Abstract
The 21-gene reverse transcriptase-polymerase chain reaction assay with a patented algorithm is validated as a good predictor of prognosis and potential benefit from adjuvant chemotherapy for lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer, while its high cost raises concern about how to finance it. Cost-effectiveness analysis comparing prevalent National Comprehensive Cancer Network (NCCN) guideline/St Gallen recommendation-guided treatment with the assay-guided treatment is carried out with budget impact estimation in the context of Japan’s health care system. Incremental cost-effectiveness ratios are estimated as 2,997,495 ¥/QALY (26,065 US$/QALY) in the comparison between NCCN guided-treatment vs. the assay-guided treatment, and as 1,239,055 ¥/QALY (10,774 US$/QALY) in the comparison between St Gallen guided-treatment vs. the assay-guided treatment. Budget impact is estimated as ¥2,638 million (US$23 million) to ¥3,225 million (US$28 million) per year. The routine use of the assay is indicated as cost-effective. And the budget impact could be judged as within fundable level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the medical profession as well as the general public have become to have high hopes for the future of “tailor-made medicine”, which means individualised treatment according to each patient’s pathology, especially using gene diagnoses or biomarkers [1]. And this is the case with cancer care in Japan, as well [2].
Regarding breast cancer care, the role of adjuvant chemotherapy for lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer (LN−, ER+, ESBC) in order to prevent or delay distant recurrence after primary surgery has been debated [3–6], while the use of hormonal therapy with tamoxifen or aromatase inhibitors in those cases is established by several large randomised clinical trials [7, 8]. Efforts to aggregate available evidences have been made in order to best guide the clinical decision of whether to add chemotherapy or not, which result in the development of consensus guidelines, such as National Comprehensive Cancer Network (NCCN) guideline [9, 10] or St Gallen recommendation [5]. These guidelines evaluate patient’s risk of recurrence based on factors such as age, tumour size and histology, and then suggest the indication for adjuvant chemotherapy to higher risk patients based on a judgement that the benefit of survival from chemotherapy overweighs the disbenefit of adverse effects and medical risks [11]. However, the risk classification which underlies this judgement has been considered as not certain nor specific enough, so that it leaves a room for the development of a more accurate and individualised predictor of the risk of recurrence.
A multigene assay of resected breast cancer tumour tissue was implemented in order to realise more informed and individualised decision for adjuvant chemotherapy indication, which resulted in the development of the 21-gene reverse transcriptase-polymerase chain reaction (RT-PCR) assay with a patented algorithm (Oncotype DX® Breast Cancer Assay). It gives an individual case of LN−, ER+, ESBC Recurrence Score (RS) that represents individualised risk of recurrence. The accuracy of RS as criteria in assessing the risk of recurrence was validated by a prospective study of historical clinical trial data from National Surgical Adjuvant Breast Cancer Project (NSABP) B-14 study with the gene assay of preserved tumour tissue [12]. Furthermore, the accuracy of RS in predicting the magnitude of chemotherapy benefit was validated by a similar study including data from NSABP B-20 study with the gene assay [13]. In other words, patients classified as high risk of recurrence by RS criteria are likely to be highly responsive to chemotherapy, which implies that the assay is clinically efficient in identifying those who could benefit from adjuvant chemotherapy.
This development is deemed as a pathway geared towards tailor-made medicine in breast cancer care, which anticipates a similar innovative assay like 70-gene signature (MammaPrint®) [14]. Yet another significant characteristic of the 21-gene RT-PCR assay is its high price, ¥450,000 (US$3,913; US$1 = ¥115), while the reimbursement for a conventional gene diagnosis test of malignant tumour is set at ¥20,000 (US$174) in the social health insurance system of Japan. Needless to say, a valuable innovation of technology deserves patent protection and accompanying financial rewards as its own right. However, from the viewpoint of economics, it is imperative to appraise the “value for money” of such highly priced new technology [15]. The proportion of LN−, ER+ cases among breast cancer is large, 28.7% [16], and the incidence of breast cancer is estimated as 41,494 in 2005 and increasing continuously [17]. Therefore, once the assay becomes a standard procedure within social insurance benefit package, more than 12,000 assays are expected to be implemented in a year. This leads to a concern about its implication for health financing. From the viewpoint of health manager, it is also imperative to appraise the “budget impact” [18], which basically correlates to the product of the price and the quantity of health services provided.
