Abstract
Purpose: A negative selection method for the enumeration and characterization of circulating epithelial/cancer cells (CCC) in Breast Cancer (BC) patients is described. This manual procedure yields reproducible results of high sensitivity and selectivity suitable for research laboratories. Patients and methods: We conducted a prospective blood sampling study in 105 women with stage 1–4 BC attending clinics at the University of Maryland Greenebaum Cancer Center to define the prevalence of CCC utilizing our sensitive double gradient centrifugation and magnetic cell sorting CCC detection and enumeration method. CCC were isolated and enumerated from 15 to 20 ml of venous blood drawn before the start of systemic therapy and periodically thereafter for up to 24 months. One or more CCC/sample was considered a positive result. Results: We analyzed 487samples for the presence of CCC; the median number of samples/patient was 4 (range 1–8). CCC were detected in 56% of patients, 19%—stage 1; 43%—stage 2; 46%—stage 3; 83%—stage 4. The probability of being positive for the presence of CCC is significantly associated with the stage of cancer (P < 0.0001). The frequency of CCC positive patients and samples increased with the advancing stage of disease. Presence of more than 10 CCC/sample was associated with the decreased survival and increased probability of having metastatic disease P = 0.001. Conclusions: Increasing number of CCC/sample correlates with the adverse outcome and poorer survival (P < 0.0001). Our CCC test based on the negative selection procedure may provide valuable prognostic information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of Breast Cancer (BC) patients are diagnosed with early stage localized disease, but many eventually relapse despite seemingly adequate local and systemic therapies. Various prognostic factors are utilized to predict which early stage patients are at risk for relapse and therefore will require adjuvant systemic therapy to lower their risk of recurrence. Patients with metastatic disease present another management problem in that the majority of these patients respond to the initial first-line chemotherapy or endocrine therapy but subsequently develop progressive disease, require additional therapies and eventually develop resistant disease. Most patients with metastatic disease remain on some form of systemic anticancer therapy for the rest of their lives and it is therefore clinically relevant to know when the progression occurs and when to initiate new therapy. There is evidence that circulating epithelial/cancer cells (CCC) may serve as a surrogate marker for the disease status while receiving therapy. CCC could potentially herald disease progression or response to therapy in patients with advanced BC [1–3].
Several approaches have been reported in the literature for the enrichment, isolation and identification of circulating tumor/cancer cells [1–13]. In a “positive” selection system, the epithelial surface antigens provide the basis for selection of CCC with reagents, e.g., monoclonal antibodies directed against these antigens on circulating epithelial cells (CEC). This selection process is also augmented by the removal of normal blood cells from the blood sample and utilizing an imaging system to evaluate the circulating tumor cells (CTC) individually to increase the sensitivity and specificity of the system. The Cell Search System; a commercially available system [1–3] is an example of such an approach and in fact is a combination of both positive and “negative” cell selection approach. This system is semi-automatic since the final selection requires an assessment by an experienced operator.
In a “negative” selection approach, normal blood cells are removed through density gradient sedimentation and magnetic cell sorting [14, 15]. The CCC are individually selected, identified, and characterized from the pool of remaining blood cells by an experienced operator with the aid of positive control slides containing cancer cells from cultures. The negative selection approach can be readily adaptable by a medical research laboratory and is especially convenient for the characterization of the isolated CCC with computerized fluorescence microscopy. The negative selection process also offers an opportunity to establish cell cultures of the viable selected CCC. Wang et al. previously published the results of the study of CCC in prostate cancer patients in which the negative selection approach was utilized [14, 15].
Much has been reported about the CTC in cancer patients, particularly based on the Cell Search System of isolation [1–3, 5, 9]. The work reported here is based on an alternative “negative” selection approach for renumeration of CCC. Our investigation was centered on providing answers to the following questions. We sought from a clinical perspective first to define experimentally how to characterize “CCC-positive” samples in comparison to “CCC-negative” samples. Next, we wished to assess the frequency of CCC positive samples and number of CCC in relation to the stage of the disease, particularly in relation to the presence of metastatic BC. Lastly, we were interested in a preliminary assessment of whether the number of CCC in a particular patient’s sample could be related to the risk of dying from BC. From a technical and methodological point of view, the nature of CCC as identified by our detection and enumeration technology was compared to CEC and CTC as identified by other investigators using other methods.
