Abstract
Purpose Prospective pilot study to assess patient outcome after stereotactic body radiation therapy (SBRT) for limited metastases from breast cancer. Methods Forty patients with ≤5 metastatic lesions received curative-intent SBRT, while 11 patients with >5 lesions, undergoing SBRT to ≤5 metastatic lesions, were treated with palliative-intent. Results Among those treated with curative-intent, 4-year actuarial outcomes were: overall survival of 59%, progression-free survival of 38% and lesion local control of 89%. On univariate analyses, 1 metastatic lesion (versus 2–5), smaller tumor volume, bone-only disease, and stable or regressing lesions prior to SBRT were associated with more favorable outcome. Patients treated with palliative-intent SBRT were spared morbidity and mortality from progression of treated lesions, though all developed further metastatic progression shortly (median 4 months) after enrollment. Conclusions SBRT may yield prolonged survival and perhaps cure in select patients with limited metastases. Palliative-intent SBRT may be warranted for symptomatic or potentially symptomatic metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Limited metastatic disease may be amenable to curative-intent surgery or radiation [1–3]. In 1995, Drs. Hellman and Weichselbaum hypothesized a “clinical significant state of oligometastases” in which a limited number of metastases may represent a disease state in which full metastatic potential is not reached [4].
Prior to postulating an oligometastatic state, Dr. Hellman proposed a model in which the extent of cancer progression in any given patient exists along a spectrum [5], ranging from a state of limited disease with the propensity to spread in an orderly, contiguous manner (as postulated by Halsted in the late 1800s) to a state of widespread systemic disease from clinical inception, even in the absence of clinically detectable metastases (as postulated by Fisher in the early 1980s [6, 7]). The oligometastatic state can be extrapolated from this ‘spectrum model’ to represent an early course of metastatic progression in some patients. A treatment paradigm of aggressive local therapy for limited metastases can potentially cure patients, prolong patient survival and/or improve disease control [4, 8–10].
Local treatment options for oligometastatic tumors include resection [9], radiofrequency ablation, and radiotherapy. Radiotherapy can be offered to patients unable to tolerate (or unwilling to undergo) more invasive procedures, or when tumors are situated in areas in which invasive procedures would result in unacceptable morbidity. In patients with several metastases, more invasive techniques such as resection and radiofrequency ablation are arguably not indicated. Hypofractionated (fewer fractions, with a higher dose per fraction) stereotactic body radiotherapy (SBRT) is becoming more widely used to treat oligometastases [11]. SBRT implies the use of a three-dimensional frame of reference to more accurately localize the tumor, allowing for reduced set-up uncertainty, which in turn enables aggressive fractionation to be used with acceptable toxicity [11–13].
Since 2001, the University of Rochester has used hypofractionated SBRT to treat patients with oligometastatic disease. We recently reported survival and disease control outcomes in patients treated with SBRT for liver metastases [14] lung metastases [15] and ≤5 metastatic lesions [16, 17]. We hypothesized that patients with ≤5 detectable metastases are amenable to potentially curative SBRT. The treatment of ≤5 lesions in patients with >5 lesions was considered palliative. We hypothesized that SBRT to select bulkier lesions in patients with >5 metastases can reduce metastatic disease burden, potentially resulting in a disease state more amenable to systemic therapy. Though we selected 5 lesions as the cut-off for curative-intent SBRT, the optimum number of lesions amenable to curative-intent therapy remains unknown. Certainly, there can be tumor, organ and host specific variables that can impact this number in any given patient. Nevertheless, for the purpose of protocol development, 5 lesions was chosen as a cut-off, extrapolating from a prior analysis from the University of Rochester of patients with metastases from prostate cancer, which confirmed a cut-off of ~5 lesions, a number that has also been postulated by others [18].
The present paper focuses on 51 patients with metastatic breast cancer treated with curative-intent SBRT or palliative-intent SBRT.
Methods and materials
Between February 2001 and December of 2007, 51 patients with metastatic breast cancer enrolled on one of two University of Rochester protocols investigating the use of SBRT to treat limited metastatic disease [16, 17]. The URCC 8700 protocol included patients with ≤5 detectable lesions from metastatic breast cancer. The URCC 9700 protocol allowed patients with >5 metastases to receive SBRT to ≤5 lesions which were deemed potentially life-threatening as a result of their bulk and/or location. Eligibility requirements for both protocols included age ≥18 years and Karnofsky Performance Status ≥70. These studies were approved by the University of Rochester Research Subjects Review Board, and all patients signed informed consent. The present analysis includes 2 patients enrolled after July of 2007, who were not included in our recent analysis of 121 patients [16, 17]. A patient with brain-only metastases from breast cancer, who did not receive extracranial SBRT, is excluded from the present analysis.