To date, there are two studies that look at economic aspects of the 21-gene RT-PCR assay based on validation studies in the U.S. health system. Hornberger et al. carried out an economic evaluation of the assay, and reported it as cost-saving based on a reclassification of patients’ risk using RS criteria, instead of NCCN criteria [19]. Lyman et al. also reported that RS-guided treatment could be cost-saving compared to the treatment with tamoxifen combined with chemotherapy for all patients, and cost-effective compared to the treatment with tamoxifen alone for all patients [20]. There is no report from any other countries nor yet a comparison with St Gallen-guided treatment.
This study aims to evaluate cost-effectiveness and budget impact of the 21-gene RT-PCR assay in Japan’s health care system. The results should be useful in considering the diffusion of the assay in Japan, and could inform health care policy in the era of tailor-made medicine in developed countries.
Methods
We conduct a cost-effectiveness analysis with decision trees and Markov modelling based on the validation studies of the 21-gene RT-PCR assay [12, 13, 21], and a costing under Japan’s social health insurance system including a sensitivity analysis from societal perspective. We also estimate the budget impact of the assay on Japan’s social health insurance system based on our economic model.
Scenarios and comparisons
Both Japanese clinical practice [22] and consensus guidelines [23, 24] are in accordance with NCCN guideline as well as St Gallen recommendation in a mixed way. And changing criteria from NCCN/St Gallen to RS in risk reclassifications with estimated distant recurrence free survival in 10 years (DRFS10) were reported in one of the validation studies as shown in Table 1 [21]. (Since DRFS10 of patients with intermediate risk according to St Gallen criteria was not yet published, we assume the mid-value of DRFS10 between high risk and low risk classified by St Gallen criteria.) Three scenarios are set up in this study: a hypothetical cohort of LN−, ER+, ESBC at the age of 55 undergoes NCCN-guided treatment, St Gallen-guided treatment, and RS-guided treatment. The age of 55 is chosen according to the average age of equivalent patient population in a nationwide cancer registry [16]. The former two scenarios intend to depict the status quo of Japanese practice to some extent. The last scenario intends to illustrate the situation in which the 21-gene RT-PCR assay is applied routinely.
Regarding the use of adjuvant chemotherapy, 100% of patients classified as high risk by NCCN/St Gallen criteria and 50% of patients classified as intermediate risk by St Gallen criteria are assumed to undergo chemotherapy, while 100% of patients classified as high or intermediate risk by RS criteria are assumed to undergo chemotherapy.
Then, the two pairs of scenarios are compared: NCCN-guided treatment vs. RS-guided treatment, and St Gallen-guided treatment vs. RS-guided treatment. These comparisons intend to depict the diffusion of the assay in Japanese practice. The use of chemotherapy decreases from 92 to 49% under the former comparison, and from 75 to 49% under the latter comparison by the adoption of RS criteria.
Decision tree and Markov model
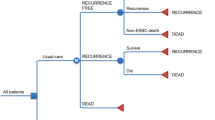
We construct decision trees with Markov model of clinical courses followed by LN−, ER+, ESBC patients, which is shown in Fig. 1.
Decision tree and Markov model. Abbreviations: Reverse transcriptase-polymerase reaction (RT-PCR), recurrence score (RS), human epidermal growth factor receptor type2 (HER2), hormonal therapy (HT), chemotherapy (CT), National Comprehensive Cancer Network (NCCN), lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer (LN−, ER+, ESBC)
The decision tree 1 shows the comparison between NCCN-guided treatment vs. RS-guided treatment; and the decision tree 2 shows the comparison between St Gallen-guided treatment vs. RS-guided treatment. Decision nodes of these trees are as to a decision whether to apply the 21-gene RT-PCR assay or not. Following chance nodes discern the cohort to different adjuvant therapies depending on the risk classification and human epidermal growth factor receptor type2 (HER2) status. Since the use of trastuzumab for HER2 positive (HER2+) cases as adjuvant therapy is about to be included in the social health insurance benefit according to the results of international clinical trials [25, 26], we set up three types of adjuvant therapies: hormonal therapy (HT), HT plus chemotherapy (CT), and HT plus CT plus trastuzumab. Branches with CT lead to subtree B via a chance node, which discern the cohort to different toxicities.
