Abstract
Background Paget’s disease of the breast is an uncommon presentation of breast malignancy, accounting for 1–3% of all the breast tumors and presents in different histopathologic patterns: in association with an underlying invasive or non invasive carcinoma, or without any underlying neoplasia. In the literature, different methods are used for the treatment. Mastectomy with or without axillary dissection has been considered as the standard treatment procedure for many years. Several studies have already shown that breast conservation with radiation therapy is an oncologically safe option. Regarding the axillary approach, several studies have documented the presence of positive sentinel lymph node even in Paget’s disease alone. The objective of this study was to retrospectively analyze outcome of patients affected by Paget’s breast disease and to define our institutional experience. Patients and methods Between May 1996 and February 2003, 114 patients with confirmed Paget’s disease of the breast were retrieved and underwent surgery at the European Institute of Oncology of Milan, Italy. The median age of the patients was 54 years at the time of the diagnosis. In our study, the histopathological examination of the operated specimen revealed one hundred seven patients with Paget’s disease associated with an underlying invasive or non invasive carcinoma, and seven patients without underlying carcinoma. Patients underwent either conservative breast surgery or mastectomy, with or without sentinel lymph node biopsy and/or axillary surgery. Each patient was evaluated after surgery at a multidisciplinary meeting to selecting systemic therapy. Results Seven patients had “pure” Paget’s disease of the breast and one hundred seven had the disease associated with an underlying carcinoma. As surgical techniques 71 mastectomies and 43 breast conserving surgeries have been performed. Complete axillary dissection was done in patients with clinically positive lymph node and/or sentinel lymph node biopsy positive. Sentinel lymph node biopsy was performed in nineteen patients with invasive component and five were positive and underwent axillary dissection. Eleven sentinel lymph node biopsies were done in patients with non invasive component and none of them was positive. Adjuvant systemic therapies were based on the final tumor, node and metastasis stage: thirty patients received adjuvant chemotherapy alone, fourteen received endocrine treatment alone, twenty-six patients were evaluated to receive both chemo and endocrine therapy. The median duration of follow up was 73 months and was updated in the last 6 months. Five patients developed local recurrence, one had regional recurrence, another two had loco-regional recurrences and fourteen had distant metastasis as a first event. Malignancy-related deaths were censored in the statistical analyses cancer for and due to another tumor in eleven patients. Additionally, deaths were not related to malignancy totally in thirteen patients. Conclusions Screening examination and imaging techniques are fundamental. Breast conserving surgery combined with breast irradiation for patients with invasive and non invasive breast carcinoma has become the treatment of first choice. All surgical conservative approaches should include the complete nipple–areolar complex and margins of resected specimen free of tumor. Thanks to the evolution of the conservative approach, good cosmetic result can be obtained. To be informed about the axillary lymph node status and to avoid the patient to have a second surgical approach, sentinel lymph node biopsy should be performed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
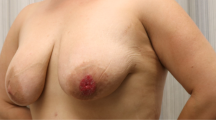
Paget’s disease of the nipple was first described in 1856 by Velpeau as an uncommon entity of the breast [1], followed by the description of Sir James Paget in 1874, as a syndrome in which ulceration of the nipple was associated with an underlying cancer [2]. Paget’s disease of the breast is an uncommon presentation of breast malignancy, accounting for 1–3% of all the breast tumors [3–5]. Clinically, it is characterized by nipple or areolar erythema, eczema, ulceration, bleeding and itching. The diagnosis of Paget’s disease is often delayed for months because of its mistaken diagnosis for a benign dermatologic disease involving the nipple. The histopathologic diagnosis is confirmed with a full-thickness biopsy of the nipple and areola.
