Abstract
A novel highly metastatic MDA-MB-231HM cells, derived from MDA-MB-231, was established in our institute. RT-PCR, real-time PCR and Western blot showed that AF1Q gene was differentially expressed between highly metastatic MDA-MB-231HM cells and its parental MDA-MB-231 cells. However, its molecular mechanisms in breast cancer metastasis remain to be characterized. To investigate the effects of AF1Q on the progression of human breast cancer cells, in the present study, recombinant expression plasmid vectors of the human AF1Q gene was transfected into MDA-MB-231 cells. We demonstrated that AF1Q overexpression enhanced the in vitro proliferation and invasive potential of breast cancer cells. Focused microarray analyses showed that 22 genes were differentially expressed between AF1Q transfected cells and its parental counterparts. Integrin α3, accompanied by up-regulation of Ets-1 and MMP-2, significantly enhanced the in vitro invasive potential of human breast cancer cells mediated by AF1Q. Estrogen-responsive ring finger protein gene (EFP), also played a role in the enhancement of in vitro proliferation of human breast cancer cells mediated by AF1Q, accompanied by down-regulation of 14-3-3δ. The association was ERα independent. These results were further demonstrated by RNA interference (RNAi) experiment in vitro. In in vivo study, we also demonstrated that AF1Q transfected breast cancer cells grew much faster and had more pulmonary metastases than vector-transfected or its parental counterparts. On the contrary, AF1Q knockdown cells grew slower and had less pulmonary metastasis. Similar effects of AF1Q on integrin α3, Ets-1, MMP-2, EFP, and 14-3-3δ expression observed in vitro studies were also found in the in vivo study. Taken together, these results provide functional evidences that overexpression of AF1Q leads to a more progression in human breast cancer, at least in part, through regulating the integrin α3, Ets-1, MMP-2, EFP, and 14-3-3δ expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy affecting women and the second leading cause of cancer death in US and some big cities in China, surpassed only by lung cancer [1]. Metastasis is a major challenge in cancer management and it is critical to determine molecular markers that definitively distinguish tumors of nonmetastatic potential from those with metastatic potential. To identify such markers, a clearer understanding of the metastatic progression in breast cancer is required. It is now widely accepted that the progression of a non-neoplastic cell to a hyperplastic cell and eventually to one that is capable of metastasis requires the stepwise accumulation of many genetic alterations [2].
However, the molecular mechanisms contributing to breast cancer progression are poorly understood. In our previously study, MDA-MB-435HM, a highly pulmonary metastatic variant of parental MDA-MB-435 cells, was derived within the presented model system by six cycles of the pulmonary metastasis implantation to the mammary fat pad (MFP) [3]. Recently another novel highly metastatic MDA-MB-231HM cells, derived from MDA-MB-231, was also established and maintained using the same methods. The differences in pulmonary metastatic potential of the genetically related cell lines in the two models make them valuable systems for understanding the molecular mechanisms underlying breast cancer metastasis. With the application of a cDNA microarray of 8000 genes, we identified 60 candidate genes, that were differentially expressed between highly metastatic MDA-MB-435HM cells and its parental counterparts, and the ALL1-fused gene from chromosome 1q (AF1Q) was one of the leading genes [4].
The human AF1q gene is located on chromosome band 1q21 and codes for a 9-kD protein with no similarity to any other known proteins. So far, the function of AF1Q gene remained unclear. Previous studies have suggested that the overexpression of AF1Q gene might play roles in leukaemia, myelodysplastic syndrome and thyroid tumor [5–8]. Our institute for the first time found that AF1Q might be a mediator of metastasis promotion in MDA-MB-435 cells [4]. But there are some doubts whether this gene is involved in the metastasis of other breast cancer cell metastasis and its functional roles in this complex and multistep process.
Materials and methods
Cell lines and animals
Human MDA-MB-231 breast cancer cell line was bought from ATCC. The highly metastatic MDA-MB-231HM cell line was established by our institute. MDA-MB-231 cells were routinely maintained in Leibovitz’s L-15 medium with 2 mM L-glutamine at 37°C in a humidified atmosphere containing 5% CO2. The medium was supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The medium was changes every 2–3 days, and cells were subcultured by treatment with 0.25% trypsin/0.53 mM EDTA solution. Female athymic BALB/c-nu/nu mice, 4–6 wk old, were obtained from Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Shanghai, China), and housed in laminar-flow cabinets under specific pathogen-free conditions with food and water ad libitum. All experiments on mice were conducted in accordance with the guidelines of National Institutes of Health (NIH) for the Care and Use of Laboratory Animals. The study protocol was also approved by Shanghai Medical Experimental Animal Care Committee.
Plasmids
The recombinant human AF1Q expression vector pcDNA3.1-AF1Q was constructed and stored in TE buffer (10 mM Tris, pH8.0/1 mM EDTA) at −30°C by our institute.
RT-PCR and Western blotting analysis
Total RNA was extracted from cultured cells using TRIzol® Reagent (Invitrogen, San Diego, CA), and then quantitated by spectrophotometry at 260 nm. RT-PCR was performed according to the manufacturer’s instructions. (MBI Fermentas, Vilnius, Lithuania) Gene-specific primers for human AF1Q gene (forward: 5′-ATGAGGGACCCTGTGAGTA-3′, reverse: 5′-TTAGAGCAAGTCCATTCGA-3′; 273 bps) were synthesized at Shanghai Sangon Biological Engineering & Technology services Co. (Shanghai, China). The cycling conditions were 94°C for 5 min, followed by 28 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 30 s and a final extension of 72°C for 7 min. Negative controls were run in parallel to exclude genomic DNA contamination. The templates for negative controls of RT-PCR were not reverse-transcribed. PCR products were separated on a 1.2% agarose gel and imaged on an Alpha Image 950 documentation system (Alpha Innotech, San Leandro, CA). Densitometry of images was performed using NIH Image version 1.62. All experiments were repeated at least three times, and GAPDH (forward: 5′-GGGAGCCAAAAGGGTCATCATCTC-3′, reverse: 5′-CCATGCCAGTGAGCTTCCCGTTC-3′; 353 bp) was chosed as an internal control.