The Markov model shows the clinical course once the adjuvant therapy is completed. Five stages are modelled here: (1) LN−, ER+, ESBC after criteria-guided adjuvant therapy; (2) Distant recurrence with response to treatment; (3) Distant recurrence with no response to treatment; (4) Progression of disease after distant recurrence; and (5) Death. Transitions between the stages are indicated with arrows. Patients follow various courses after recurrence, so conditions other than these five stages and transitions not described with arrows here are possible. However, we model the course in this way based on available reports of prognosis model of metastatic breast cancer, which is calibrated with the results of several randomised trials [19, 27]. Patients with recurrence undergo drug treatment with HT, CT, and/or trastuzumab depending on their status.
The span of each stage is set up at 1 year. Markov process is repeated up to 10 years, since the transitional probabilities of recurrence are calculated from DRFS10 and most of recurrences are expected to occur within this time horizon. After the 10-year, survived patients without recurrence are assumed to have a life expectancy for Japanese female at age 65 [28], and those with recurrence are to have a life expectancy of 2 years.
Outcome estimation
Outcomes by the scenario in terms of years of life saved (YOLSs) and quality adjusted life years (QALYs) are estimated by assigning probabilities and utility weights to the decision trees and Markov model from the literature.
Probabilities of risk classification, attached to the first chance nodes of each branch, are adopted from one of the validation studies of the 21-gene RT-PCR assay [21] shown in Table 1. Table 2 shows the other probabilities and utility weights used. A probability of HER2+, 9.3%, attached to the second chance nodes, is adopted from a nationwide breast cancer registry [16]. Probabilities of adjuvant chemotherapy toxicity, attached to the chance node in the subtree B, are assumed to be 60% for minor toxicity, 5% for major toxicity and 0.5% for fatal toxicity from a report of efficacy and cost-effectiveness of adjuvant chemotherapy in breast cancer [29].
Regarding the Markov model, transitional probabilities of recurrence with adjuvant HT are calculated from DRFS10 in Table 1. The effectiveness of adding adjuvant CT and trastuzumab are incorporated as risk reduction of recurrence. Relative risk reductions resulted from CT among patients classified as high risk and intermediate risk by RS criteria are fixed at 74 and 39%, respectively, which are adopted from one of the validation studies of the 21-gene RT-PCR assay [13]. A relative risk reduction resulted from trastuzumab among HER2+ patients are assumed to be 36% for up to 2 years according to the results of clinical trial [26]. As mentioned earlier, transitional probabilities between stages after recurrence are adopted from prognosis model of metastatic breast cancer [19, 27]. It is assumed that the response to treatment and the prognosis after recurrence differ depending on HER2 status. Probabilities of the response to treatment for recurrence are fixed at 38.0% among HER2− patients and 54.0% among HER2+ patients [27]. Probabilities of the progression of disease after recurrence are also fixed at: 59.7% if HER2− and having responded to treatment, 53.7% if HER2+ and having responded to treatment, 98.3% if HER2− and not having responded to treatment and 88.5% if HER2+ and not having responded to treatment [19]. Probabilities of death after the progression of disease are fixed at 40.0% among HER2− patients and 37.2% among HER2+ patients [19].
In order to estimate the outcome in terms of QALYs, utility weights are chosen for various health statuses during the clinical course which patients follow. A weight for health status after adjuvant therapy without any toxicity or distant recurrence is chosen to be 0.98 [30]. Weights for toxicities are 0.90 for minor toxicity, and 0.80 for major toxicity [29], of which duration is assumed as 6 months. Health status during chemotherapy against the distant recurrence or the progression of disease weighs 0.50 [31], of which duration is assumed as 6 months. Health statuses after the chemotherapy weigh 0.84 if responded, 0.70 if stable and 0.49 if progressive [27].
Outcome is discounted at a rate of 3% [32].
Costing
From societal perspective, costing should cover the opportunity cost borne by various economic entities in the society. In the context of this study, costs borne by social insurers and patients are considered, since these two entities are major payers to health care providers under Japan’s social health insurance system. The amount of direct payments by these entities, mostly according to the national medical care fee schedule, are estimated as costs, while costs to sector other than health and productivity losses are left uncounted in this study. This choice of scope in costing allows the following budget impact estimation.
