in MDA-MB-231 cells albeit greater extends with cisplatin (Fig. 5a–b). HA14-1 drastically down-regulated Bcl-xL expression in our experimental model (Fig. 5a–b). Therefore, altered ratio of pro-apoptotic and anti-apoptotic Bcl-2 family members might be an important key question to understand the sensitizing effect of HA14-1 in MDA-MB-231 cells. Of note, phosphorylation of Bcl-2 has been implicated as an important regulatory mechanism of its function and is a common event in response to anti-mitotic drugs. Recent studies have revealed that this post-translational modification may be necessary for Bcl-2’s full and potent anti-apoptotic function. Paclitaxel treatment activates members of the JNK/SAPK MAPK family, which then disable Bcl-2 through phosphorylation within its flexible loop region. Although paclitaxel can also bind to the loop of Bcl-2 protein, its significance is unclear. It is possible that alterations in the loop region of Bcl-2 could account for some cases of paclitaxel-resistance contrast to other mitotic blockers [23]. Thereby, further investigation of paclitaxel induced Bcl-2 phosphorylation and related up-stream events might be informative.
Previous studies pointed out that, lack of Bax might cause failure to respond to HA14-1 [8]. Hence, determination of the Bax expression and related events in HA14-1-exposed cell lines might be significant in characterization of drug specific sensitizing effect of HA14-1. We herein demonstrated that Bax was markedly up-regulated by pre-treatment of HA14-1 followed by cisplatin or paclitaxel (Fig. 5a–b). Moreover, pre-treatment of HA14-1 followed by cisplatin induced Bax translocation to mitochondria MDA-MB-231 cells (unpublished data).
Considering the finding that cisplatin or paclitaxel induced apoptosis in MDA-MB-231 cells which have lacking functional p53, we can conclude that apoptosis induction by both drugs do not require p53 transcriptional activity in consistent with previous reports. Additionally, Puma, a BH3-only protein, is a transcriptional target of p53-mediated apoptosis signaling. It is also an important mediator of apoptosis and induced by a number of chemotherapeutics and radiation [24, 25]. Puma acts by modulating Bax activity through binding to Bcl-xL and dissociate the interaction of Bax and Bcl-xL to facilitate cytochrome c release from the mitochondria, thereby triggering the apoptotic cascade [26]. It was previously demonstrated that p53-independent Puma up-regulation might be playing role in cisplatin-induced apoptosis in colon cancer cells [27]. Over-expression of Puma induces disorganization/disruption of the cellular microtubule network in apoptotic fibroblasts [28]. Therefore, microtubules displayed de-polymerized and dispersed structures throughout the cytoplasm. In contrast to the mechanistic action of Puma, paclitaxel and docetaxel induce stabilization of microtubule formation and lead to subsequent cell death. As shown in Fig. 5a–b, while cisplatin (30 μM) was up-regulating Puma mRNA and protein expression, paclitaxel (20 nM) did not up-regulate Puma expression in MDA-MB-231 cells. Based on this result, we conclude that cisplatin and HA14-1 induce p53-independent Puma up-regulation in MDA-MB-231 cells, whereas paclitaxel failed to up-regulate Puma. Interestingly, cisplatin-induced apoptosis was not observed in Bcl-2 over-expressing MDA-MB-231 cells. Similarly, resistance to cisplatin was determined in p53 deficient cells which have up-regulated Bcl-2 or Bcl-xL [29]. Hence, we conclude that p53-independent mechanisms which were activated by cisplatin in MDA-MB-231 cells could be successfully abrogated by Bcl-2. However, pre-treatment of HA14-1 did not increase the apoptotic effect of cisplatin in Bcl-2 over-expressing MDA-MB-231 cells (Fig. 6b). In previous reports, the synergistic effect of HA14-1 with SP600125 which is a specific inhibitor of c-Jun N-terminal kinase in U937 Bcl-2 over-expressing cells was found to be dose dependent [16]. Therefore, higher doses of HA14-1 might be more effective to enhance cisplatin-induced apoptosis in Bcl-2 over-expressing cells.