SBRT technique
The technique of SBRT employed at the University of Rochester has been previously described [14–17, 19], and is briefly outlined here. The Novalis ExacTrac ® patient positioning platform (BrainLAB AG, Heimstetten, Germany) in conjunction with a relaxed end-expiratory breath hold was used to reproducibly position the patient. The BrainSCAN (BrainLAB AG, Heimstetten, Germany) system was used for treatment planning. The gross target volume (GTV) was identified and contoured on axial CT images; MRI and PET scans, when available, were fused with the planning CT scan for more accurate delineation of the GTV. The PTV was generated with a minimum GTV expansion of 10 mm in the craniocaudal direction, and 7 mm in other directions, which allows for coverage of 2 to 3 standard deviations of motion [19]. SBRT was delivered using conformal arcs. Treatment was prescribed to the isocenter, with the 80% isodose line covering the PTV.
Follow-up
Patients were followed through July 2008. Follow-up visits were planned 1 month after completing SBRT and every 3 months subsequently for 2 years. Thereafter, intervals ranged from 3 to 6 months, based on physician preference. Patients underwent diagnostic imaging studies prior to all follow-up visits after the initial 1 month visit.
Endpoints
Overall survival (OS) and progression free survival (PFS) were calculated using Kaplan-Meier actuarial survival analyses, with survival and failure times defined from the day of enrollment until an event or last follow-up. Local failure was scored as an event if any treated lesion grew by ≥20%, based on the Response Evaluation Criteria In Solid Tumors (RECIST) criteria [20], or was confirmed pathologically. For patients treated with curative-intent SBRT, distant failure was scored as an event if a patient progressed with distant metastases beyond that which could be treated with curative-intent (i.e. ≥5 metastases or incurable disease). This definition considers the state of oligometastases to be a potentially chronic disease, in which distant progression occurs when metastases are not amenable to curative-intent. For patients treated with palliative-intent, distant failure was defined as the progression of untreated metastases and/or development of new metastases not amenable to stereotactic radiation. Progression was defined as local or distant failure. Stata version 9.2 was used for all data analysis.
Results
Patient characteristics: those treated with palliative-intent
The characteristics of patients treated with palliative-intent SBRT are summarized in Table 1. Patients enrolled 25–210 months (median 64) after the initial diagnosis of breast cancer, 5–49 months (median 22) after the initial diagnosis of metastatic disease and 1–26 months (median 13) after the development of the metastases treated on protocol (with 2 of the 11 patients having had developed the metastases treated on protocol at the time of the initial presentation of metastatic disease). Five patients had undergone curative-intent local therapy for oligometastatic disease prior to enrollment, including resection (n = 3), radiofrequency ablation (n = 1), radiation therapy (n = 1) and SRS (n = 2). Prior to enrollment, all patients received chemotherapy for metastatic disease; 5 patients also received hormonal therapy. Nine patients were referred for SBRT after progressive growth of lesions on chemotherapy (based on RECIST criteria) and 1 patient had stable disease. One patient was referred for SBRT 1 month after developing the metastases treated on protocol, and thus was not assessable for response to systemic therapy. Seven patients had innumerable (>25) lesions, and 4 patients had 6–13 detectable lesions. In all patients, the bulkier lesions, deemed potentially life threatening, were they to continue to grow, were selected for SBRT. Most patients were asymptomatic from their lesions, though 1 patient had abdominal pain from liver metastases. Although all but 2 patients presented with multi-organ involvement of metastatic disease (see Table 1), all patients underwent SBRT to lesions confined to one organ; thus, the lesions in other organs were not felt to be life-threatening at the time of enrollment. The characteristics of the individual treated lesions are summarized in Table 2.