Cost items are identified along the decision trees and Markov model: the 21-gene RT-PCR assay, adjuvant therapies, treatments for toxicity, monitorings, treatments for distant recurrence, and end-of-life treatments as shown in Table 3. As already mentioned, the cost of the assay is ¥450,000 (US$3,913), according to the price offered by Japanese supplier of Oncotype DX® Breast Cancer Assay. Costs of treatments except the end-of-life treatments are estimated by combining a model of breast cancer care and the national medical care fee schedule. The care model is developed based on both a nationwide survey of Japanese expert practice [22] and consensus guidelines [23, 24].
Adjuvant hormonal therapy includes outpatient care with tamoxifen, aromatase inhibitors, and LH–RH analogues depending on patient’s status, and is assumed to continue up to 5 years, which costs ¥534,610 (US$4,649) per year. Adjuvant chemotherapy includes various regimens. Anthracycline-based combination chemotherapy is used for about half of the cases, and oral fluorinated pyrimidine and CMF (cyclophosphamide, methotrexate and 5-fluorouracil) therapy are frequently used among other regimens. These cost ¥343,001 (US$2,983). Adjuvant trastuzumab costs ¥3,105,120 (US$27,001) per year, of which administration is assumed to continue for 1 year.
There are three levels of toxicity in the decision tree. However, only the cost of major toxicity is estimated as ¥173,352 (US$1,507), which includes unplanned 1 month hospitalisation in two-fifths of the cases and rescue treatment at outpatient clinic in three-fifths of the cases [33, 34]. The cost of minor toxicity, from which 60% of patients suffer, is included in the cost of adjuvant chemotherapy, since prophylactic use of antiemetic, for example, is applied routinely these days. And the clinical course of fatal toxicity is diverse and not fit to costing by modelling here, so its cost is estimated later coupled with the cost of end-of-life treatment.
Patients who complete adjuvant therapy are assumed to visit a clinic twice a year for the purpose of monitoring, which costs ¥25,340 (US$220) per year.
There are various options of treatments for the distant recurrence depending on regimens used in adjuvant therapy. Yet, we assume crossover hormonal treatments followed by capecitabine within the first year as typical first line and second line therapies for our hypothetical cohort, which cost ¥558,458 (US$4,856) per year. We further assume that this cost is applicable to second year and afterwards. For HER2+ patients, trastuzumab is additionally administered, of which cost is the same as one during the adjuvant therapy.
The end-of-life treatments are diverse in contexts and lack consensus guidelines or survey data. Its practice reflects other factor than medical judgements, for example, patients’ and their family’s preference. Therefore, we do not try to build care model of these cases but exercise an insurance claim review on 80 recent fatal cases in breast cancer at Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital. This results in ¥1,315,143 (US$11,436) per year, which is also used as the cost of treating fatal toxicity.
Costs are also discounted at a rate of 3% [32].
Comparison of scenarios
Incremental cost-effectiveness ratios (ICER) are calculated for the purpose of comparing the scenarios:
Sensitivity analysis
In order to appraise the stability of ICERs against assumptions and uncertainty of adopted values of probabilities, utility weights, and costs in our economic model, one way sensitivity analyses are performed. The age of cohort is changed to 45 and 65 years old. DFRS10s shown in Table 1 are changed by ±50%, which embrace the relaxation of mid-value assumption of DRFS10 of patients with intermediate risk according to St Gallen criteria into both end values. The use of adjuvant chemotherapy in NCCN-guided treatment is changed from 50% of high risk cases only to 100% of high risk cases and 50% of low risk cases; and from 0 to 100% of intermediate risk cases in St Gallen-guided treatment. Propensity to alter treatment among patients classified as intermediate risk by RS criteria reclassification is changed from 100 to 50%. As shown in Table 2, probabilities other than relative risk reductions are changed by ±50%, while the relative risk reductions are changed according to the reported 95% confidence intervals of each value. The effectiveness of adjuvant trastuzumab is extended to 5 years. Utility weights are all changed by ±20%. And as shown in Table 3, costs are all changed by ±50%. Discount rate is also changed from 0 to 5%.
Budget impact estimation
Budget impact is defined as a forecast of rates of use (or changes in rates of use) with their consequent short- and medium-term effects on budgets and other resources to help health service managers [35]. The budget in this study is defined as funds held by social insurers. We estimate the budget impact with our economic model assuming that all new LN−, ER+, ESBC in Japan undergo RS-guided treatment instead of NCCN/St Gallen-guided treatment from 2008 to 2012. The incidence of breast cancer is adopted from a forecast [17], and a share of LN−, ER+, ESBC is fixed at 28.7% [16]. A share of the budget in costs is assumed to be 70% according to the co-payment ratio in Japan’s social health insurance system.
