Abstract
Background
In the treatment of extensive liver metastasis of breast cancer (LMBC), locally administered Mitomycin C (MMC) to the liver might be an effective approach with limited toxicity.
Patients and methods
We retrospectively reviewed the records of 30 patients with LMBC treated with intra-hepatic MMC at our institution. MMC (12 mg) was administered by transcatheter bolus infusion into the hepatic arteries every 4 weeks. Tumour response according to RECIST criteria, progression free survival (PFS), overall survival (OS) and duration of response (DR) were used to evaluate efficacy.
Results
There was a local response in the liver and a global response in respectively 33 and 26%. The median PFS, DR and OS were 3, 4 and 7 months, respectively. There was more benefit in patients without documented metastases outside the liver and without severe liver dysfunction. Thrombocytopenia, leucocytopenia and an allergic reaction were observed after MMC administration in 20 (67%), 12 (40%) and 4 patients (13%), respectively.
Conclusion
Intra-hepatic MMC bolus infusion as treatment of extensive LMBC is associated with limited toxicity and has a significant response rate in the liver. Prospective investigations are required to define the place of this modality for treating patients with breast cancer liver metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common female cancer in Western countries, affecting one-eighth of women. Despite appropriate adjuvant therapy, metastatic disease develops in ∼20–30% of all patients and in half of them liver metastases occur at some point in their clinical course [1, 2]. Based on studies dating from two decades ago, the presence of liver metastases of breast cancer (LMBC) has long been seen as a poor prognostic factor, with a median survival of less than 6 months [2, 3]. More recent data have questioned these findings, as patients treated in the past 10 years seem to have a much better outcome, with median survivals in the range of 1–2 years [1–4].
Metastatic breast cancer is generally viewed as disseminated disease that requires systemic rather than local therapy. The benefit of systemic chemotherapy and endocrine therapy for metastatic disease has been established [4]. For systemic chemotherapy, systemic toxicity is usually a dose-limiting factor. This makes local approaches with reduced systemic toxicity an attractive alternative for patients who only have localized metastatic disease [2, 5, 6]. The benefit of local treatment options for isolated or limited LMBC such as surgery and radiofrequency ablation has been suggested by several small retrospective studies with highly selected patients [7–13].
In patients with liver metastases too extensive for resection or ablation, an alternative approach may be to deliver regional chemotherapy intra-arterially. The rationale behind this approach is based on the fact that liver metastases derive most of their blood supply from the hepatic artery [14]. When a drug with a high first pass hepatic extraction is administered, it is possible to achieve high intra-hepatic drug concentrations with a reduced systemic toxicity [15]. Mitomycin C (MMC) is a prototype of a bioreductive alkylating agent, undergoing biotransformation to an active species that crosslinks DNA. It may also generate oxygen-free radicals, highly reactive species who may cause substantial tissue damage through lipid peroxidation and protein and nucleic acid attack. MMC has been a standard chemotherapy for metastatic breast cancer for many years [16, 17]. Some rare but serious side effects, most importantly the Haemolytic Uremic Syndrome (HUS), have limited its widespread use. The last decade, the advent of new, more effective and safer chemotherapeutics such as taxanes, vincalakaloids, oral fluoropyridimes, has pushed MMC somewhat into the background, but it is still a reasonable option for patients. However, MMC is an excellent candidate for regional administration because of the high first pass hepatic extraction that assures high local drug concentrations with a reduced systemic toxicity [18, 19]. In a rat model of liver metastases from colorectal cancer, isolated liver perfusion with MMC induced a marked DNA synthesis inhibition and even complete tumour remission [20]. MMC has been used intra-arterially as regional chemotherapy for metastatic colorectal cancer and has given good responses [15, 17]. In the treatment of LMBC, there have been several reports with promising results of the intra-hepatic administration of MMC containing regimens [14, 21–26]. Nevertheless, the number of reported series has remained small and heterogeneous. With only a few cases in each series and a great variety in the chemotherapeutic regimens used, interpretation of the results of these studies is difficult. Therefore, the place of intra-hepatic MMC administration in the treatment of patients with liver metastases from breast cancer remains unclear. We analysed retrospectively efficacy and safety of intra-hepatic MMC administration through bolus infusion in patients with extensive LMBC treated at our institution.