To quantitatively determine the expression level of the AF1Q, the cDNA templates of the cells were amplified by real-time quantitative PCR. The reaction was performed using the DNA Engine Opticon™ 2 real-time PCR detection system (MJ Research, USA) with SYBR Green I. GAPDH was chosed as internal control. The Opticon 2 apparatus was used to measure the fluorescence of each sample in every cycle at the end of the extension, and the comparative threshold cycle (2−ΔΔcT) method was used to allow the quantification of the mRNA of these genes. After PCR, a melting curve was obtained by increasing the temperature from 65 to 95°C with a temperature transition rate of 0.1°C /s. The melting curves of all final PCR products were analyzed. The differences in melting temperature of PCR products were allowed to distinguish genuine products from nonspecific products and primer dimers. To ensure that the correct product was amplified in the reaction, all samples were also separated on 1.2% agarose gel electrophoresis. All PCR conditions and primers were optimized to produce a single product of the correct size.
Western blotting analysis was performed according to the published method with some modifications [9]. Briefly, Equal amounts of protein (50 μg) from different cells were separated by 10% SDS-PAGE and then incubated with mouse anti-human monoclonal antibodies against AF1Q (Abnova Corporation). Target proteins were detected by enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech) and exposure to Biomax ML film (Eastman Kodak, Rochester, NY). Images were captured by Alpha Image 950 documentation system and analyzed by NIH Image version 1.62. Relative protein in different cell lines was normalized to the signal intensity of β-actin as an internal control.
Stable transfections of AF1Q
Exponentially growing MDA-MB-231 cells were transfected with pcDNA3.1-AF1Q using Lipofectamine™ 2000 Transfection Reagent (Invitrogen, San Diego, CA) according to the manufacturer’s instructions. The stably transfected colonies were isolated and identified by RT-PCR and Western blotting analysis. In this study, two clones were chosed and named of MDA-MB-231/AF1Q-1 and MDA-MB-231/AF1Q-2. The cells only transfected with pcDNA3.1 vector was named of MDA-MB-231/vector. The transfectants were used before passage 20 in all cases to minimize the impacts of clonal diversification and phenotypic instability. For all functional and biological assays, cells between 70 and 90% confluence were used with viability >95%.
Immunofluorescence staining assays
Immunofluorescence staining assays were performed to analyze the distribution and expression of AF1Q in parental and AF1Q transfectants cells. Cells were cultivated on glass coverslips and incubated with the primary antibodies (mouse anti-human monoclonal antibodies against AF1Q). After washed with 1% BSA-TBST, the cells were incubated with a fluorescein-labeled goat anti-mouse IgG (American Qualex, San Clemente, CA) and stained with 0.5 μg/ml DAPI in TBS for 1 min. Then the coverslips were sealed onto a glass slide using aqueous mounting medium and checked under microscope.
Colony formation assay
The colony formation assay was performed to measure growth promotion by AF1Q gene transfection on cells according to the previously reported protocol [10] with some modifications. Identical numbers of parental cells, empty vector, and AF1Q transfectants cells were seeded in 6-well tissue culture plates to form colonies. After 10 days incubation the number of colonies (≥20 cells) was counted within a field at ×200 under a light microscope. For each test, a total of five fields were selected at random, and the numbers were averaged. The assay was repeated at least three independent experiments with 0.5 × 104, 1 × 104, and 2 × 104 of the cells seeded, respectively.
Cell proliferation assay
Cell proliferation assays were performed to analyze the proliferation potential of parental, empty vector, and AF1Q transfectants cells by using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). The cells were harvested and plated in 96-well plates at 1 × 103 per well in 100 μl cell culture medium and maintained at 37°C in a humidified incubator containing 5% CO2. At the indicated time points, 10 μl of the CCK-8 solution was added into the triplicate wells and incubated for 1.5 h, then absorbance at 450 nm was measured to calculate the numbers of vital cells in each well.
In vitro invasion assays
In vitro invasion assays were performed to analyze the invasive potential of parental MDA-MB-231, MDA-MB-231/vector, and AF1Q transfectants with a Matrigel invasion chamber (Becton Dickinson Labware, Bedford, MA) as described previously with some modifications [11]. Each well insert was coated with 100 μl of a 1:3 dilution of Matrigel in serum-free culture medium. Then, a mixture of 200 μl L15 medium with 10% FBS, 200 μl supernatant of corresponding cell culture, and 200 μl supernatant of NIH/3T3 cell culture was added to the lower chambers as a chemoattractant, and 1 × 105 cells in 250 μl of serum-free L15 medium were added to the top of this Matrigel layer. The cells were incubated at 37°C for 24 h. The cell suspension was aspirated, and excess Matrigel was removed from the filter using a cotton swab. Invasion was assessed by counting the cells that had traveled across the filter and were attached to the bottom side of the filter. Then, the filters were fixed in 10% formalin and stained with H&E. Cells which had invaded through the Matrigel and reached the lower surface of the filter were counted under a light microscope at a magnification of ×200. Five fields should be counted for each sample.