Paget’s disease of the breast presents in different histopathologic patterns: in association with an underlying invasive or non invasive carcinoma, or without any underlying neoplasia. The underlying carcinoma can be located in any part of the breast. The incidence of the multifocality or multicentricity of the associated underlying carcinoma in Paget’s disease has been reported in 32% to 41% of patients [6, 7]. It is characterized histopathologically by the infiltration of the nipple epidermis with large round and ovoid tumor cells with abundant pale cytoplasm and vesicular nuclei with hyperchromatic nuclei with prominent nucleoli [8]. The pathogenesis of Paget’s disease still remains debatable: there are two theories about the origin of the Paget’s cells. Transformation theory proposes that Paget’s cells are transformed in situ keratinocytes of the epidermis of the nipple [9] and, whereas the most widely accepted second theory assumes that Paget’s cells are ductal carcinoma cells that have migrated from the underlying mammary ducts to the epidermis of the nipple [10, 11]. Lagios et al. in their experience reported although Paget’s disease of the nipple, without associated underlying breast carcinomassociated underlying tumors without or with minimal extent of underlying breast carcinoma. [12]. The second theory on the pathogenesis of Paget’s disease is supported by the presence of associated underlying breast carcinoma in nearly all patients [3, 4, 13].
In the literature, different methods are used for the treatment of Paget’s disease of the breast: nipple excision alone, radiotherapy alone, central lumpectomy or quadrantectomy with or without radiation, total mastectomy. Mastectomy with or without axillary dissection has been considered as the standard treatment procedure for these patients for many years [3, 4, 11, 14, 15]. However, it is reasonable to consider applicability of breast-conserving surgery: it has demonstrated similar long term survival rates than mastectomy in long-term follow-up studies [8, 16–18]. Several studies have already shown that breast conservation with radiation therapy is an oncologically safe option [19]. Regarding the axillary approach, several studies have documented the presence of positive sentinel lymph node even in Paget’s disease alone; sentinel lymph node biopsy should be considered to evaluate the axilla in all patients with Paget’s disease [20, 21]. This report may be supported by the fact that there is almost always an associated underlying carcinoma.
The objective of this study was to retrospectively analyze outcome of patients affected by Paget’s breast disease and to define our institutional experience.
Patients and methods
Between May 1996 and February 2003, 148 patients underwent surgery for Paget’s disease of the breast at the European Institute of Oncology of Milan, Italy, and were included into a specific database: the information was completed using data from the Institutional Breast database and additional specific data directly from medical records. Patients with recurrent breast carcinoma, other primary tumor, metastatic disease, those who received neoadjuvant treatment, and male patients were excluded from the analysis. Finally, hundred fourteen patients with confirmed Paget’s disease of the breast were retrieved, and Table 1 shows the patient’s characteristics and stratification for invasive/non invasive tumors.
The median age of the patients was 54 years (range 27–88) at the time of the diagnosis. A preoperative mammogram was performed at presentation in 106 patients with clinical findings of Paget’s disease. In our study, the histopathological examination of the operated specimen revealed one hundred seven (94%) patients with Paget’s disease associated with an underlying invasive or non invasive carcinoma, and seven (6%) patients without underlying carcinoma. As a surgical approach, patients underwent either conservative breast surgery (including a complete excision of the nipple-areola complex) or mastectomy, with or without sentinel lymph node biopsy and/or axillary surgery.
Each patient was evaluated after surgery at a multidisciplinary meeting to selecting systemic therapy. The presence of invasive carcinoma was a recommendation for systemic adjuvant chemotherapy treatment. Endocrine therapy was indicated for patients with endocrine responsive disease. Thirty-five out of forty-three patients in the group of the breast conserving surgery were evaluated to receive breast irradiation, including boost, intraoperative and standard radiotherapy, and four patients of the mastectomy group received chest wall irradiation.
Statistical methods
Cancer specific survival (CSS) was measured from the date of resection to the date of death due to breast cancer, with patients alive at the end of observation or those who died from causes other than breast cancer being censored in survival analyses. Disease free survival (DFS) was measured from the date of resection to the date of any first event, with patients free of disease at their last follow-up visit being censored in survival analyses. The Log-rank test was used to assess survival differences between groups in the univariate analysis. Multivariate analysis was not performed due to the limited number of events. All analyses were performed with the SAS software (SAS Institute, Cary, NC).