Patients and methods
Patients
We reviewed the records of all (n = 30) patients consecutively treated with intra-hepatic MMC between October 2000 and November 2006 at the Leuven University Hospital. These patients were eligible for MMC admission if they had breast cancer with progressive and life-threatening liver disease as a major site of metastasis, independent of previous systemic chemotherapeutic regimens. We studied their clinical and laboratory records to obtain their oncologic history, liver function at first admission (represented by liver transaminase levels: AST and ALT), the course of the MMC admission and overall survival (OS). OS was measured from the date of the first admission until death. We evaluated toxicity according to the CTC3.0 criteria (http://ctep.info.nih.gov/reporting/ctc.html), and used the laboratory counts at each MMC admission time to determine the haematologic toxicity. No Nadir haematologic counts were taken between cycles. Through radiological imaging the tumour response in and outside the liver was measured, using the RECIST criteria. A CT scan was performed every two cycles, or every three cycles when there was a biochemical sign of response. A response needed to be confirmed after at least 4 weeks. The progression free survival (PFS) and duration of response (DR) were calculated. PFS was defined as the survival time between the first admission date and disease progression or death. DR was measured from the first documentation of response to disease progression. The follow-up ended in April 2007.
MMC administration
MMC was administered by transcatheter bolus infusion into the right and left hepatic artery. All procedures were performed in a dedicated angiosuite by an experienced interventional radiologist (SH and GM). Under local anaesthesia, percutaneous vascular access was made to the right femoral artery. After selective catheterization of the common hepatic artery, both right and left hepatic arteries were cannulated with use of a microcatheter (Progreat 2.7, Terumo Europe, Leuven, Belgium or Renegade, Boston Scientific Coorperation, Natick, MA, USA) and a bolus injection of respectively 8 mg of MMC in 70 cm3 saline (right hepatic artery) and 4 mg of MMC in 30 cm3 saline (left hepatic artery) was administered. The procedure is shown in Fig. 1.
(A) Selective injection of the celiac trunk. Left hepatic artery (arrow) branching from the common hepatic artery. (B) Superselective injection of the left hepatic artery with use of a microcatheter. (C) Selective injection of the superior mesenteric artery. Right hepatic artery branching (arrowhead) from the proximal superior mesenteric artery (‘right replaced hepatic artery’). (D) Superselective injection of the right hepatic artery with use of a microcatheter
Statistics
Data are presented with median and range. Kaplan–Meier curves for PFS and OS were determined by Statistica 7.0 program.
Results
Patient characteristics
A total of 30 patients were treated because they had progressive and life-threatening liver disease as a major site of metastasis of their breast cancer. All had multiple liver metastases. Patient characteristics at onset of therapy are depicted in Table 1. The median age was 54 years (ranging between 30 and 85 years). Nineteen patients had documented metastatic sites outside the liver. Bone metastasis was most frequent (14 out of 19), followed by lung metastasis (6 out of 19). For all but one patient, there was a previous administration of other systemic chemotherapeutic regimens before the start of MMC, with a median of five regimens (ranging between 0 and 7). All patients with hormone-sensitive tumours previously received hormonal therapy (24 out of 30) and all those who had HER2/Neu positivity previously received Trastuzumab (5 out of 30). During therapy, no concomitant systemic chemotherapy was given. Trastuzumab was continued in the five patients who received it previously. At first admission, half of the patients had liver dysfunction grade 1 (15 out of 30), 16.7% had grade 2 (5 out of 30) and 13.3% had grade 3 dysfunction (4 out of 30). Normal liver function tests were present in 20% of patients (6 out of 30).