Focused microarray analysis
For the analysis of the differential expression of multiple genes involved in human metastasis, we used the GEArray Q Series Human Tumor Metastasis Gene Array and Human Cancer Signal Transduction Pathway Finder (SuperArray Bioscience, Frederick, MD). Each GEArray membrane contained 96 specific cDNAs known to be involved in human tumor growth, signal transduction and metastasis. 14 cDNAs is duplicated between two arrays. For every cell line, 5 μg total RNA was extracted and resuspended in RNAse-free water. Biotinylated complementary DNA (cDNA) probes were then generated from RNA isolates using linear polymerase chain amplification. The Nonrad GEArray Ampolabeling kit (SuperArray Inc) was used to generate biotin-labeled probes. The cDNA probes generated by linear amplification of the messenger RNA (mRNA) were denatured, and hybridization was performed according to the manufacturer’s protocol (SuperArray Inc.). The relative abundance of a particular transcript was estimated by comparing its signal intensity to the signal derived from the housekeeping genes. Regulated genes were identified by ratio more than 3.0 or less than 0.33, which indicates at least three-fold up-regulation or 67% reduction.
Real-time PCR analysis
To confirm the above microarray results, the six genes (EFP, IL-2, P53, integrin α3, LAMB2 and TRAF1) identified by microarray analysis were randomly chosen for further validation by real-time quantitative PCR as described previously. The primers were designed using Primer 3 software (Table 1).
RNA interference (RNAi) experiments
To further demonstrate the role for the AF1Q gene in the progression of human breast cancer, we utilized the RNA interference (RNAi) technique to down-regulate the AF1Q gene expression using the BLOCK-iTTM Pol II miR RNAi Expression Vector Kits (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions.
Plasmid construction: The engineered pre-miRNA sequence structure is based on the murine miR-155. The pcDNATM6.2-GW/EmGFP-miR plasmid with spectinomycin resistance gene was used for the cloning of small synthetic oligonucleotides. Three different miR155-based AF1Q (GenBank Accession No: NM_006818) targeting sequences were designed by using Invitrogen’s RNAi design algorithm on line. Basic local alignment search tool (BLAST) was used on all miRNA sequences to avoid off-target gene silencing. These sequences are shown in Table 2. Based on the computer analysis, these inserted oligonucleotides would specifically bind to homologous sites of AF1Q mRNA, and thus might interfere with AF1Q expression in cultured cells. In order to identify successful construction of recombinant plasmids, the EmGFP forward sequencing primer (5′-GGCATGGACGAGCTGTACAA-3′) and miRNA reverse sequencing primer (5′-CTCTAGATCAACCACTTTGT-3′) were designed to perform PCR. After PCR the plasmids which contained the miRNA insert fragments were verified by DNA sequencing. Finally, the mutants were excluded from this experiment and the right one was purified using QIAGEN Plasmid Maxi (tip 500) Kit according to the manufacturer’s instructions.
Transfection of stable miRNA plasmids: The pcDNATM6.2-GW/EmGFP-miR expression vectors containing either the AF1Q miRNA insert (pCMV-AF1Q miRNA-980, pCMV-AF1Q miRNA-1082, or pCMV-AF1Q miRNA-1128) or the pcDNATM6.2-GW/EmGFP-miR-neg control plasmid (Invitrogen, Carlsbad, CA, USA) was transfected into target cells with the Lipofectamine 2000 reagent according to the manufacturer’s instruction, respectively. After 48 h of transfection, Western blot and RT-PCR were performed to assess the selectivity of AF1Q knockdown as described above. Successfully transfected cells clones were obtained by a long-term culture in a selection medium containing 4 μg/ml Blasticidin. 3 months later, Western blot and RT-PCR were performed to confirm the knockdown of AF1Q.
In vitro invasion assay: The effects of reduced AF1Q expression on the invasion capacity in breast cancer cells were determined by in vitro invasion assay as described previously. All experiments were performed in triplicate.
RT-PCR and Western blotting analysis
To further explore the molecular mechanisms underlying AF1Q mediated progression of breast cancer by integrin α3 and EFP, expression of several related genes, such as AF1Q, integrin α3, MMP-2, Ets-1, MMP-9, EFP, ERα, and 14-3-3δ, were investigated in the parent, vector transfected, AF1Q transfected, and AF1Q knockdown cells by RT-PCR, real-time PCR and Western blotting analysis, respectively. The primers were designed using Primer 3 software (Table 1). All experiments were performed in three times.
Flow cytometry and proliferation assays
To confirm the roles of EFP in progression of breast cancer by AF1Q, cell proliferation assays and flow cytometry were utilized to investigate the proliferation potential. The control, AF1Q transfected, and AF1Q knockdown cells at logarithmic growth phase were harvested and single-cell suspensions containing 1 × 106 cells were made, respectively. The target cells were treated following the standardized protocol and cell cycle analyses were performed by flow cytometry. Cell proliferation assays was also conducted as described previously.