Results
Regarding the age classification, the number of patients affected is higher between 35 and 49 years old in cases of Paget’s disease associated with an invasive carcinoma and after 65 years old in cases of Paget’s disease associated with a non invasive carcinoma As expected, the rate between the presence and absence of an associated underlying tumor was significantly different: seven patients (6%) had “pure” Paget’s breast disease and one hundred seven (94%) had the disease associated with an underlying carcinoma. Table 2 shows the histopathologic findings at definitive surgery. There were seven patients without underlying carcinoma, thirty-nine (34%) patients with Paget’s disease associated with an in situ carcinoma: solid (n = 13), comedo (n = 9), cribriforme (n = 7), micropapillary (n = 2), apocrine (n = 2) and others (n = 6). The disease of sixty-eight (60%) patients was associated with an underlying invasive carcinoma: ductal (n = 64), lobular (n = 1), mucinous (n = 1) and invasive Paget’s disease (n = 2). Pathological assessment included evaluation of histological type and size of the tumor, lymph node status, tumor grading, estrogen (ER) and progesterone receptor (PR) status, Ki-67 labeling index, c-erbB-2 overexpression and vascular invasion. We noticed that patients with Paget’s disease had more likely ER and PR negativity, a high histologic grade and an overexpression of c-erbB-2 oncoprotein.
As surgical techniques we have performed 71 (62%) mastectomies and 43 (38%) breast conserving surgical treatments: a more detailed description is 50 (74%) mastectomies and 18 (27%) breast conserving surgery for disease associated with an underlying invasive component, 21 mastectomies (46%) and 25 (54%) breast conserving surgeries for Paget’s disease associated with an underlying non invasive carcinoma or pure Paget’s nipple disease. Complete axillary dissection was done in patients with clinically positive lymph node and/or sentinel lymph node biopsy positive. Of 85 patients who had axillary lymph nodes examination, 35 patients had axillary metastasis. Sentinel lymph node biopsy was performed in nineteen patients with invasive component and five were positive and underwent axillary dissection. Eleven sentinel lymph node biopsies were done in patients with non invasive component and none of them was positive.
Adjuvant systemic therapies were based on the final tumor, node and metastasis stage and the multidisciplinary evaluation was so: thirty patients received adjuvant chemotherapy alone, fourteen received endocrine treatment alone, twenty-six patients were evaluated to receive both chemo and endocrine therapy. In the non invasive group, twelve patients received endocrine therapy. Seventeen of eighteen patients who underwent breast conserving surgery because of presence of underlying carcinoma received breast irradiation: thirteen (72%) were evaluated for boost radiotherapy followed by post-operative breast radiotherapy, four patients (22%) received standard breast irradiation and one patient did not receive radiotherapy. Due to specific histological findings making us suspect a higher risk of local relapse of disease, an external radiotherapy to the chest wall was delivered in four patients treated with mastectomy. In the non invasive group with breast conserving surgery, two patients (8%) received intraoperative radiotherapy with electrons (ELIOT), fourteen received the standard breast irradiation, other two were evaluated for boost radiation and the remaining seven patients (28%) did not receive radiotherapy.
Table 3 describes the events related to breast cancer: five patients developed local recurrence, one had regional recurrence, another two had loco-regional recurrences and fourteen had distant metastasis as a first event. Among forty-three patients who underwent conservative surgery, after a median follow-up of 73 months, we observed six loco-regional recurrences, four invasive and two non invasive, and no distant metastasis. Regarding the breast conserving surgery group, the three local disease recurrences indicated in the pathologic findings for the non invasive group were: non invasive component in one patient (treated with mastectomy and lymph node biopsy), invasive component in another patient (treated with conservative breast surgery and lymph node biopsy), and in the remaining patient, Paget’s disease reappearance on the surgical scar (underwent conservative surgery). One patient who had received mastectomy suffered of local recurrence with invasive component and underwent soft tissue excision of chest wall. In the invasive group, only one local recurrence occurred and the patient underwent mastectomy.