Patients and methods
Study design
We initiated a blood sampling protocol at the University of Maryland Marlene and Stewart Greenebaum Cancer Center (UMGCC) in June of 2003. The objectives of the study were to identify the population of BC patients with detectable CCC by our negative selection approach. Consecutive subjects with histologically proven stage 1-4 BC signed an UM IRB approved consent. Participants were required to have no systemic therapy within the 4-week period prior to the first sampling and were starting new systemic therapy for BC at the time of initiation of blood sampling. We did not control for the type of systemic or local therapy patients received at the time of blood sampling, except that in all cases the first sample was drawn before the initiation of systemic therapy (chemotherapy or endocrine therapy). Follow up samples were drawn intermittently without fixed schedule during the ensuing 24 months, but always before subsequent chemotherapy treatments and for patients on hormonal therapy during the routine follow up clinic visits all patients were treated and followed according to the accepted standards of care. Although patients were sampled repeatedly to determine if presence and number of CCC correlate with the status of their disease no additional staging or testing was required per protocol. As this was a prospective observational study, the levels of CCC were not utilized to make treatment decisions; but changes in systemic therapies were made based on widely accepted standards for management of BC patients. We also planned to determine if certain biomarkers such as Her-2/neu and ER could be identified and quantified by immunofluorescent staining methods on individual cancer cells isolated from our patients’ venous blood. Those aspects will be described in a separate paper.
Blood collection and cell detection and enumeration
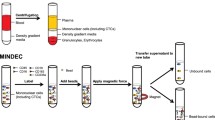
A total of 15–20 ml of venous blood/sample were drawn into Vacutainer tubes containing acid citrate dextrose and processed at room temperature within 24 h of the blood draw. Each sample was diluted with phosphate-buffered saline. Tubes were capped and mixed gently by inversion 3–6 times. Ten milliliter of a 1.068 g/ml density gradient were slowly added to the bottom of the sample tube followed by another 10 ml of a 1.083 g/ml gradient beneath the 1.068 g/ml density gradient. The tube was centrifuged at 400 × g for 30 min at 20°C. The upper gradient interface (1.068 g/ml) and the lower gradient interface (1.083 g/ml) were collected separately along with the gradient. The two collections were washed and centrifuged. The supernatant in each tube was then gently removed by careful aspiration and cell pellets were collected.
Magnetic cell sorting and slide preparation
To remove excess leukocytes and thus enrich the target cancer epithelial cells in the blood, a proprietary enrichment system using CD-45 mouse-antihuman immunoglobulin G magnetic beads was employed. The cell pellet from the 1.083 g/ml gradient collection was resuspended and placed on ice for the magnetic cell sorting. Two hundred microliters of magnetic Dynal beads were added to a 5-ml polypropylene tube. The beads were washed in 3.0 ml of 0.1% BSA to remove sodium azide. The cell suspension from the 1.083 g/ml gradient (about 1.0 ml) was transferred to the tube containing the washed beads. Remaining cells were washed from the wall of the original centrifuge tube with another ml of 0.1% BSA and transferred to the tube containing the magnetic beads for a total volume of 2.0 ml. The cells were incubated with the beads at 4–8°C for 30 min at 10 rpm on an Orbitron Rotator. The tube was then placed in a magnetic particle concentrator for 2 min to allow for maximum leukocyte depletion. The 1.068 g/ml cell suspension was transferred and kept on ice during incubation of the 1.083 g/ml suspension with anti-CD45 magnetic beads. After leukocyte depletion, the supernatants from the CD45 negative collection of the 1.083 g/ml cell suspension and the 1.068 g/ml cell suspension were combined and washed with 40 ml 1 × PBS using a centrifugation step at 250 × g for 10 min. The cell pellet was resuspended in 300 μl of 0.1% BSA and then loaded evenly onto a Pap-pen outlined area (20 × 20 mm) on a slide. Slides were air dried for at least 3 h or placed on Slide Warmer (Fisher) set at 41°C for 1 h before staining.