Outcome: patients treated with palliative-intent
All patients treated with palliative-intent SBRT developed worsening distant metastases shortly (3–16 months, median 4) after enrollment. All patients died with progressing disease at 4–24 months after enrollment, resulting in a mean and median survival of 13 months. Three patients with no known CNS metastases at enrollment and 1 other patient with known CNS metastases died from new CNS metastases; one patient died from new liver metastases. The other 6 patients progressed with new extracranial metastases, which was presumably the cause of their death. Albeit at short follow-up due to patient death, only 1 lesion (with a GTV of 19 ml) failed locally in a patient treated to 4 liver metastases; this patient died from brain metastases. Thus, no patient died from local progression of their treated lesion(s). Two patients, with innumerable metastases involving at least 3 organs, who received SBRT to 1–2 liver metastases with a net GTV of 65 and 463 ml, survived >18 months.
The 7 patients with innumerable metastases, compared to the 4 patients with 6–13 metastases, had a worse overall survival (median survival of 6 vs. 16 months, P = 0.064 on UVA) and similar progression free survival (median progression free survival of 4 vs. 3 months, P = 0.2 on UVA).
Patient characteristics: those treated with curative-intent
The characteristics of patients treated with curative-intent SBRT are summarized in Table 3. Patients enrolled 2–155 months (median 56) after the initial diagnosis of breast cancer, 1–96 months (median 12) after the initial diagnosis of metastatic disease, and 1–96 months (median 7) after the development of the metastases treated on protocol (with 36 of the 40 patients having had developed the metastases treated on protocol at the time of the initial presentation of metastatic disease). Six patients had undergone curative-intent local therapy for oligometastatic disease prior to enrollment, including resection (n = 2), radiofrequency ablation (n = 2), radiation therapy (n = 2) and SRS (n = 1).
Only 4 patients did not receive systemic therapy for metastatic disease prior to enrollment: one patient had cryptogenic cirrhosis and was medically unfit for systemic therapy, and 3 patients refused systemic therapy. The remaining 36 patients received chemotherapy (n = 16), hormonal therapy (n = 10) or both (n = 10) after the diagnosis of metastatic breast cancer and prior to enrollment. Of these 36 patients, 12 were referred for SBRT after progressive lesion growth on systemic therapy, while 16 were referred for consolidative SBRT for stable disease (n = 8) or disease response (n = 8) to systemic therapy. Seven patients were referred for radiation shortly (1 to 2 months) after starting (or continuing) systemic therapy for new metastases, and thus response to systemic therapy could not be assessed. In 1 patient, response to systemic therapy could not be assessed because the patient underwent a debulking resection in addition to systemic therapy prior to radiation.
The characteristics of the individual lesions treated are summarized in Table 4. The size of the individual lesions, stratified by response to chemotherapy is summarized in Table 5. There was a statistically significant difference in lesion GTV between the 3 groups (P = 0.003 with ANOVA). Lesions treated with SBRT after progression were significantly larger than lesions that were stable or responding (P = 0.0009 on t-test).
Outcome: patients treated with curative-intent
Seven patients with a total of 10 treated lesions experienced a local failure(s) 5–17 months (median 12) after completion of SBRT. Eight of the 10 treated lesions in these patients progressed after radiation. All 7 local failures were within the radiation field. Three of these 7 patients underwent curative-intent salvage therapy. Table 6 summarizes the oligometastatic tumor characteristics and patient outcome for these 7 patients who experienced a local failure.
After SBRT, 32 patients received adjuvant systemic therapy, including hormonal therapy (n = 13), chemotherapy (n = 10) or both (n = 9). Sixteen of these 32 patients eventually developed widespread metastases, of whom 13 received further systemic therapy: hormonal therapy (n = 1), chemotherapy (n = 6) or both (n = 6). In 3 of these 16 patients, this information was not available. Among the 8 patients who did not receive systemic therapy after SBRT, 3 are alive without evidence of disease at 9, 21 and 71 months and 4 patients (all ER/PR negative) died at 6, 18, 19 months and 34 months (<1 month after developing widespread metastases in 2 patients and 15–16 months after developing widespread metastases in 2 others). One of these 8 patients is alive at 27 months with metastatic progression in the bones only; she began hormonal and bisphosphante therapy after developing new bone metastases.