Tumorigenicity and metastasis assays in nude mice
The tumorigenicity and spontaneously metastatic capability of the different cells were determined using an orthotopic xenograft tumor model in the athymic mice as described previously [12]. Animals were divided into four groups, including the parental, empty vector, AF1Q transfectant, and AF1Q knockdown groups. Each group had eight mice. 2 × 106 cells were injected orthotopically into the exposed axillary MFP of anesthetized athymic mice. The cells in AF1Q knockdown group were inoculated in 6 weeks after transfection. Animals were monitored every 2 days for up to 6 weeks for tumor growth and general health. The rate of primary tumor growth of different cells was determined by plotting the means of two orthogonal diameters of the tumors, measured at 7-day intervals. Animals were killed and autopsied at 6 weeks of post-inoculation. The metastasis deposits of the lung were assessed by macroscopic observation to evaluate the metastatic potential. To confirm the presence of lung metastases, sections were cut at 50 μm intervals and H&E staining were performed.
Tissue samples harvested for Western blot analysis was snap-frozen in liquid nitrogen. The protein expression of AF1Q, integrin α3, Ets-1, MMP-2, EFP, and 14-3-3δ in xenograft tumor tissues of different groups was investigated by Western blot.
Statistical analysis
Statistical analysis was performed using the software of Statistical Package for the Social Sciences (SPSS) Version 11.5 for Windows, and P < 0.05 was considered as statistically significant.
Results
Expression of AF1Q in MDA-MB-231 and MDA-MB-231HM cells
To confirm the roles of AF1Q, RT-PCR, real-time PCR and Western blot analysis of AF1Q were performed in MDA-MB-231 and MDA-MB-231HM cells. As shown in Fig. 1, analysis of replicate samples from MDA-MB-231HM and MDA-MB-231 cells suggested a close correlation between the expression of AF1Q and the metastatic potential of MDA-MB-231 cells.
RT-PCR, real-time PCR, and Western blot analysis of AF1Q differentially expressed in MDA-MB-231HM (lane 2) and parental MDA-MB-231 cell line (lane 1). A and B, Differential expression of AF1Q in both cell lines revealed by RT-PCR (A) and quantitative real-time PCR (B) analysis. GAPDH is shown as an internal control. (C) Representative immunodetection of AF1Q is shown. (D) Relative protein expression of AF1Q in different cell lines was normalized to the signal intensity of β-actin
Stable transfection and expression analysis
To investigate the effects of AF1Q on the metastatic phenotype of MDA-MB-231 cells, stable AF1Q transfectants was established and named of MDA-MB-231/AF1Q-1 and MDA-MB-231/AF1Q-2. As shown in Fig. 2, RT-PCR, real-time PCR and Western blotting analysis revealed that levels of AF1Q expression were found much higher in both MDA-MB-231/AF1Q-1 and MDA-MB-231/AF1Q-2 compared with the parental or vector control-transfected cells.
Expression of AF1Q in AF1Q transfectants and control cells. Lanes 1–4: MDA-MB-231, MDA-MB-231/vector, MDA-MB-231/AF1Q-1 and MDA-MB-231/AF1Q-2. (A) and (B), RT-PCR (A) and quantitative real-time PCR (B) analysis of AF1Q expression in different MDA-MB-231 cells. (C) Western blot analysis of AF1Q expression in different MDA-MB-231 cells. (D) The relative expression of AF1Q protein in different cell lines was normalized to β-actin
In vitro effects of AF1Q overexpression on MDA-MB-231 cells
Immunofluorescence staining assays
As shown in Fig. 3A, positive fluorescence staining for AF1Q was recognized in the cytoplasm of different cells. Compared with the parent cells, thicker fluorescence staining for AF1Q expression was observed in transfectant cells
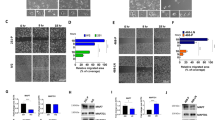
In vitro effects of AF1Q overexpression on MDA-MB-231 cells. (A) Representative fluorescent microscopic photographs of MDA-MB-231 transfected with AF1Q. The fluorescence was found to distribute in cytoplasm. Compared with the parent cells, thicker fluorescence staining for AF1Q expression was observed in transfectants cells. (B) Colony formation assay for MDA-MB-231 in the group of 1 × 104 cells. (C) The growth curves of different MDA-MB-231 cells. (D) In vitro matrigel invasion assay, the AF1Q transfected cells were more invasive than its parental counterparts (P < 0.05)
Colony formation assay
In all of the four groups, recombinant vector-transfected cells formed more colonies than the control, but the statistically significant difference was only observed in the group of 1 × 104 cells (Fig. 3B) (P < 0.05).
Proliferation assay
To investigate whether overexpression of AF1Q could modulate the proliferation rate of MDA-MB-231, we assessed the growth of the parent, AF1Q transfected and vector transfected cells. As shown in Fig. 3C, AF1Q was able to increase the proliferation of MDA-MB-231 cells significantly at 4th and 5th day (P < 0.05).
In vitro invasion assays
In vitro invasion assay were performed to determine the effects of AF1Q on cell invasion. The AF1Q transfectants showed higher invasive capacity compared with either parental or vector control-transfected cells (Fig. 3D) (P < 0.05).
According to the above results, MDA-MB-231/AF1Q-1 was used as AF1Q transfectant (MDA-MB-231/AF1Q) in the next further study for its higher AF1Q expression and invasive potential.
Focused microarray analysis
To gain insight into the molecular mechanisms associated with the enhanced metastatic potential by AF1Q, microarray analyses were performed to determine the differences in gene expression profile between the AF1Q transfectant and its parental counterpart. We analyzed the expression of 178 genes known to be involved in human tumor growth, signal transduction and metastasis using this technology. Nine genes were found to be up-regulated and 13 genes were down-regulated (as judged by a >3-fold change in expression; Table 3).