Results
Cost-effectiveness
Table 4 shows the result of the cost-effective analysis. The cost of RS-guided treatment, ¥4,135,279 (US$35,959), exceeds that of NCCN-guided treatment, ¥3,845,923 (US$33,443), which results in a positive incremental cost of ¥289,355 (US$2,516). The effect in YOLSs of RS-guided treatment, 19.895 years, exceeds that of NCCN-guided treatment, 19.812 years, which results in a positive incremental effect of 0.083 year. The effect in QALYs of RS-guided treatment, 19.405 years, exceeds that of NCCN-guided treatment, 19.309 years, which results in a positive incremental effect of 0.097 year.
Similarly, the cost of RS-guided treatment, ¥4,134,791 (US$35,955), exceeds that of St Gallen-guided treatment, ¥3,841,580 (US$33,405), which results in a positive incremental cost of ¥293,211 (US$2,550). The effect in YOLSs of RS-guided treatment, 19.900 years, exceeds that of St Gallen-guided treatment, 19.679 years which results in a positive incremental effect of 0.221 year. The effect in QALYs of RS-guided treatment, 19.410 years, exceeds that of St Gallen-guided treatment, 19.173 years, which results in a positive incremental effect of 0.237 year. The cost and effects of RS-guided treatment scenario in this comparison are slightly different from those in the former comparison because of a difference in the risk reclassification from counterpart scenarios.
In both comparisons, the routine use of the 21-gene RT-PCR assay gains more but costs more at the same time. Incremental cost-effectiveness ratios (ICERs) of the former comparison are 3,465,713 ¥/YOLS (30,137 US$/YOLS) and 2,997,495 ¥/QALY (26,065 US$/QALY), and those of the latter comparison are 1,328,975 ¥/YOLS (11,556 US$/YOLS) and 1,239,055 ¥/QALY (10,774 US$/QALY).
Stability of ICER
Figure 2 shows the results of one way sensitivity analyses. Items are listed in the order of the magnitude of ICER change in terms of yen per QALY, while those change ICER less than 200,000 ¥/QALY (1,739 US$/QALY) are not reported.
Between NCCN-guided treatment vs. RS-guided treatment, ICER is most sensitive to the change of the cost of the 21-gene RT-PCR assay, which ranges from ¥672,402 (US$5,847) to ¥5,322,588 (US$46,283). It is also sensitive to the change of the utility weight for a health status after adjuvant therapy without distant recurrence, which ranges from ¥2,861,163 (US$24,880) to ¥5,725,775 (US$49,789). The changes of ICER by the change of all items fall in a range from ¥672,402 (US$5,847) to ¥5,725,775 (US$49,789). Among the values used in the outcome estimation, DRFS10 of patients who are reclassified as intermediate risk by RS criteria from low risk by NCCN criteria, has the largest impact on the result. Among costs of treatments, the cost of adjuvant chemotherapy is most influential to the result.
Between St Gallen-guided treatment and RS-guided treatment, ICER is most sensitive to the change of the assumption on the use of adjuvant chemotherapy among patients classified as intermediate risk by St Gallen criteria, which ranges from ¥788,230 (US$6,854) to ¥2,989,020 (US$25,991). It is also sensitive to the change of the cost of the 21-gene RT-PCR assay, which ranges from ¥290,593 (US$2,527) to ¥2,187,518 (US$19,022). The changes of ICER by the change of all items fall in a range from ¥290,593 (US$2,527) to ¥2,989,020 (US$25,991). Among values used in the outcome estimation, DRFS10 of patients who are reclassified as high risk by RS criteria from intermediate risk by St Gallen criteria, has the largest impact on the result. Among costs of treatments, the cost of adjuvant chemotherapy is most influential to the result.
Overall, the change of ICERs by the change of assumptions and values is limited from ¥290,593 (US$2,527) to ¥5,725,775 (US$49,789).
Budget impact
Table 5 shows the result of the budget impact estimation. Annual costs per case by the scenario are calculated from our economic model. RS-guided treatment accompanies high costs in the first year, which probably reflects that the high price of the 21-gene RT-PCR assay is not cancelled out by the reduction of adjuvant chemotherapy.