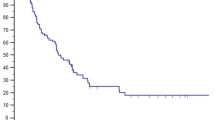
The OS and PFS curves for the 40 patients undergoing curative intent SBRT are depicted in Fig. 1. The 2-year and 4-year OS are 76 and 59%, respectively; the median survival has not yet been reached. The 2-year and 4-year PFS are 44 and 38%, respectively; the median PFS is 23 months. The ultimate PFS at 4 years (incorporating salvage therapy for local failure) is 43%. The 2-year and 4-year distant control (DC) is 50 and 43%, respectively. The 2-year and 4-year patient local control (LC) is 80%. The survival of the 26 patients alive at last follow-up is 9–91 months (median 50). The survival of the 19 patients alive with no evidence of active disease is 9–91 months (median 51). The survival of the 7 patients alive with metastatic disease is 27–60 months (median 49), and 12–51 months (median 25) after covert distant progression.
Outcome: lesions in patients treated with curative-intent
The 4-year tumor LC is 89%; no lesions failed locally after 18 months. Among the 8 lesions that failed locally, the mean and median GTV was 57 and 51 ml, respectively; in contrast, the mean and median GTV of lesions with no documented LF was 19 and 6 ml, respectively. This difference was significant on a two-tailed t-test (P = 0.036). Excluding 7 locally controlled lesions in 4 patients with short follow-up (5 lesions in 2 patients who died from distant metastases at 6 and 7 months, and 2 lesions in 2 living patients with <12 months follow-up) did not impact these results. Excluding these lesions, the mean and median GTV of locally controlled lesions was 18 and 6 ml, respectively (P = 0.020 compared to local failures).
Univariate and multivariate analyses of patients treated with curative-intent
As part of this hypothesis-generating analysis, the metastatic sites involved, number of metastases treated, and response to chemotherapy were assessed on UVA for OS, PFS, DC and patient LC.
Presenting with an isolated metastasis versus >1 lesion was associated with better PFS (P = 0.028), DC (P = 0.004) and OS (P = 0.087). Patients with bone metastases (n = 11) experienced an improved PFS (P = 0.037), DC (P = 0.079) and LC (P = 0.065). Patients with bone-only metastases (n = 8) fared better with respect to OS, PFS, DC and patient LC. All 8 patients with bone-only metastases are alive, and 7 of 8 are with no evidence of disease recurrence at 22–89 months (median 50). One patient developed new bone metastases 22 months after SBRT; prior to developing widespread disease, this patient had initially refused all systemic therapy, including bisphosphanate therapy. For the other 32 patients, the outcome was as follows: 4-year OS of 49% (P = 0.033 versus those with bone-only disease), 4-year PFS of 25% (P = 0.007), 4-year DC of 31% (P = 0.015), and 4-year patient LC of 71% (P = 0.13).
PFS and OS were worse in patients treated with progressing lesions versus stable or responding lesions (2-year PFS of 13 vs. 53%, P = 0.026 and 2-year OS of 63 vs. 81%, P = 0.061). Of those patients with progressing lesions, two patients are alive and free of disease at 12–18 months, and another is alive at >6 years after surgical salvage (and is scored as a PFS failure; see Table 6).
MVA models for OS, PFS and DC were run including the following variables: bone-only disease, progression versus no progression prior to SBRT, 1 vs. >1 treated lesion and net GTV (continuous variable of sum of GTVs from the contoured target volumes on planning CT scan). A MVA was also run for patient LC. No variable proved significant or borderline significant with the exception of net GTV for patient LC (P = 0.033). For OS, PFS and DC, there was a non-significant trend towards worse outcome with greater net-GTV, but this was not significant (0.12 > P values > 0.1).
The metastatic sites involved and the response to chemotherapy was assessed on UVA for lesion LC. Thoracic lymph node involvement was borderline significant on UVA (P = 0.070). On MVA, only lesion GTV was significant (P = 0.045) for lesion local control.
Discussion
Systemic therapy remains the standard of care for patients with metastatic breast cancer, and can prolong survival. However, with rare exception, systemic therapy is not considered a curative option for patients with grossly evident disease [21]. Breast cancer patients with isolated metastases treated with systemic therapy in conjunction with surgery and/or radiation appear to fare well with respect to survival and disease control [21–27]. Patients with >1 metastases [22, 24–26] or limited bone marrow positivity [25, 26] may also benefit from curative-intent therapy.
In theory, patients with truly limited metastatic disease may be amenable to curative-intent local therapy, in lieu of systemic therapy. However, there is no clinically reproducible manner in which to determine the extent of microscopic metastatic disease. Most women presenting with limited visceral metastases do develop additional metastases after resection alone [1, 21]. Thus, systemic therapy should remain a standard option for patients with oligometastatic disease. In these patients, local therapy can be offered as either consolidative treatment after assessment of response to systemic therapy, or as upfront treatment.