Real-time PCR confirmation of microarray results
The microarray analyses were validated by real-time PCR. EFP, IL-2, P53, integrin α3, LAMB2 and TRAF1 were randomly chosen to confirm the above microarray results. As shown in Fig. 4, analysis of replicate samples from AF1Q transfectants and the control cells confirmed the level of expression as revealed by microarray analysis. Two of these genes, integrin α3 and EFP, was further investigated the molecular mechanisms associated with the enhanced progression of breast cancer by AF1Q.
RNA interference (RNAi) experiments
After DNA sequencing, three plasmids constructed (pCMV-AF1Q miRNA-980, pCMV-AF1Q miRNA-1082, and pCMV-AF1Q miRNA-1128) were transfected into the MDA-MB-231/AF1Q cells. To valuate inhibition of AF1Q expression, the expression levels of AF1Q were compared among the MDA-MB-231/AF1Q, pCMV-AF1Q miRNA-980 transfected, pCMV-AF1Q miRNA-1082 transfected, pCMV-AF1Q miRNA-1128 transfected and the pCMV-AF1Q miRNA-neg transfected cells at 48 h after transfection. As shown in Fig. 5A–D, AF1Q were strongly inhibiteded in pCMV-AF1Q miRNA-1128 transfected cells (P < 0.05).
pCMV-AF1Q miRNA-1128 efficiently silences AF1Q expression in AF1Q transfected breast cancer cells. Lanes 1–5: MDA-MB-231/AF1Q, pCMV-AF1Q miRNA-980 transfected, pCMV-AF1Q miRNA-1082 transfected, pCMV-AF1Q miRNA-1128 transfected, and pCMV-AF1Q miRNA-neg transfected cells. A and B, RT-PCR (A) and real-time PCR (B) illustrated that pCMV-AF1Q miRNA-1128, rather than other groups, reduces AF1Q mRNA in MDA-MB-231/AF1Q Cells. C, A representative Western blot image illustrated that pCMV-AF1Q miRNA-1128, rather than other groups, reduced AF1Q protein. (D) The relative expression of AF1Q protein in above different cells was normalized to the signal intensity of β-actin. E and F, RT-PCR (E) and real-time PCR (F) showed that pCMV-AF1Q miRNA-1128 decreased the AF1Q mRNA in MDA-MB-231/AF1Q cells at 3 months after initial transfection. (G) Western blot analysis showed that pCMV-AF1Q miRNA-1128 decreased the AF1Q protein in MDA-MB-231/AF1Q cells at 3 months after initial transfection. (H) The relative expression of AF1Q protein in above different MDA-MB-231/AF1Q cells was normalized to the signal intensity of β-actin
In order to assess the phenotype of tumor cells in which AF1Q expression was inhibited over a long-term period, one of clones from pCMV-AF1Q miRNA-1128 transfected cells was chose and cultured for a long-term period in a selection medium containing Blasticidin. At 3 months, RT-PCR, real-time PCR and Western blotting analysis of the clone cells demonstrated decreased AF1Q expression compared with MDA-MB-231/AF1Q cells (Fig. 5E–H) (P < 0.05).
AF1Q knockdown inhibited breast cancer cell invasion in vitro and down-regulation of integrin α3, MMP-2, and Ets-1
The pCMV-AF1Q miRNA-1128 was used to knockdown the AF1Q gene in MDA-MB-231/AF1Q cells. As shown in Fig. 6A, reduced AF1Q expression in MDA-MB-231 cells significantly inhibits the invasive capacity in vitro as compared with the control cells (P < 0.05). Integrin α3 is one of up-regulation genes in the AF1Q transfected cells and its expression is related to the invasive potential of cancer cells. Integrin α3 was reported to be implicated in the expression of Ets-1, MMP-2 and MMP-9. RT-PCR, real-time PCR and Western blot analysis showed that AF1Q transfectants constitutively expressed high levels of AF1Q, integrin α3, Ets-1 and MMP-2, and the AF1Q knockdown cells expressed low levels of these genes (Fig. 6B–E). However, we didn’t find the significant differences levels of MMP-9 among the above cells (data not show).
Down-regulation of AF1Q in breast cancer cells decreased the invasion capacity in vitro, accompanied with inhibition of integrin α3, MMP-2, and Ets-1. Lanes 1–4: the parents, empty vector, AF1Q transfected, and AF1Q knockdown (MDA-MB-231/AF1Q pCMV-AF1Q miRNA-1128 transfected) cells. (A) In vitro invasion assays of the invasive potential of AF1Q knockdown cells and its controls. AF1Q knockdown cells was less invasive than its controls (P < 0.05). B and C, RT-PCR (B) and quantitative real-time PCR (C) analysis of AF1Q, integrin α3, MMP-2, and Ets-1 expression is shown. (D) Western blot analysis of AF1Q, integrin α3, MMP-2, and Ets-1 expression in different cells. (E) The relative expression of AF1Q, integrin α3, MMP-2, and Ets-1 protein in different cell lines was normalized to the signal intensity of β-actin
AF1Q mediates the proliferation potential of breast cancer cells by 14-3-3δ through EFP in vitro
To further explore the molecular mechanisms enhanced proliferation potential of breast cancer by AF1Q in vitro, we focused on EFP gene. As shown in Fig. 7A–D, RT-PCR, real-time PCR and Western blot analysis showed that AF1Q transfectants constitutively expressed high levels of AF1Q and EFP, and the AF1Q knockdown cells expressed low levels of AF1Q and EFP. Western blot analysis also showed the down-regulation of 14-3-3δ in AF1Q transfectants and up-regulation in AF1Q knockdown cells. However, RT-PCR and real-time PCR did not show any significant differences in 14-3-3δ mRNA expression between them. The reasons might be that EFP ubiquitinated and degraded the 14-3-3δ protein by the proteasome. So we could not find the difference expression in mRNA levels.