Costs treating LN−, ER+, ESBC incidence with NCCN/St Gallen/RS-guided treatment are calculated by the year taking mortality into account, and incremental costs are also calculated by the year according to comparisons. Calculated with these costs, the budget impact of the diffusion of the assay in Japan is estimated as ¥2,638 million (US$23 million) to ¥3,225 million (US$28 million).
Discussion
We evaluate the cost-effectiveness of the 21-gene RT-PCR assay in Japan’s health care system with two scenarios depicting status quo and one scenario of the routine use of the assay for LN−, ER+, ESBC. Our economic model indicates that the diffusion of the assay gains more in terms of outcome but costs more at the same time. The estimated ICERs, 2,997,495 ¥/QALY (26,065 US$/QALY) and 1,239,055 ¥/QALY (10,774 US$/QALY), comparing NCCN/St Gallen-guided treatment with RS-guided treatment, respectively, are not more than a suggested social willingness-to-pay for one life year gain from an innovative medical intervention in Japan, 6,000,000 ¥/QALY (52,174 US$/QALY) [36]. Sensitivity analyses show that this result is plausibly robust, since ICERs do not exceed the threshold by various changes of assumptions made or values employed. In this sense, the assay has good value for money.
Incremental effects in terms of QALY are longer than those in terms of YOLS; and ICERs in terms of yen per QALY are smaller than those in terms of yen per YOLS in both comparisons. These imply that the assay is not only efficient in prolonging survival but also improving quality of life.
Our sensitivity analyses also reveal that the price of the assay is one of the major determinants of cost-effectiveness as expected. An intuitive comparison with the price of a conventional gene diagnosis test of malignant tumour in Japan, ¥450,000 (US$3,913) vs. ¥20,000 (US$174), seems to make a health manager feel it difficult to reimburse the cost of the assay by the social insurance, because there may be an incompatibility to an incremental manner of revising fee schedule. Our study, however, implies that the price offered by Japanese supplier of Oncotype DX® Breast Cancer Assay still makes ICER an acceptable level from the viewpoint of welfare economics.
We estimate the budget impact of the assay on the social health insurance system. The policy implication of the budget impact is not prescriptive [37]. Yet, the estimated impact, ¥2,638 million (US$23 million) to ¥3,225 million (US$28 million) per year for the coming 5 years, is substantially less than the estimated budget impact of adjuvant trastuzumab, which is about to be included into social insurance benefit, ¥16,000 million (US$139 million) to ¥32,000 million (US$278 million) [38]. The characteristics of the assay of which application is limited to only once per case probably contribute to this difference, since the cost of trastuzumab amounts through its repeated administration. This implies that the diffusion of the assay through listing as an approved diagnostic test by the social health insurance could be justifiable.
The past economic evaluation of the assay reported from the U.S. considers a change from NCCN-guided treatment to RS-guided treatment [19], while our model allows a comparison between NCCN-guided treatment and St Gallen-guided treatment as an ex ante scenario. We find a notable difference in ICERs in this comparison. The ICER of the change from St Gallen-guided treatment is more favourable than that from NCCN-guided treatment. This is interesting because the reduction of use of adjuvant chemotherapy according to the reclassification from St Gallen criteria, 26%, is smaller than that from NCCN, 43%. The difference in ICER is due to more gain in the outcome. Although caution is needed in transferring the findings from economic models to any different context [39], our model might indicate that the assay has better value for money in countries where St Gallen-guided treatment is widely used.
However, this study has its own limitations. First, our outcome estimation depends on the validation studies carried out in the U.S. Although the evidences adopted are considered as the best available knowledge, it is needless to say that there are differences in population, as well as in cancer care practice between the U.S. and Japan. With this in regard, another validation study employing Japanese historical clinical trial data with the gene assay of preserved tumour tissue is launched [40]. A further economic evaluation incorporating new evidences is necessary to confirm the findings of this study. Second, utility weights adopted here are also derived from Western countries due to an unavailability of data from Japan. Third, our model does not include potentially costly clinical stages such as local recurrence or contralateral breast cancer due to the lack of data in validation studies. Regarding these shortcomings, reports and data that refines the model are awaited. Fourth, consensus guidelines are renewed continuously by incorporating newly available evidences [11, 41], so that the relative usefulness of the assay may be diminished in the near future, or the assay may be incorporated in the guidelines in a long run.