In the 11 patients treated with palliative-intent SBRT, all developed progression of metastatic disease at a relatively short time interval after enrollment (median of 4 months). Thus, our results do not compellingly support the hypothesis that decreasing disease bulk in patients with breast cancer has the potential to reduce distant metastatic progression. Nevertheless, SBRT to asymptomatic metastases in patients with many lesions may be warranted if the clinician believes that select lesions would cause symptoms with further growth. Indeed, 2 patients, with innumerable metastases involving at least 3 organs, who received SBRT to 1–2 liver metastases (with a net GTV of 65 and 463 ml), lived >18 months; arguably their liver disease could have caused symptoms or proved fatal earlier.
The patients treated with curative-intent SBRT were diagnosed with metastatic disease a median of 12 months prior to enrollment, compared to 22 months for patients treated with palliative-intent SBRT. The majority (36 of 40) of patients treated with curative-intent SBRT were treated to lesions that developed at the time of the initial presentation of metastatic disease, compared to only 2 of 11 patients treated with palliative-intent. Thus the patients treated with curative-intent SBRT were generally treated earlier in their disease course, and were more likely to undergo treatment to the initially presenting metastatic lesions. Patients treated with palliative-intent had progressed further along their natural disease course, generally with new metastases more life threatening than the lesions with which they initially presented.
Other than a greater GTV adversely affecting local control, no patient or tumor related variable proved significant in the MVAs of outcome among curatively treated patients, albeit in a small population possibly underpowered to detect significant differences. Although bone-only disease was significant on UVA for OS, PFS and DC, this variable did not achieve statistical significance or borderline significance on any MVA model. This likely reflects the instability of the MVA regression analysis with small patient numbers and only one distant failure event (and no deaths) in the bone-only patient group. Certainly, further studies are needed to assess the impact of SBRT for oligometastatic breast cancer lesions, and what variables influence outcome. The Southwest Oncology Group is proposing a study to investigate SBRT for patients with ≤5 lesions from breast cancer.
As discussed in the Introduction, the number of lesions amenable to curative-intent SBRT remains unknown, and perhaps is impacted by many variables. In our analysis of 121 patients treated with curative-intent SBRT, the number of lesions was not significant for any measured outcome. Whether or not the number of metastatic lesions involved predicts outcome in breast cancer patients is unclear from our data. The number of curatively treated lesions, stratified by 1 lesion or >1 lesions, was significant on UVA for survival and disease control, though did not remain significant on MVA. Among breast cancer patients treated with palliative-intent, those with innumerable metastases tended to live longer than those with ‘countable’ lesions. Thus, breast cancer patients with more than 5 ‘countable’ lesions may potentially be amenable to curative-intent therapy, though we do not have enough patients to determine this. Arguably, if the proposed SWOG study finds that lesion number is not a significant correlate with outcome, further studies can investigate outcome in patients with a greater number of lesions.
In summary, breast cancer patients with ≤5 clinically apparent metastases generally fare well after SBRT to oligometastatic lesions. Breast cancer can have a prolonged disease course, and thus longer follow-up is needed to confirm the hypothesis that oligometastatic disease is potentially curable with multimodality therapy incorporating local treatment. Nevertheless, our survival and progression free survival numbers are promising. Patients treated with palliative-intent appear to experience control of their lesions, albeit at short follow-up due to metastatic progression and death in these patients. Additional studies are needed to further explore SBRT for oligometastatic disease from breast cancer.