AF1Q promoted cell progression in vitro, up-regulated EFP and down-regulated 14-3-3δ protein expression. Lanes 1–4: the parents, empty vector, AF1Q transfected, and AF1Q knockdown cells. A and B, RT-PCR (A) and quantitative real-time PCR (B) analysis of AF1Q, EFP, and 14-3-3δ expression is shown. (C) Western blot analysis of AF1Q, EFP, and 14-3-3δ expression in different cells. (D) The relative expression of AF1Q, EFP, and 14-3-3δ protein in different cell lines was normalized to the signal intensity of β-actin. (E) Representative results of flow cytometric analysis. (F) The growth curves for the different cells in vitro proliferation assay
The proliferation potential mediated by AF1Q was confirmed by cell cycle analysis using flow cytometry and proliferation assays. Flow cytometry analysis showed that a high percentage of AF1Q transfectants cells and low percentage of AF1Q knockdown cells were in the proliferating stages, particularly in S phase (Fig. 7E). Proliferation assays also confirmed that AF1Q mediated the proliferation potential of breast cancer cells by 14-3-3δ through EFP in vitro (Fig. 7F).
As we all known, MDA-MB-231 cells is ERα negative and EFP gene is one of the estrogen receptor (ERα) target genes. To explore the difference expression of ERα in the above parent, AF1Q transfectant, and AF1Q knockdown cells, RT-PCR, real-time PCR and Western blot analysis were used. However, we couldn’t find the any increased levels of ERα in the AF1Q transfected cells (data not show).
Tumor growth and pulmonary metastasis in the athymic mice were regulated by the expression of AF1Q
The effect of AF1Q expression on tumor growth and metastasis was further assayed in an athymic mice model. Results revealed that AF1Q transfectants tumor grew faster than either vector-transfected or parental group and AF1Q knockdown group grew slower than the other three groups in the athymic mice (Fig. 8A). To study pulmonary metastasis, at the experimental endpoint, lungs were examined physically at autopsy and the overt surface metastases were observed. The surface metastases were found in 37.5% of mice in AF1Q transfected group, 12.5% of the parent groups and none of the AF1Q knockdown group. The difference between the AF1Q transfected group and the AF1Q knockdown group was significant. (P < 0.05) Furthermore, lungs were subjected to microscopic examination for morphological evidence of tumor cells by light microscopy on H.E-stained paraffin sections. The most micrometastases were observed in AF1Q transfected mice. Only a few micrometastases or no micrometastase were observed in mice of parental, vector-transfected, or knockdown groups (Fig. 8B). In addition, similar effects of AF1Q on integrin α3, Ets-1, MMP-2, EFP, and 14-3-3δ expression observed in the in vitro studies were also found in the in vivo studies (Fig. 8C and D).
Regulation of the growth and pulmonary metastasis of MDA-MB-231 cells by AF1Q in in vivo studies. (A) The growth curves for the parents, empty vector, AF1Q transfected, and AF1Q knockdown cells in in vivo proliferation assay. (B) Photomicrographs of micrometastases in lung sections (arrow) obtained from mice bearing the parents (a), vector transfected (b), AF1Q transfected (c), and AF1Q knockdown (d) tumors for MDA-MB-231 cells (H.E, ×200). (C) The protein levels of AF1Q, EFP, 14-3-3δ, integrin α3, MMP-2, and Ets-1 were detected by Western blot in xenografts of different groups. Lanes 1–4: the parents, empty vector, AF1Q transfected, and AF1Q knockdown. (D) The relative expressions of AF1Q, EFP, 14-3-3δ, integrin α3, MMP-2, and Ets-1 protein in above different groups was normalized to the signal intensity of β-actin
Discussion
Until recently, the specific mechanisms that actually promote breast cancer metastasis still remain unclear. In the previous study, our institute employed the high-throughput cDNA microarray and comparative protemic system to identify a number of genes and proteins, such as AF1Q, peroxiredoxin 6 and so on, that showed significantly increased expression levels in the highly metastatic MDA-MB-435HM cells compared with its parental counterparts [3, 4].
The AF1q gene was identified as a mixed lineage leukaemia (MLL) fusion partner in an infant with acute myeloid leukaemia (AML) carrying a t(1; 11) (q21; q23) translocation [13]. Tse et al. found that high AF1q expression indicated a poor prognosis in children with AML and its levels might provide a marker for risk stratification in patients with MDS [7]. Jacques et al. reported the AF1Q might be involved in tumorigenesis of thyroid oncocytic tumors [5]. Our institute also demonstrated that AF1Q has a role in metastatic potential of MDA-MB-435 cells. However, the function of AF1q remains to be defined.
In the present study, we also found that AF1Q was up-regulation in MDA-MB-231HM cells and it suggested that AF1Q played a role in the metastatic potential of MDA-MB-231HM cells. We generated stable MDA-MB-231/AF1Q transfectants and tested them for any alterations in the invasive and proliferation potential in vitro. As expected, our results showed that AF1Q also could enhance the invasive and proliferation potential of MDA-MB-231 cells in vitro. Furthermore fluorescence staining for AF1Q was recognized in the cytoplasm, which appears to contradict to published literature regarding AF1Q location. Lin et al. described that intact wild type human AF1Q cDNA encodes a transmembrane protein [14]. Subsequent analysis is needed to explain the disparity in subcellular locations of AF1Q.