Table 4 depicts univariate survival analysis. Vascular invasion was the only statistically significant prognostic factor for both CSS and DFS. Even if not statistically significant, early age, tumor diameter larger than 2 cm and nodal involvement were consistently associated with worse CSS and DFS. CSS was significantly poorer for patients with invasive disease (P = 0.03; Fig. 1). DFS was similar in the two groups (P = 0, 64; Fig. 2)
The median duration of follow up was 73 months and was updated in the last 6 months. No patient was lost to follow-up. Malignancy-related deaths were censored in the statistical analyses cancer for and due to another tumor in eleven patients. Additionally, deaths were not related to malignancy totally in thirteen patients.
Discussion
Paget’s disease, as a special form of breast cancer, remains unusual. In our study, the median age of the patients was 54 years old, similar with the given literature where the peak incidence of is between 50 and 60 years old, a population of women older than breast cancer without Paget’s disease. The most frequently reported symptoms are nipple discharge, scaling or eczematous changes, like erythema, eczema or ulceration. Most of our patients had two or more symptoms at presentation. The eczematous reaction almost always appears first on the nipple, subsequently spreading to the areola [22, 23].
Paget’s disease of the breast is diagnosed primarily by clinical exam, so it should be diagnosed at the moment of the nipple changes when no palpable mass in the breast is present. In patients whose disease is confined to the nipple, prognosis is better than in those accompanied by a palpable mass in the breast and suspicious mammogram [24].
The presence of a palpable breast mass or suspicious mammogram is with a high probability accompanied with an underlying cancer, usually an invasive carcinoma, and prognosis is determined by the stage of the malignancy. In our series, we reported 21 cases (19%) without radiologic findings [6 (9%) in invasive group and 15 (33%) in non invasive group] and 85 cases (77%) with radiologic findings [57 (86%) in invasive group and 28 (62%) in non invasive group]. In a recent study including 40 women with a benign mammogram and no palpable mass, the majority (68%) had non invasive ductal carcinoma and invasive cancer occurred in 5% of the patients [24]. Magnetic resonance imaging and breast ultrasound have been considered for screening and diagnosis [25]: in case of non invasive breast cancer, magnetic resonance imaging (MRI) has a sensitivity of 95% compared to 70% with mammography [26] and seems able to differentiate the normal from the abnormal nipple and tumors confine to the retroareolar tissue from tumor involving the nipple/areolar complex [25]. As underlying carcinoma is common even in women with a benign mammogram and no palpable mass, breast ultrasound and MRI may be useful in detecting distant lesions specially in young women.
Compared with those of the given literature, the rate of associated underlying carcinoma in Paget’s disease of the breast in our series (94%) was similar as the other studies (82–100%) [3, 6, 7, 11]. Other series concluded that an underlying breast tumor is present in nearly 96–100% of these patients, reflecting the prognosis of the disease [3, 4, 8]. The majority of the patients have underlying cancer and their prognosis depends primarily by the presence or absence of an invasive component [3, 5]. In our study, even if not statistically significant, early age, tumor diameter larger than 2 cm and nodal involvement were consistently associated with worse prognosis.