Immunofluorescence staining
Circulating epithelial cells were not fixed at any time during the isolation procedure after which they were dried on microscope slides and fixed in 2% paraformaldehyde for 10 min at 4–8 °C and then rinsed two times with 1 × PBS. Slides were placed in PBS for 10 min at room temperature and blotted dry. Cells were then incubated with a fluorescently labeled pananticytokeratin mAb cocktail (500 ng until after 0.1% BSA PBS) CAM 5.2 (Becton Dickinson), 200 ng of Multi CK mAb and 100 ng of CK-19 mAb (NeoMarkers) mixed in 100 μm of 0.1% BSA/PBS at 4 °C for at least 1 h and then washed twice in PBS at room temperature for 10 min. The slides were blotted dry and mounted with DAPI-containing medium under a coverslip (Fig. 1).
Positive controls
The MCF-7, T47D, HCC2218, and SK-BR-3 BC cell lines were used for validation of tests for tumor cell recovery and immunofluorescent staining. Approximately 100 cancer cells were spiked into 15 ml of blood from healthy controls. To accurately estimate the number of cells spiked into the blood, two control slides were prepared from the same volume of the cell suspension and cells were verified by cytokeratin-positive staining and counted. The average number of the cells from these control slides was used to calculate the cell recovery. The spiked blood was subjected to the same complete detection and enumeration and staining procedure as described above for patient samples.
Negative controls
Blood samples of healthy blood donors from BRT Laboratories Inc. (Baltimore, MD) and other healthy volunteers served as negative-control samples for our studies. A total of 10–20 ml of the whole blood from each of the donors was subjected to the complete detection and enumeration and staining procedure as described above. From1998 to 2005 blood samples (N = 207) from 92 healthy blood donors aged 21–75 (46 females, 46 males) tested negative for presence of CCC. Sixty-two donors were tested 2–3 times within a 3-year time period.
Fluorescence microscopy
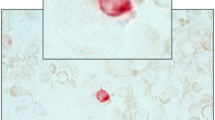
Stained cells were examined on a Leica DM RXA microscope (Leica Microsystems, Exton, PA, USA) equipped with a MicroMax Digital CCD Camera System, model 1300YHS, utilizing filter cubes which allow for differentiation of up to the two fluorescence signals. Excitation, dichroic, and emission filters in each cube were 360 nm/400 nm/470 nm for DAPI and 470 nm/497 nm/522 nm for FITC. Digital images of stained cells were acquired with a 40 × objective using Image-Pro Plus software ( Fig. 2).
Fluorescence microscope images of the cells obtained from a blood sample of a BC patient that was processed by negative selection. Images of the same field of cells were acquired with filter cubes that can discriminate FITC and DAPI fluorescence. Left, image of an epithelial CCC stained with anti-pan cytokeratin-FITC. Right, image of the nuclear DNA in the CCC and leucocytes stained with DAPI
Cell recovery and assay specificity
For purpose of quality control, one control sample was carried out each time a patient sample was processed; on average we used one control/three patient samples (range 1–7 patient samples) to ensure the recovery in the enrichment process and the proper staining procedure. A total of 188 positive control samples were carried out for 487 patient samples from 105 patients. The tumor cells targeted for recovery by the assay stain positive for cytokeratin peptides and were considered CD45-negative by the virtue of the fact that they were not removed from the blood sample when incubated with anti-CD45 magnetic beads during the leukocyte depletion step. The mean CCC/sample recovery rate was 65.4% (SD 10.3%) and is consistent with our previous results and similar to other enrichment and detection procedures [8]. A total of 188 control samples were run in conjunction with the patient blood samples during this study and the recovery rate never fell below 53%. Assuming that the cell recovery in patients’ blood samples is similar to the positive control data; the absence of a CCC in a sample means that the number of CCC has to be one or less.