Focused microarray analyses were utilized to investigate the molecular mechanisms of AF1Q in breast cancer metastasis. Our results showed that nine genes were found to be up-regulated and integrin α3 is one of up-regulation genes in the AF1Q transfected cells. Integrin α3 belongs to the integrin family members and the integrin family serves as adhesion receptors for extracellular matrix proteins and cellular counterligands. These adhesion receptors are heterodimers of transmembrane glycoproteins (α and ß subunits); various combinations of α and ß subunits produce polymorphisms of ligand specificity. The expression of integrin α3ß1 was demonstrated to be positively correlated with the increased invasiveness of the tumors [15–17]. These studies suggest that integrin α3 is also involved in the invasion of cancer cells. The mechanisms that integrins modulate cancer cell migration and invasion are not entirely known, but some reports have shown that the integrin is implicated in the production and/or activation of MMP-9 and MMP-2 [18–20]. Kato et al. found that the Ets family of transcription factors regulated the expression of integrin α3 [21]. The Ets transcription factors were also reported to be involved in tumor metastasis through the promotion of angiogenesis and the expression of MMPs [12, 22, 23]. These observations suggest that integrin α3, Ets and MMPs cooperatively promote adhesion and invasion of cancer cells during the metastatic processes.
In the present study, we demonstrated that overexpression of AF1Q could up-regulate the expression of integrin α3, Ets-1, and MMP-2 accompanied with the increased in vitro invasive potential in MDA-MB-231 cells. The results were confirmed in AF1Q knockdown cells. However, we could not find any difference expression of MMP-9 levels. More studies should be done to explain the disparity.
In the present study, a miRNA expression vector based on murine miR-155 sequence was used to construct AF1Q targeting RNAi Expression Vector. One construction, chosen from three different miR155-based AF1Q targeting pre-miRNA pol II Vectors, was used to decrease the expression of AF1Q and generate the stable transfectants of AF1Q knockdown in MDA-MB-231 cells. The stable inhibition of AF1Q was observed in three months, which provide the confirmation of its utilization in vivo. The ability of invasion was analyzed in AF1Q knockdown breast cancer cells and the obviously decreased in vitro invasion was observed. All the results confirmed the roles of AF1Q in the invasive potential of breast cancer.
The estrogen-responsive ring finger protein (EFP) is another up-regulation gene in the AF1Q transfected cells from the results of microarray analysis. The human EFP gene, one of the estrogen receptor (ER) target genes, is located at chromosome 17q23.1 and codes a 67KD protein [24]. It has an estrogen-responsive element (ERE) at the 3′-untranslated region (UTR) and could mediate estrogen-induced cell growth. Some studies [25–27] showed that high expression of EFP was correlated with the cancer development, which was mediated by the down-regulation of 14-3-3δ. 14-3-3δ is a p53-inducible protein that sequesters mitotic Cdks in the cytoplasm to inhibit entry into mitosis. To allow cell-cycle progression, estrogens stimulate production of EFP, which binds and ubiquitinates 14-3-3δ. 14-3-3δ marked with ubiquitin chains is recognized and degraded by the proteasome. Released Cdks are free to escape into the nucleus and promote mitotic entry.
In the present study, we demonstrated that up-regulation of EFP were accompanied with the inhibition of 14-3-3δ protein in AF1Q transfected cells. Cell cycle analysis also showed that EFP overexpression could increase the percentage of cells in the proliferation stage of the cell cycle. However, we could not find any difference expression in 14-3-3δ mRNA levels between the AF1Q transfected and its control cells. The reasons might be that EFP ubiquitinated and degraded 14-3-3δ protein by the proteasome.
MDA-MB-231 cell is ERα negative. However, we couldn’t found any increased levels of ERα in the AF1Q transfected cells. How was EFP up-regulated? One plausible explanation is there might be some relationship between AF1Q and EFP that is estrogen independence. Urano et al. [28] have found that EFP could promote a switch from estrogen-dependent to estrogen-independent proliferation of breast cancer cells. They demonstrated that estrogen-dependent MCF-7 breast cancer cells forced to express EFP gained the ability to form tumors in nude mice in the absence of estrogen.