The use of the 21-gene RT-PCR assay has just begun to have an impact on clinical recommendations made by the U.S. oncologists and patients’ choice [42]. It is easy to imagine that similar change in practice will occur in Japan soon, because patients have strong preference to innovation such as tailor-made medicine [1]. As the prognostic usefulness of the 21-gene RT-PCR assay in guiding treatment for lymph-node-positive cases is recently reported [43], the indication of the assay will expand. Further economic evaluation that responds to this contextual change may become imperative.
Once the usefulness of the assay is confirmed by the Japanese validation study, Japanese health manager inevitably needs to decide how to fit the assay to the health care system. The results of this study imply the possibility of coverage by the social insurance. If health manager gives much importance to fiscal policy or cost containment, the selective indication of the assay for higher risk patients, which results to avoid additional use of adjuvant chemotherapy, might be a potential option. Further analysis incorporating such scenarios may be useful.
In conclusion, the routine use of the 21-gene RT-PCR assay for LN−, ER+, ESBC is indicated as cost-effective with a fundable level of budget impact in Japan. The results could inform health managers in developed countries where NCCN-guided treatment as well as St Gallen-guided treatment are practiced.
References
Kondo M, Toi M (2006) Cost-effective treatment options in first-line therapy for advanced breast cancer in Japan. Expert Rev Anticancer Ther 6(2):197–204
Miya T (2007) Current status and problems of tailor-made medicine in anticancer therapy. Gan To Kagaku Ryoho 34(4):515–519
Goldhirsch A, Wood WC, Gelber RD et al (2003) Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 21(17):3357–3365
Senn HJ, Thürlimann B, Goldhirsch A et al (2003) Comments on the St. Gallen consensus 2003 on the primary therapy of early breast cancer. Breast 12(6):569–582
Goldhirsch A, Glick JH, Gelber RD et al (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16(10):1569–1583
Goldhirsch A, Coates AS, Gelber RD et al (2006) First – select the target: better choice of adjuvant treatments for breast cancer patients. Ann Oncol 17(12):1772–1776
Early Breast Cancer Trialists’ Collaborative Group (2001) Tamoxifen for early breast cancer. Cochrane Database Syst Rev 2001(1):CD000486
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Carlson RW, McCormick B (2005) Update: NCCN breast cancer clinical practice guidelines. J Natl Compr Canc Netw 3(Suppl 1):S7–S11
Carlson RW, Brown E, Burstein HJ et al (2006) NCCN task force report: adjuvant therapy for breast cancer. J Natl Compr Canc Netw 4(Suppl 1):S1–S26
Carlson RW, Hudis CA, Pritchard KI et al (2006) Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw 4(10):971–979
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Lacal JC (2007) How molecular biology can improve clinical management: the MammaPrint experience. Clin Transl Oncol 9(4):203
Drummond MF, Sculpher MJ, Torrance GW et al (2005) Methods for the economic evaluation of health care, 3rd edn. Oxford University Press, Oxford
The Japanese Breast Cancer Society (2007) Zenkoku nyugan kanja touroku chousa houkoku – kakuteiban—dai 35 gou 2004 nenji shourei (National breast cancer patient registry survey report – final report—No. 35 2004 cases). The Japanese Breast Cancer Society, Tokyo
Ohno Y, Nakamura T, Murata K et al (2004) Nihon no ganrikan no shoraisuikei – Bayes gata Poisson cohort model niyoru kaiseki ni motozuku 2020 nen madeno yosoku – (Future estimate of cancer incidence in Japan – Bayesian Poisson cohort model estimate until 2020). In: Oshima A, Ishiguro A, Tajima K (eds) Gan toukei hakusho – rikan, shibo, yogo – 2004 (Cancer statistics white paper – incidence, mortality, prognosis – 2004). Shinohara Shuppan Shinsha, Tokyo
van Oostenbruggen MF, Jansen RB, Mur K, Kooijman H (2005) Penny and pound wise: pharmacoeconomics from a governmental perspective. Pharmacoeconomics 23(3):219–226
Hornberger J, Cosler LE, Lyman GH (2005) Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11(5):313–324. Erratum in: Am J Manag Care 11(8):476
Lyman GH, Cosler LE, Kuderer NM et al (2007) Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109(6):1011–1018