To evaluate the specificity of the cell recovery and the staining procedures, a total of 207 blood samples from 92 healthy individuals were examined as negative controls. These negative control tests were conducted either side by side with the patient’s blood sample or with the positive control tests. None of these negative control samples was found to contain cytokeratin-positive cells. The negative control data indicate that these samples from the healthy blood donors must contain zero or no more than one positive cell. These results lead to the conclusion that samples containing one cell or more would be considered CCC positive and samples containing zero positive cells would be considered CCC negative.
Statistical methodology
Summary statistics including median and range are provided for continuous factors while frequency tables are presented for categorical factors. Exact tests (Fisher’s or χ2) are used to analyze presence or absence of metastases in different patient groups. The Joncheere-Terpastra test is used to compare groups with ordinal categorization. Wilcoxon rank sum test is used for group comparisons with continuous outcomes such as the amount of CCC. Logistic regression was used to assess the association of the presence of CCC with stage of the BC and other risk factors. The forward selection was applied to identify risk factors that differentiate patients with or without the presence of CCC. The Cox regression model was used to assess whether potential risk factors are associated with patient survival. Kaplan–Meier’s survival curves are used to describe the survival distributions on patients in specific group (Fig. 3).
Patient characteristics
One hundred and five women with stage 1–4 histologically proven BC participated in our clinical trial. During the 24 months sampling period, three stage three patients and one stage two patient developed metastatic disease. The tumor characteristics such as histological type, size, number of positive lymph nodes, grade, ER, PGR, Her2/neu were obtained from the historical pathology reports, usually from the initial surgery (lumpectomy or mastectomy). Patient characteristics are shown in Table 1.
Results
CCC in stage 1–4 Breast Cancer patients’ blood samples
We considered presence of one or more CCC per blood sample as a CCC positive sample. During the 24-month monitoring period 487 venous blood samples were drawn from 105 BC patients and tested for presence of CCC. Patients were sampled repeatedly; but resampling was not directed by the status of their disease such as progression. Eleven patients (9 stage 4, 1 stage 1, and 1 stage 3) were sampled only once. Median number of samples per patient was 4 (range 1–8). During the 24-month sampling period CCC were detected in 59 of 105 patients; 35 stage 4, 12 stage 3, 9 stage 2, and 3 stage 1. The frequency of CCC positive samples increased with the increasing stage of the disease and was 4% for stage 1, 9% for stage 2, 20% for stage 3, and 56% for stage 4 patients, respectively. In the first sample drawn, CCC were detected in 32 out of 105 patients; 1 out of 16 stage 1, 6 out of 47 stage 2, and 3 and 25 out of 42 stage 4 patients (Table 2). The maximum number of CCC/sample was, 2 for stage 1, 37 for stage 2, 19 for stage 3, and 1283 for stage 4 patients.
The median follow-up time for the 105 patients was 1.74 years (range 1.52–1.94). All patients with stage 1 disease were alive and free of metastatic disease at the time of the final analysis, while one stage 2 and three stage 3 patients developed metastatic disease. The median overall survival with the corresponding 95% CI has not been reached yet for stage 3 patients. For the stage 4 patients, the median overall survival was 1.41 years with the corresponding 95% CI (0.73–2.05 years).
We studied the possible impact of the distribution of CCC positive blood samples in our patients. Patients were categorized according to CCC status, i.e., those who have become CCC positive at some point while on study, and those who have not. Patients who tested positive for CCC anytime during the sampling period were more likely to have metastatic disease. There is a strong association between the stage of cancer and the probability of being positive or negative for the presence of CCC in samples (P < 0.0001 Joncheere-Terpstra test).
Presence of CCC in blood samples therefore was associated with the stage of disease. Only 19% stage 1 patients tested positive for CCC during the sampling period while, 43% stage 2 patients, 46% stage 3 patients, and 83% of stage 4 patients tested positive for CCC at least once during the sampling period (Table 2).