In this study, we also demonstrated that AF1Q could enhance the orthotopic tumor growth and pulmonary metastasis in vivo. On the contrary, AF1Q knockdown cells showed the decreased ability in breast cancer progression. These changes were associated with regulation of EFP, integrin α3, Ets-1, MMP-2, and 14-3-3δ protein levels in xenograft tumors. All these results indicate that AF1Q promote breast cancer progression, partially via regulation of EFP, integrin α3, Ets-1, MMP-2, and 14-3-3δ expression. It might provide a potential therapeutic way for breast cancer development.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57(1):43–66
Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG (1997) Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med 75(6):429–439
Li DQ, Wang L, Fei F, Hou YF, Luo JM, Zeng R, Wu J, Lu JS, Di GH, Ou ZL et al (2006) Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics 6(11):3352–3368
Li DQ, Hou YF, Wu J, Chen Y, Lu JS, Di GH, Ou ZL, Shen ZZ, Ding J, Shao ZM (2006) Gene expression profile analysis of an isogenic tumour metastasis model reveals a functional role for oncogene AF1Q in breast cancer metastasis. Eur J Cancer 42(18):3274–3286
Jacques C, Baris O, Prunier-Mirebeau D, Savagner F, Rodien P, Rohmer V, Franc B, Guyetant S, Malthiery Y, Reynier P (2005) Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J Clin Endocrinol Metab 90(4):2314–2320
So CW, Ma SK, Wan TS, Chan GC, Ha SY, Chan LC (2000) Analysis of MLL-derived transcripts in infant acute monocytic leukemia with a complex translocation (1;11;4)(q21;q23;p16). Cancer Genet Cytogenet 117(1):24–27
Tse W, Meshinchi S, Alonzo TA, Stirewalt DL, Gerbing RB, Woods WG, Appelbaum FR, Radich JP (2004) Elevated expression of the AF1q gene, an MLL fusion partner, is an independent adverse prognostic factor in pediatric acute myeloid leukemia. Blood 104(10):3058–3063
Tse W, Joachim Deeg H, Stirewalt D, Appelbaum FR, Radich J, Gooley T (2005) Increased AF1q gene expression in high-risk myelodysplastic syndrome. Br J Haematol 128(2):218–220
Chang XZ, Wang ZM, Yu JM, Tian FG, Jin W, Zhang Y, Yu J, Li LF, Liu XF, Li ZW et al (2007) Isolation of a human gallbladder cancer cell clone with high invasive phenotype in vitro and metastatic potential in orthotopic model and inhibition of its invasiveness by heparanase antisense oligodeoxynucleotides. Clin Exp Metastasis 24(1):25–38
Zhang Z, Futamura M, Vikis HG, Wang M, Li J, Wang Y, Guan KL, You M (2003) Positional cloning of the major quantitative trait locus underlying lung tumor susceptibility in mice. Proc Natl Acad Sci USA 100(22):12642–12647
Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47(12):3239–3245
Hou YF, Yuan ST, Li HC, Wu J, Lu JS, Liu G, Lu LJ, Shen ZZ, Ding J, Shao ZM (2004) ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene 23(34):5799–5806
Tse W, Zhu W, Chen HS, Cohen A (1995) A novel gene, AF1q, fused to MLL in t(1;11) (q21;q23), is specifically expressed in leukemic and immature hematopoietic cells. Blood 85(3):650–656
Lin HJ, Shaffer KM, Sun Z, Jay G, He WW, Ma W (2004) AF1q, a differentially expressed gene during neuronal differentiation, transforms HEK cells into neuron-like cells. Brain Res Mol Brain Res 131(1–2):126–130
Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A (2000) The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer 87(3):336–342
Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M (1996) Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in the peritoneal implantation of scirrhous gastric carcinoma. Br J Cancer 74(9):1406–1412
Pochec E, Litynska A, Amoresano A, Casbarra A (2003) Glycosylation profile of integrin alpha 3 beta 1 changes with melanoma progression. Biochim Biophys Acta 1643(1–3):113–123
Baum O, Hlushchuk R, Forster A, Greiner R, Clezardin P, Zhao Y, Djonov V, Gruber G (2007) Increased invasive potential and up-regulation of MMP-2 in MDA-MB-231 breast cancer cells expressing the beta3 integrin subunit. Int J Oncol 30(2):325–332
Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, Pozzi A (2005) Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer 116(1):52–61
Fouchier F, Penel C, Pierre Montero M, Bremond P, Champion S (2007) Integrin alphavbeta6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn(2+). Eur J Cell Biol 86(3):143–160
Kato T, Katabami K, Takatsuki H, Han SA, Takeuchi K, Irimura T, Tsuji T (2002) Characterization of the promoter for the mouse alpha 3 integrin gene. Eur J Biochem 269(18):4524–4532
Taki M, Verschueren K, Yokoyama K, Nagayama M, Kamata N (2006) Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int J Oncol 28(2):487–496
Yamamoto H, Horiuchi S, Adachi Y, Taniguchi H, Nosho K, Min Y, Imai K (2004) Expression of ets-related transcriptional factor E1AF is associated with tumor progression and over-expression of matrilysin in human gastric cancer. Carcinogenesis 25(3):325–332
Inoue S, Orimo A, Hosoi T, Kondo S, Toyoshima H, Kondo T, Ikegami A, Ouchi Y, Orimo H, Muramatsu M (1993) Genomic binding-site cloning reveals an estrogen-responsive gene that encodes a RING finger protein. Proc Natl Acad Sci USA 90(23):11117–11121
Horie-Inoue K, Inoue S (2006) Epigenetic and proteolytic inactivation of 14–3-3sigma in breast and prostate cancers. Semin Cancer Biol 16(3):235–239
Ikeda K, Inoue S (2004) Estrogen receptors and their downstream targets in cancer. Arch Histol Cytol 67(5):435–442
Suzuki T, Urano T, Tsukui T, Horie-Inoue K, Moriya T, Ishida T, Muramatsu M, Ouchi Y, Sasano H, Inoue S (2005) Estrogen-responsive finger protein as a new potential biomarker for breast cancer. Clin Cancer Res 11(17):6148–6154
Urano T, Saito T, Tsukui T, Fujita M, Hosoi T, Muramatsu M, Ouchi Y, Inoue S (2002) Efp targets 14–3-3 sigma for proteolysis and promotes breast tumour growth. Nature 417(6891):871–875
Acknowledgements
This research was supported in part by the grants from 973 (2006CB0D0901), National Natural Science Foundation of China (30371580, 30572109 to ZM. S.); Shanghai Science and Technology Committee (03J14019, 06DJ14004, 06DZ19504; to ZM. S.); China Postdoctoral Science Foundation (20060390141 to XZ. C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, XZ., Li, DQ., Hou, YF. et al. Identification of the functional role of AF1Q in the progression of breast cancer. Breast Cancer Res Treat 111, 65–78 (2008). https://doi.org/10.1007/s10549-007-9761-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9761-y