In different series, the most associated underlying non invasive carcinoma is the “comedo” type [3, 4]. In our study, the”solid” type (33%) was higher than the “comedo” type (23%). Invasive cancer associated with Paget’s disease was more commonly estrogen and progesterone negative, with high pathological grade [17]. We confirmed this report in our study: in the invasive disease group, we found 38 of 68 (56%) patients with estrogen receptor negative, 44 of 68 (65%) with progesterone receptor negative, and 41 of 66 (62%) cases were histologically Grade III and 25 of 66 (38%) were Grade II. A more pronounced affirmation of these data is seen in the non invasive disease group (Table 2). A high positivity for c-erbB-2 in Paget’s breast disease with underlying invasive and non invasive carcinoma was demonstrated in several studies [27–29]. c-erbB-2 overexpression in breast carcinoma is known to be associated with an aggressive disease potential and with worse prognosis [30–32]. In our findings 71 of 81 patients (88%) had an overexpressed c-erbB-2 oncoprotein.
To achieve adequate local control in a disease in which almost always an underlying carcinoma is associated, mastectomy has been the standard treatment for the Paget’s disease of the breast. Several studies have shown that 20–40% of mastectomy specimens from patients with Paget’s disease have multifocal or multicentric component and were often underestimated in mammography [4–6]. In these cases a breast conserving surgical approach without mastectomy is problematic. Dixon et al. and Polgar et al. reported a high local recurrence rate in patients with underlying carcinoma treated with local excision alone and concluded that local excision alone was not an appropriate treatment [5, 33]. Among our forty-four patients who underwent conservative surgery, after a median follow-up of 73 months, we observed six loco-regional recurrences, four invasive and two non invasive, and no distant recurrence. Breast conserving surgery is safe in patients with Paget’s disease, as it provides acceptable local control when the disease is adequately excised. Regarding our series, the non invasive Paget group undergoing breast conserving surgery, local or loco-regional relapse was seen at three of five patients who did not receive breast irradiation. Stockdale et al. reviewed 28 patients with Paget’s disease of the nipple treated with nipple biopsy and radiotherapy alone and three of them having an underlying mass developed recurrence and died of metastatic disease [34]. Invasive recurrence is associated with poor prognosis after breast-conserving surgery in patients with Paget’s disease of the nipple [5, 6]. Three of our five recurred patients of the non invasive group developed invasive breast carcinoma and two of them were of the high Grade III and associated with axiller metastasis too. The first results of the current study were so, although long term follow-up will be required. However, few studies have suggested in recent years a role for breast-conserving therapy in selected groups of patients, including patients without the presence of palpable mass or mammographic changes. Pierce et al. reported experience on selected patients that the combination of conservative breast surgery and radiation treatment is an alternative to mastectomy [18]. Data from Marshall et al. confirm excellent rates of local control, disease-free survival, and overall survival at 10 and 15 years following breast conserving therapy and radiotherapy for Paget’s disease of the breast and this study continues to support the recommendation of local excision and definitive breast irradiation as an alternative to mastectomy in the treatment of selected patients [35]. Chen et al. in their recent large analysis concluded that patients who underwent central lumpectomy and mastectomy for Paget’s disease of the breast had similar outcomes [17].
Breast conserving surgery and sentinel lymph node biopsy are the treatment of choice for most women with early-stage breast cancer [36, 37]. Since its introduction in the mid-1990s, sentinel lymph node biopsy has been rapidly adopted for axillary staging of clinically node-negative breast cancer patients [38]. As confirmed in the definitive histopathologic specimen, Paget’s disease of the breast is almost always associated with an underlying breast cancer, even in absence of clinical and/or radiological findings. So patients should be treated similar to other breast cancer. Sentinel lymph node biopsy should be performed to evaluate the axilla when invasive breast cancer and in selected patients with associated ductal intraepithelial neoplasia [21, 39]. Sukumvanich et al. described in their “Paget only”cohort including 39 patients, that invasive cancer was found in 27% of cases and the frequency of positive sentinel lymph node biopsy was found in 11% patients [20].
The rarity of Paget’s disease of the breast does not allow for randomized trials comparing treatment modalities, with the aid of improved mammographic and radiotherapy techniques, the breast-conserving approach has been considered as a treatment option in selected patients [24, 35]. Although long term follow-up will be required, data from previous studies suggest that the type of surgical treatment did not result in differences in DSS and RFS. Due to the limited small number of patients, different classification of selected patients and required long term follow-up, there are no randomized trials to choose to best treatment for each case.