We used logistic analysis to model the probability of detecting CCC in BC patients. Logistic regression model was fit to assess the probability of detecting CCC in blood samples for stage 1–3 patients and neither the tumor size, nor the number of axillary lymph nodes involved with tumor had any significant effect on the presence of CCC in blood samples with P-values; 0.32 and 0.57, respectively. In addition, the forward selection was applied to identify the effects that differentiate these two patients’ categories, and the following plausible risk factors were examined in the forward selection model: ER, PR, HER2/neu, metastatic status, and tumor grade. The resulting model has two variables, metastatic status, and tumor grade that are statistically significantly associated with the presence or absence of CCC, P-values <0.0001 and 0.02. It is evident that lower tumor grade and absence of metastatic disease dramatically lower the probability of developing CCC. For stage 4 patients ER, PR, Her2/neu, tumor grade, number of prior chemotherapies, and hormonal therapies were not correlated with the presence of positive or negative CCC samples.
Several factors were examined for their association with survival. Cox regression model was used to assess whether the following factors are predictive of the hazard of death: median number of CCC/sample, ER, PR, Her2/neu status, and the site of metastatic disease. An estimated relative risk of dying of BC is associated with the 6.2-fold increase in patients with visceral disease (Cox regression, P-value = 0.017); also the estimated risk of death in patients who are HER2/neu positive (N = 29) is 4.1 times that of patients who are HER2/neu negative (N = 68) (P-value = 0.020), holding other factors constant.
The median number of CCC/sample was compared between several categories of stage 4 patients with disease dominant sites in the following locations (bone only, liver, lung, soft tissue, and CNS). Patients with liver dominant disease tend to have larger number of CCC/sample than other patients with stage 4 BC (P-values; P = 0.008 and 0.016, respectively, Table 3).
Patients were divided into four groups based on the numbers of CCC/sample anytime during the 24 months sampling period; Group A = 0 CCC/sample (N = 46), Group B 1-10 CCC/sample (N = 40), Group C 11-100 CCC/sample (N = 13), Group D > 100 CCC/sample (N = 6) (Table 4). BC patients with more than 100 CCC/sample at some point during the sampling period were at the highest risk of dying from metastatic disease. The probability of having metastatic disease and mortality due to BC were associated with the presence and the level of CCC/sample (Exact χ2-test for presence or absence of metastases in A, B, C, D groups, P = 0.001; Fisher’s exact tests for B vs. C + D, P = 0.001; B vs. C, P = 0.02; A vs. B, P = 0.04; A + B vs. B + D, P < 0.0001). Overall survival correlates with the number of CCC (P-value < 0.0001) as shown in Table 4 and Fig. 3. In addition, although the number of CCC positive samples is smaller we performed similar analysis looking at the survival of our patients based on the number of CCC in the first sample drawn. The numbers of patients in groups A (0), B (0–10), C (11–100), and D (>100) according to their CCC status in the first blood sample are as follows: 73, 23, 4, and 5. The log-rank test, and the Wilcoxon test results showed that these patients also have dramatically different survival experience, both P-values < 0.0001.