Briefly, we demonstrated that down-regulation of Bcl-2 by siRNA potentiated cytotoxic effects of cisplatin and paclitaxel in both cell lines, whereas chemical inhibition of Bcl-2 promoted cisplatin and to a much lesser extend paclitaxel-induced cell death in MDA-MB-231 cells. The sensitizing effect of HA14-1 through the induction of apoptosis was found to be drug and cell type specific. The treatment of MDA-MB-231 cells by cisplatin activated p53-independent mitochondrial apoptotic pathway through induction of caspase-3 and overcame Bcl-2-mediated protection in functional p53 lacking cells. However, Bcl-2 over-expression confers resistance to cisplatin. Pre-treatment with HA14-1 followed by cisplatin markedly down-regulated Bcl-2 and Bcl-xL and up-regulated BH3-only protein Bax and Puma. As pro- and anti-apoptotic Bcl-2 proteins are in balance for controlling the mitochondrial apoptotic pathway, their ratio is indicative for apoptotic response. Previous findings and those in our study provide a strong biological rationale for using HA14-1 in combination with therapeutic options. According to these all findings, pre-treatment of HA14-1 followed by cisplatin has potential as a novel therapeutic approach in breast carcinoma.
References
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17(3):393–403. doi:10.1016/j.molcel.2004.12.030
Reed JC (2003) Apoptosis targeted therapies for cancer. Cancer Cell 3:17–22. doi:10.1016/S1535-6108(02)00241-6
Letai A (2005) Pharmacological manipulation of Bcl-2 family members to control cell death. J Clin Invest 115:2648–2655. doi:10.1172/JCI26250
Wang JL, Zhang ZJ, Choksi S, Shan S, Lu Z, Croce CM et al (2000) Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res 60:1498–1502
Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM et al (2000) Structure based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 97:7124–7129. doi:10.1073/pnas.97.13.7124
Manero F, Gaultier F, Gallenne T, Cauquil N, Gree D, Cartron PF et al (2006) The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res 66:2757–2764. doi:10.1158/0008-5472.CAN-05-2097
Zimmermann AK, Loucks FA, Le SS, Butts BD, Florez-McClure ML, Bouchard RJ et al (2005) Distinct mechanisms of neuronal apoptosis are triggered by antagonism of Bcl-2/Bcl-x(L) versus induction of the BH3-only protein Bim. J Neurochem 94:22–36. doi:10.1111/j.1471-4159.2005.03156.x
Chen J, Freeman A, Liu J, Dai Q, Lee RM (2002) The apoptotic effect of HA14-1, a Bcl-2 interacting small molecular compound, requires Bax translocation and is enhanced by PK11195. Mol Cancer Ther 1:961–967
Tian D, Das SG, Doshi JM, Peng J, Lin J, Xing C (2008) sHA14-1, is a stable and ROS free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer Lett 259:198–208. doi:10.1016/j.canlet.2007.10.012
Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y (2001) Discovery of small-molecule inhibitors of Bcl-2 through structure based computer screening. J Med Chem 44:4313–4324. doi:10.1021/jm010016f
An J, Chervin AS, Nie A, Ducoff HS, Huang Z (2006) Overcoming the radioresistance of prostate cells with a novel Bcl-2 inhibitor. Oncogene 26(5):652–661. doi:10.1038/sj.onc.1209830
Oliver L, Mahe B, Gree R, Vallette FM, Juin P (2007) HA14-1, a small molecule inhibitor of Bcl-2 bypasses chemoresistance in leukemia cells. Leuk Res 31(6):859–863. doi:10.1016/j.leukres.2006.11.010
Pei X, Dai Y, Grant S (2003) The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor, HA14-1 in multiple myeloma cells. Leukemia 17:2036–2045. doi:10.1038/sj.leu.2403109
Sinicrope FA, Penington RC, Tang XM (2004) Tumor necrosis factor related apoptosis inducing ligand induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA14-1 in human colon cancer cells. Clin Cancer Res 10:8284–8292. doi:10.1158/1078-0432.CCR-04-1289