Paik S, Shak S, Tang G et al (2004) Risk classification of breast cancer patients by the recurrence score assay: comparison to guidelines based on patient age, tumor size, and tumor grade. In: Abstracts of 27th annual San Antonio breast cancer symposium, Texas, 8–11 December 2004
Iwata H, Saeki T (2006) Current practices in breast cancer treatment in Japan – a questionnaire survey. Jpn J Breast Cancer 21(3):311–322
Japan Society of Clinical Oncology (2005) Kouganzai tekiseishiyou no gaidorain: nyuugan (Guideline of appropriate use of anti cancer drugs: breast cancer). Int J Clin Oncol 10(Suppl.):15–55
Japanese Breast Cancer Society (2006) Kagakuteki konkyo ni motozuku nyuugan sinryo gaidorain: 1 yakubutsu ryouhou 2006 nenban (Evidence-based breast cancer care guideline: 1 drug treatments 2006 version). Kanehara Shuppan, Tokyo
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Smith I, Procter M, Gelber RD et al (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369(9555):29–36
Elkin EB, Weinstein MC, Winer EP et al (2004) HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 22(5):854–863
Ministry of Health, Labour, Welfare (2007) The 20th life tables. Health and Welfare Statistics Association, Tokyo
Hillner BE, Smith TJ (1991) Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. A decision-analysis model. N Engl J Med 324(3):160–168
Earle CC, Chapman RH, Baker CS et al (2000) Systematic overview of cost-utility assessments in oncology. J Clin Oncol 18(18):3302–3331
Cole BF, Gelber RD, Gelber S et al (2001) Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet 358(9278):277–286
Gold MR, Siegel JE, Russell LB et al (eds) Cost-effectiveness in health and medicine. Oxford University Press, New York
Iwata H, Nakamura S, Toi M et al (2005) Interim analysis of a phase II trial of cyclophosphamide, epirubicin and 5-fluorouracil (CEF) followed by docetaxel as preoperative chemotherapy for early stage breast carcinoma. Breast Cancer 12(2):99–103
Papaldo P, Ferretti G, Di Cosimo S et al (2006) Does granulocyte colony-stimulating factor worsen anemia in early breast cancer patients treated with epirubicin and cyclophosphamide? J Clin Oncol 24(19):3048–3055
Culyer AJ (2005) The dictionary of health economics. Edward Elgar, Cheltenham
Ohkusa Y (2003) Empirical research for the critical value of expenditure per QALY. Iryou to Shakai 13(3):121–130
Trueman P, Drummond M, Hutton J (2001) Developing guidance for budget impact analysis. Pharmacoeconomics 19(6):609–621
Shiroiwa T, Fukuda T, Shimozuma K et al (2007) The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat (in press). doi:10.1007/s10549-007-9679-4
Drummond M, Pang F (2001) Transferability of economic evaluation results. In: Drummond M, McGuire A (eds) Economic evaluation in health care: merging theory with practice. Oxford University Press, Oxford
Toi M (2007) Update on the confirmatory Japanese study utilizing Oncotype DX. In: Abstracts of the 15th annual meeting of the Japanese breast cancer society, Kanagawa, 29–30 June 2007
Goldhirsch A, Wood W, Gelber R et al (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18(7):1133–1144
Lo SS, Norton J, Mumby PB et al (2007) Prospective multicenter study of the impact of the 21-gene recurrence score (RS) assay on medical oncologist (MO) and patient (pt) adjuvant breast cancer (BC) treatment selection. J Clin Oncol 25(18S):577
Goldstein LJ, Gray R, Childs BH et al (2007) Prognostic utility of 21-gene assay in hormone receptor (HR) positive operable breast cancer and 0–3 positive axillary nodes treated with adjuvant chemohormonal therapy (CHT): an analysis of intergroup Trial E2197. J Clin Oncol 25(18S):526
Acknowledgements
This study is funded by Japan’s Ministry of Health, Labour and Welfare research grant, a study on the construction of algorithm of multimodality therapy with biomarkers for primary breast cancer by a formulation of decision making process, led by Masakazu Toi (H18-3JIGAN-IPPAN-007, H19-3JIGAN-IPPAN-007). Authors appreciate Dr Hiroji Iwata at Aichi Cancer Center for providing his survey data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, M., Hoshi, S.L., Ishiguro, H. et al. Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat 112, 175–187 (2008). https://doi.org/10.1007/s10549-007-9842-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9842-y