Conclusions
In Paget’s disease of the breast, screening examination and imaging techniques are fundamental. Comparing our findings to those of published studies, we believe that breast conserving surgical therapy combined with breast irradiation for patients with invasive and non invasive breast carcinoma has become the treatment of first choice. To reduce the risk of local recurrence in Paget’s disease, all surgical conservative approaches should include the complete nipple–areolar complex and margins of resected specimen free of tumor. Nowadays, thanks to the evolution of the conservative approach to breast cancer, nipple reconstruction and nipple tattoo, good cosmetic result can be obtained. To be informed about the axillary lymph node status and, in case of associated underlying invasive or microinvasive carcinoma and to avoid the patient to have a second surgical approach, sentinel lymph node biopsy should be performed, even if clinical and radiological exams are negative.
References
Velpeau A (1856) On disease of the mammary areola preceding cancer of the mammary region (trans: Mitchell H). Sydenham Society, London
Paget J (1874) On disease of the mammary areola preceding carcinoma of the mammary gland. St Bartholomews Hosp Rep 10:87–89
Ashikari R, Park K, Huvos AG, Urban JA (1970) Paget’s disease of the breast. Cancer 26:680–685
Chaudary MA, Millis RR, Lane EB, Miller NA (1986) Paget’s disease of the nipple: a ten-year review including clinical, pathological, and immunohistochemical findings. Breast Cancer Res Treat 8:139–146
Dixon AR, Galea MH, Ellis IO, Elston CW, Blamey RW (1991) Paget’s disease of the nipple. Br J Surg 78:722–723
Kothari AS, Beechey-Newman N, Hamed H, Fentiman IS, D’Arrigo C, Hanby AM (2002) Paget disease of the nipple: a multifocal manifestation of higher-risk disease. Cancer 95:1–7
Fu W, Mittel VK, Young SC (2001) Paget disease of the breast: analysis of 41 patients. Am J Clin Oncol 24:397–400
Bijker N, Rutgers EJ, Duchateau L, Peterse JL, Julien JP, Cataliotti L et al (2001) Breast-conservating therapy for Paget’s disease of the nipple. Cancer 91:472–477
Muir R (1935) Pathogenesis of Paget’s disease of the nipple and associated lesions. BR J Surg 22:728–737
Inglis K (1946) Paget’s disease of the nipple, with special reference to changes in the ducts. Am J Pathol 22:1–33
Yim JH, Wick MR, Philpott GW, Norton JA, Doherty GM (1997) Underlying pathology in mammary Paget’s disease. Ann Surg Oncol 4:287–292
Lagios MD, Westdahl PR, Rose MR, Concannon S (1984) Paget’s disease of the nipple. Alternative management in cases without or with minimal extent of underlying breast carcinoma. Cancer 54:545–551
Kollmorgen DR, Varanasi JS, Edge SB, Carson WE III (1998) Paget’s disease of the breast: a 33–year experience. J Am Coll Surg 187:171–177
Paone JF, Baker RR (1981) Pathogenesis and treatment of Paget’s disease of the breast. Cancer 48:825–829
Freud H, Maydovnik M, Laufer N, Durst A (1977) Paget’s disease of the breast. J Surg Oncol 9:93–98
Kawase K, Dimaio DJ, Tucker SL, Buchholz TA, Ross MI, Feig BW et al (2005) Paget’s disease of the breast: there is a role for breast-conserving therapy. Ann Surg Oncol 1:21–27
Chen C, Sun L, Anderson B (2006) Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer 107:1448–1458
Pierce LJ, Haffty BG, Solin LJ, McCormick B, Vicini FA, Wazer DE et al (1997) The conservative management of Paget’s disease of the breast with radiotherapy. Cancer 80:1065–1072