Discussion
Several publications have appeared previously concerning the significance of the circulating tumor cells or CTC in BC patients [1–3, 5–9, 16–20]. The uniqueness of our study in part rests on our approach and methodology. Our study was based on the negative circulating epithelial cell selection approach. After removal of 99% or more of the RBC and WBC from the blood samples, the remaining cells were scanned visually to recognize the DNA stain and the pancytokeratin stain, as well as the morphological intactness of the epithelial/cancer cells [14, 15]. This visual selection step is essential to assure the reliability of the assay. In addition, extensive positive controls (188 samples) and negative controls (207 samples from 92 healthy blood donors) were done. The positive controls accompanying all tests of patients’ samples assured that the recovery rate for each CCC test was at least 1 CCC/sample, and that the staining procedure was conducted properly each time the CCC test was performed. Another distinction of our study is the long duration of the sampling period (up to 24 months); with a large number of samples/patient, median of 4 (range 1–8); although the number of patients in our study (105 patients) is comparable to the other reports in the literature. From Table 2, we were able to conclude that the probability of the being CCC positive versus CCC negative is strongly associated with the stage of BC (Jancheeve-Terpstra Test, P < 0.000l). In addition, for stage 1–3 patients, neither the tumor size nor the number of positive axillary lymph nodes have a significant correlation with the CCC presence in the blood samples in the logistic regression model (P-values are 0.32 and 0.57, respectively). When ER, PR, HER2/neu, metastatic status, and tumor grade were selected in the logistic regression model, only metastatic status and tumor grade were statistically significant predictors for having CCC positive samples (P-values are <0.0001 and 0.02, respectively). The odds ratio estimates for the probability of having CCC positive samples are strongly associated with the presence of metastatic disease and the tumor grade.
Interestingly when using the “negative” selection methodology, the number of CCC/sample correlates with patients categorized as being afflicted with disease in any visceral sites (liver, lung, and CNS), but this conclusion is clearly driven by the presence of liver metastasis, as the strength of the correlation is larger in patients with the liver as site of metastasis than with the number of CCC in patients with metastasis in other areas (Wilcoxon 2-sided test P-value, 0.008 (overall visceral) and 0.016 (liver), respectively), with at best a positive trend (P = 0.088) for the correlation of CCC with the presence of bone only metastases. While larger numbers of patients would be needed to establish this trend with greater certainty, others have not reported this difference [1, 2, 16, 21].
When our patients were separated into four groups based on the number of CCC/sample and this was correlated with the risk of dying due to BC (Table 4) we saw an impressive association between the overall survival and the rising number of CCC/sample (P-value < 0.0001). This is graphically shown in the Kaplan–Meier Curve of survival function (Fig. 3) indicating the negative influence of rising CCC numbers on survival.
A comparison between our study and the studies from three other groups is shown in Table 5. In summary the major conclusions regarding the association of the CCC numbers in blood samples of patients with metastatic BC with their prognosis are shared with the other studies that were based on different methodology of detection and enumeration/enrichment of circulating cancer/tumor/epithelial cells. Thus, the clinical association of the higher number of CCC/sample with poorer prognosis in metastatic BC continues to be supported by the data in this study. From our study, we cannot make any definitive conclusions regarding the significance of the presence of CCC in blood samples of patients with stage 1–3 BC as only 1 stage 2 and 3 stage 3 patients progressed. Previously others reported on the significance of the CTC in patients with metastatic BC based on the Cell Search method and showed that there is a significant relationship between the progression-free and overall survival and the CTC numbers of <5 cells/7.5 ml sample versus ≥5 cells [1–3, 9]. The “negative” selection methodology may have the potential to dissect more finely the prognoses of patients with zero and different “levels” of positivity up to 100 CCC/sample. Larger numbers of samples with low numbers of CCC would be necessary to more thoroughly explore this question.
Looking to the immediate future, we have two urgent and fundamental questions arising from these initial investigations. First, what is the nature of these circulating cells in our BC patients? We have identified them as epithelial cells by virtue of pancytokeratin markers. In a previously reported study of CCC in prostate cancer patients we have identified them additionally by abnormal chromosome numbers (chromosome 7, 8, and 18 based on centromere counting), and increased DNA content compared to normal leucocytes, and their abnormal morphology compared to leucocytes [14, 15]. Fehm et al. [22, 23] analyzed CEC in breast, kidney, prostate and colon cancer patients and concluded that the vast majority of CEC are aneuploid and derived from the primary tumor.
In our study, we show statistically significant association of the presence of the CCC with the presence of metastases, and the risk of dying due to BC. Thus, it is consistent that the CCC identified by our methodology are cancer cells, although we did not formally evaluate their cytologic features. Whether these cells are clonogenic, able to propagate, divide and metastasize is unknown but is of considerable interest to establish. Additional cancer cell biomarkers would be very valuable for further clarification of this issue.