References
Rubin P, Green J (1968) Solitary metastases. Charles C. Thomas, Springfield, IL
Milas L, Hunter N, Withers HR (1976) Concomitant immunity to pulmonary metastases of a murine fibrosarcoma: influence of removal of primary tumor by radiation or surgery, of active specific immunization and treatment with Corynebacterium granulosum. Int J Radiat Oncol Biol Phys 1:1171–1178
Rosenberg SA (1987) Surgical treatment of metastatic cancer. Lippincott Williams & Wilkins, Philadelphia, PA
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13:8–10
Hellman S (1994) Karnofsky memorial lecture. Natural history of small breast cancers. J Clin Oncol 12:2229–2234
Fisher B (1980) Laboratory and clinical research in breast cancer—a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res 40:3863–3874
Fisher B, Redmond C, Fisher ER (1980) The contribution of recent NSABP clinical trials of primary breast cancer therapy to an understanding of tumor biology—an overview of findings. Cancer 46:1009–1025. doi :10.1002/1097-0142(19800815)46:4<1009::AID-CNCR2820461326>3.0.CO;2-H
Mehta N, Mauer AM, Hellman S et al (2004) Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol 25:1677–1683
Singletary SE, Walsh G, Vauthey JN et al (2003) A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 8:241–251. doi:10.1634/theoncologist.8-3-241
Tait CR, Waterworth A, Loncaster J et al (2005) The oligometastatic state in breast cancer: hypothesis or reality. Breast 14:87–93. doi:10.1016/j.breast.2004.10.003
Carey Sampson M, Katz A, Constine LS (2006) Stereotactic body radiation therapy for extracranial oligometastases: does the sword have a double edge? Semin Radiat Oncol 16:67–76. doi:10.1016/j.semradonc.2005.12.002
Timmerman RD, Kavanagh BD, Cho LC et al (2007) Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 25:947–952. doi:10.1200/JCO.2006.09.7469
Kavanagh BD, McGarry RC, Timmerman RD (2006) Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol 16:77–84. doi:10.1016/j.semradonc.2005.12.003
Katz AW, Carey-Sampson M, Muhs AG et al (2006) Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 67:793–798. doi:10.1016/j.ijrobp.2006.10.025
Okunieff P, Petersen AL, Philip A et al (2006) Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 45:808–817. doi:10.1080/02841860600908954
Milano MT, Katz AW, Schell MC et al (2008) Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys (in press)
Milano MT, Katz AW, Muhs AG et al (2008) A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 112:650–658. doi:10.1002/cncr.23209
Singh D, Yi WS, Brasacchio RA et al (2004) Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 58:3–10. doi:10.1016/S0360-3016(03)01442-1
O’Dell WG, Schell MC, Reynolds D et al (2002) Dose broadening due to target position variability during fractionated breath-held radiation therapy. Med Phys 29:1430–1437. doi:10.1118/1.1485977
Therasse P, Arbuck SG, Eisenhauer EA (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216. doi:10.1093/jnci/92.3.205
Rivera E, Holmes FA, Buzdar AU et al (2002) Fluorouracil, doxorubicin, and cyclophosphamide followed by tamoxifen as adjuvant treatment for patients with stage IV breast cancer with no evidence of disease. Breast J 8:2–9. doi:10.1046/j.1524-4741.2002.08002.x
Buzdar AU, Blumenschein GR, Montague ED et al (1984) Combined modality approach in breast cancer with isolated or multiple metastases. Am J Clin Oncol 7:45–50. doi:10.1097/00000421-198402000-00006
Holmes FA, Buzdar AU, Kau S-W (1994) Combined-modality approach for patients with isolated recurrences of breast cancer (IV-NED): the M.D. Anderson experience. Breast Dis 7:7–20
Blumenschein GR, DiStefano A, Caderao J et al (1997) Multimodality therapy for locally advanced and limited stage IV breast cancer: the impact of effective non-cross-resistance late-consolidation chemotherapy. Clin Cancer Res 3:2633–2637
Nieto Y, Cagnoni PJ, Shpall EJ et al (1999) Phase II trial of high-dose chemotherapy with autologous stem cell transplant for stage IV breast cancer with minimal metastatic disease. Clin Cancer Res 5:1731–1737
Nieto Y, Nawaz S, Jones RB et al (2002) Prognostic model for relapse after high-dose chemotherapy with autologous stem-cell transplantation for stage IV oligometastatic breast cancer. J Clin Oncol 20:707–718. doi:10.1200/JCO.20.3.707
Hanrahan EO, Broglio KR, Buzdar AU (2005) Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas M.D. Anderson Cancer Center and assessment of prognostic factors. Cancer 104:1158–1171. doi:10.1002/cncr.21305
Acknowledgements
The authors would like to thank the family of Barbara Fogarty for their support. We also thank Laura Brumbaugh for editorial assistance. Paul Okunieff, M.D., received grant support from BrainLAB AG (Heimstetten, Germany) between April 1, 2000, and March 31, 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milano, M.T., Zhang, H., Metcalfe, S.K. et al. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat 115, 601–608 (2009). https://doi.org/10.1007/s10549-008-0157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0157-4