Su Y, Zhang X, Sinko PJ (2007) Exploitation of drug induced Bcl-2 overexpression for restoring normal apoptosis function: a promising new approach to the treatment of multidrug resistant cancer. Cancer Lett 253:115–123. doi:10.1016/j.canlet.2007.01.018
Moon D, Kim M, Choi YH, Kim ND, Chang JH, Kim G (2008) Bcl-2 overexpression attenuates SP600125 induced apoptosis in human leukemia U937 cells. Cancer Lett 264:316–325. doi:10.1016/j.canlet.2008.02.011
Lickliter JD, Wood NJ, Johnson L, McHugh G, Tan J, Wood F et al (2003) HA14-1 selectively induces apoptosis in Bcl-2 overexpressing leukemia/lymphoma cells, and enhances cytarabine induced cell death. Leukemia 17:2074–2080. doi:10.1038/sj.leu.2403102
Pei XY, Dai Y, Grant S (2004) The small-molecule Bcl-2 inhibitor HA14-1 interacts synergistically with flavopiridol to induce mitochondrial injury and apoptosis in human myeloma cells through a free radical dependent and Jun NH2 terminal kinase dependent mechanism. Mol Cancer Ther 3:1513–1524
Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A et al (2002) Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood 99:3461–3464. doi:10.1182/blood.V99.9.3461
Okani JI, Rustgi AK (2001) Paclitaxel induces prolonged activation of the Ras/MEK/ERK pathway independently of activation of the programmed cell death machinery. J Biol Chem 276:19555–19564. doi:10.1074/jbc.M011164200
Ling Y, Zhong Y, Perez-Soler R (2001) Disruption of cell adhesion and caspase-mediated proteolysis of beta- and gamma-catenins and APC protein in paclitaxel-induced apoptosis. Mol Pharmacol 59:593–603
Skommer J, Wlodkowic D, Matto M, Eray M, Pelkonen J (2006) HA14-1, a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular B lymphoma B cells. Leuk Res 30:322–331. doi:10.1016/j.leukres.2005.08.022
Srivastava RK, Mi QS, Hardwick JM, Longo DL (1999) Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA 96(7):3775–3780. doi:10.1073/pnas.96.7.3775
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) Puma induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7(3):673–682. doi:10.1016/S1097-2765(01)00213-1
Wang X, Li M, Wang J, Yeung CM, Zhang H, Kung HF, Jiang B, Lin MC (2006) The BH3-only protein, PUMA, is involved in oxaliplatin-induced apoptosis in colon cancer cells. Biochem Pharmacol 71(11):1540–1550. doi:10.1016/j.bcp.2006.02.011
Ming L, Wang P, Bank A, Yu J, Zhang L (2006) Puma dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem 281:16034–16042. doi:10.1074/jbc.M513587200
Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, Chen X, Liu KJ, Hu CA (2005) Puma overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res 65(5):1647–1654. doi:10.1158/0008-5472.CAN-04-1754
Lowe SW, Ruley HE, Jacks T, Housman DE (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967. doi:10.1016/0092-8674(93)90719-7
Stewart DJ (2007) Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 63(1):12–31. doi:10.1016/j.critrevonc.2007.02.001
Acknowledgment
This work was partially supported by Turkish Association for Cancer Research and Control, Terry Fox Cancer Research Grant-35-D/882.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2009_343_MOESM1_ESM.pdf
Quantitative mRNA levels were determined by quantitative real time PCR assay following transfection of MCF-7 and MDA-MB-231 cells with Bcl-2 siRNA and scramble siRNA for 48 h. Bcl-2 mRNA copy number was calculated using standard curve of β-actin. These values are normalized by dividing with β-actin. Columns represent mean (±SEM) of independent two experiments and each experiment was repeated three times (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Arisan, E.D., Kutuk, O., Tezil, T. et al. Small inhibitor of Bcl-2, HA14-1, selectively enhanced the apoptotic effect of cisplatin by modulating Bcl-2 family members in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat 119, 271–281 (2010). https://doi.org/10.1007/s10549-009-0343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0343-z