The results of our investigation as well as other published studies show no clear consensus about the basic mechanism(s) that affect the presence and number of CCC/CTC in blood samples of BC patients. For patients with metastatic BC the increasing or decreasing CCC number while on chemotherapy is an independent predictor of progression-free and overall survival and continues to be a research direction of interest to several groups. The use of CCC/CTC numbers to predict therapeutic outcomes in early stage BC patients can only be established by a strong association with clinical outcomes such disease free and overall survival and such an association requires prospective clinical studies with large numbers of patients. Nonetheless, the “negative” selection methodology described here can be an important opportunity for exploratory studies not only of clinical outcomes, but also of pharmacodynamic investigations with novel and standard therapies. In addition our negative CCC selection and enumeration procedure described here can be readily adapted without the need for expensive instrumentation by most research laboratories.
References
Cristofanilli M et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Cristofanilli M et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7):1420–1430
Cristofanilli M (2006) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 33(3 Suppl 9):S9–S14
Alvero AB et al (2004) Improved method for the detection of cytokeratin 19-positive cells in the peripheral blood of breast cancer patients. Lab Invest. 84(5):658–661
Allard WJ et al (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904
Braun S, Marth C (2004) Circulating tumor cells in metastatic breast cancer—toward individualized treatment? N Engl J Med 351(8):824–826
Braun S, Naume B (2005) Circulating and disseminated tumor cells. J Clin Oncol 23(8):1623–1626
Budd GT et al (2006) Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409
Hayes DF et al (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224
Witzig TE et al (2002) Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 8(5):1085–1091
Webster DR, Sabbadini E (1967) The prognostic significance of circulating tumor cells: a five-year follow-up study of patients with cancer of the breast. Can Med Assoc J 96(3):129–131
Sabbatini R et al (2000) Detection of circulating tumor cells by reverse transcriptase polymerase chain reaction of maspin in patients with breast cancer undergoing conventional-dose chemotherapy. J Clin Oncol 18(9):1914–1920
Ring AE et al (2005) Detection of circulating epithelial cells in the blood of patients with breast cancer: comparison of three techniques. Br J Cancer 92(5):906–912
Ts’o PO et al (1997) Detection of intact prostate cancer cells in the blood of men with prostate cancer. Urology 49(6):881–885
Wang ZP et al (2000) Identification and characterization of circulating prostate carcinoma cells. Cancer 88(12):2787–2795
Muller V et al (2005) Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 11(10):3678–3685
Muller V, Pantel K (2004) Bone marrow micrometastases and circulating tumor cells: current aspects and future perspectives. Breast Cancer Res 6(6):258–261
Muller V, Pantel K (2005) BM micrometastases and circulating tumor cells in breast cancer patients: where have we been, where are we now and where does the future lie? Cytotherapy 7(6):478–482
Tang C, Lin AY (2004) Circulating epithelial cells in breast cancer. N Engl J Med 351(23):2452–2454; author reply 2452–2454
Taback B et al (2001) Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: correlation with clinical stage of disease. Cancer Res 61(24):8845–8850
Gaforio JJ et al (2003) Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer 107(6):984–990
Fehm T et al (2002) Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 8(7):2073–2084
Fehm T et al (2005) Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy 7(2):171–185
Acknowledgement
The work done at CCC Diagnostics LLC is supported by NCI Grant #CA081903.
Author information
Authors and Affiliations
Corresponding author
Additional information
Partly presented at the 41st and 42nd Annual Meeting of the American Society of Clinical Oncology, May 2005, Orlando, FL, USA and June, 2006, Atlanta, GA, USA.
Rights and permissions
About this article
Cite this article
Tkaczuk, K.H.R., Goloubeva, O., Tait, N.S. et al. The significance of circulating epithelial cells in Breast Cancer patients by a novel negative selection method. Breast Cancer Res Treat 111, 355–364 (2008). https://doi.org/10.1007/s10549-007-9771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9771-9