Joseph KA, Ditkoff BA, Estabrook A et al (2007) Therapeutic options for Paget’s disease: a single institution long-term follow-up study. Breast J 13(1):110–111
Sukumvanich P, Bentrem DJ, Cody HS et al (2007) The role of sentinel lymph node biopsy in Paget’s disease of the Breast. Ann Surg Oncol 14(3):1020–1023
Laronga C, Nasson D, Hoover S et al (2006) Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg 192(4):481–483
Osther PJ, Balslev E, Blichert-Toft M (1990) Paget’s disease of the nipple. Acta Chir Scand 156:343–352
Jamali FR, Ricci A, Deckers PJ (1970) Paget’s disease of the breast. Am J Surg 76:365–381
Zakaria S, Pantvaidya G, Gosh K, Degnim AC (2007) Paget’s disease of the breast: accuracy of preoperative assessment. Breast Cancer Res Treat 102(2):137–142
Friedman EP, Hall-Craggs MA, Mumtaz H, Schneidau A (1997) Breast MR and the appearance of the normal and abnormal nipple. Clin Radiol 52:854–861
Soderstrom CE, Harms SE, Copit DS et al (1996) 3D RODEO breast MRI of lesions containing ductal carcinoma in situ. Radiology 201:427–432
Haerslev T, Krag JG (1992) Expression of citokeratin and erbB-2 oncoprotein in Paget’s disease of the nipple. An immunohistochemical study. APMIS 100:1041–1047
Wolber RA, Dupuis BA, Wick MR (1991) Expression of c-erb-B2 oncoprotein in mammary and extrammamary Paget’s disease. Am J Clin Pathol 96:243–247
Lammie GA, Barnes DM, Millis RR, Gullick WJ (1989) An immunohistochemical study of the presence of c-erbB-2 protein in Paget’s disease of the nipple. Histopathology 15:505–514
Winstanley J, Cooke T, Murray GD, Platt-Higgins A, George WD, Holt S et al (1991) The long term significance of c-erB-2 in primary breast cancer. Br J Cancer 63:447–450
McCann AH, Dervan PA, O’Regan M, Codd MB, Gullick WJ, Tobin BM et al (1991) Prognostic significance of c-erbB-2 and oestrogen receptor status in human breast cancer. Cancer Res 51:3296–3303
Lovekin C, Ellis IO, Locker A, Robertson JF, Bell J, Nicholson R et al (1991) c-erbB-2 oncoprotein expression in primary and advanced breast cancer. Br J Cancer 63:439–443
Polgar C, Orosz Z, Kovacs T, Fodor J (2002) Breast-conserving therapy for Paget disease of the nipple: a prospective European Organization for Research and Treatment of Cancer Study of 61 patients. Cancer 94:1904–1905
Stockdale AD, Brierley JD, White WF, Folkes A, Rostom AY (1989) Radiotherapy for Paget’s disease of the nipple: a conservative alternative. Lancet 2:664–666
Marshall JK, Griffith KA, Haffty BG, Solin LJ, Vicini FA, McCormick B et al (2003) Conservative management of Paget disease of the breast with radiotherapy: 10- and 15-year outcomes. Cancer 97(9):2142–2149
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation ort he treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Shivers S, Cox C, Leight G et al (2002) Final results of the Department of Defense multicenter breast lymphatic mapping trial. Ann Surg Oncol 9:248–255
Lucci A Jr, Kelemen PR 3rd Miller C et al (2001) National practice patterns of sentinel lymph node dissection for breast carcinoma. J Am Coll Surg 192(4):453–458
Veronesi U, Paganelli G, Viale G et al (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349:546–553
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caliskan, M., Gatti, G., Sosnovskikh, I. et al. Paget’s disease of the breast: the experience of the European institute of oncology and review of the literature. Breast Cancer Res Treat 112, 513–521 (2008). https://doi.org/10.1007/s10549-007-9880-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9880-5