Efficacy
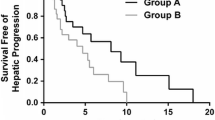
Patients received a median of three intra-hepatic MMC administrations (ranging from 1 to 11). Four patients received upfront a modified dose (3 because of liver dysfunction grade 3 at baseline, 1 because of thrombocytopenia grade 3). In four other patients, the MMC dose was decreased during subsequent cycles of MMC admission, because of thrombocytopenia, grade 1 (one patient) or grade 2 (three patients). Nine patients were still alive at the time we reviewed their records, of which two are still without progression after 5 months. Results are outlined in Tables 2 and 3 and an example of a partial response is shown in Fig. 2. Local response in the liver was 33.3%. However, when we take into account disease progression outside of the liver, the response rate was only 26.6%. Because of the retrospective nature of this study, four responses could not be confirmed after at least 4 weeks. Median DR was 4 months. Five patients had a better ‘best response’ in the liver than globally, indicating that the therapy mainly worked on the liver metastases. Kaplan–Meier curves for PFS and OS are presented in Figs. 3 and 4. The median PFS was 3 months (Inter Quartile Rate (IQR) 1.0–7.75 months). The median OS was 7.3 months (IQR 4.0–21.6 months). Analysis of the subgroup of patients who had no documented metastatic sites outside the liver at inclusion, showed a response in six patients (54.5%). One other patient without documented metastatic sites outside the liver had a good local response but developed rapidly progressive disease outside the liver. No global treatment responses and only one partial response in the liver were reported in the nine patients who had a liver dysfunction at inclusion of grade 2 or higher. The treatment response seemed not related to the number of systemic chemotherapeutic regimens administered previously. No treatment responses were reported in the documented metastatic sites outside the liver.
Safety
The most common adverse reactions observed are outlined in Table 4. No toxic death or grade 4 events were recorded in the medical files. Thrombocytopenia in some grade occurred in 66.6% of the patients. In five patients with thrombocytopenia, the percutaneous procedure for MMC admission was judged to be no longer safe, and consequently MMC admission was cancelled. Leucocytopenia occurred in 40% of the patients, but in only one patient grade 3 toxicity was reported. Four patients complained of mild nausea, while nausea grade 2 occurred in only one patient leading to the discontinuation of MMC admission after seven cycles. There were no major procedure-related complications. Some form of allergic reaction occurred in four patients during the procedure, probably due to the iodinated contrast material administered. In two patients this was a grade 3 allergic reaction with angioedema requiring an intervention with glucocorticoids and antihistaminic drugs. We reported 17 patients with anemia grade 1, but in 11 of them, anemia was already present at the time of inclusion. In one patient anemia grade 2 occurred during the course of MMC admission. There was one patient with anemia grade 4 after the first admission, requiring transfusion with 3 units of packed cells. There were no other frequently occurring relevant toxicities reported in the medical files.
Discussion
Despite of substantial therapeutic advances made over the recent years and the fact that outcome seems to have improved, prognosis in terms of survival remains poor for patients with LMBC [1–4]. Because metastatic breast cancer is considered incurable, therapy is palliative of intent, and tries to prolong survival while maintaining an optimal quality of life. In patients heavily pretreated with systemic polychemotherapy, quality of life might decrease due to the cumulative toxicity of the therapy. Thus, in patients with only or predominantly metastases in the liver, it might be interesting to use local approaches that have the theoretic advantage of lowering the tumour burden in the liver while keeping the systemic toxicity reduced. Different local treatment strategies in the management of LMBC have been proposed and investigated in the past.
Some reviews of small patient series reported good results with resection of LMBC. However, only women with isolated or limited metastases in the liver and a good performance status were eligible for surgery, and there was a high recurrence within the liver after successful resection. Nonetheless, most authors concluded that metastasectomy might be of significant benefit in the management of selected patients with operable metastatic breast cancer limited to the liver [7–12]. The use of the more recently developed, less invasive, radiofrequency ablation technique, is also limited to a very select group of women and although it can provide local control of small metastases, impact on survival has not yet been reported [13]. A Recent study by Bangash et al. [27] reported the use of 90Y radioembolisation in 27 women with LMBC, a complex and more expensive technique. They found a good radiologic response, but impact on survival was limited to patients with a high performance status and a low tumour burden.
In patients with liver metastases too extensive for resection or ablation, regional chemotherapy might represent a good alternative. We reviewed the available literature about this strategy and found many reports showing efficacy for liver metastases mainly of gastrointestinal origin. However, the experience with intra-hepatic chemotherapy for breast cancer is very limited, justifying the present report describing our experience in a group of 30 women with extensive LMBC, all treated at our institution with intra-hepatic MMC bolus infusion over the last 6 years.
Different techniques have been developed to administer MMC into the hepatic artery such as continuous intra-hepatic infusion of MMC through a surgically implanted port system [22] and bolus infusion through a percutaneously placed microcatheter cannulating the right and left hepatic artery. Because MMC is believed to be potentiated by an hypoxic environment, the intra-hepatic infusion of MMC was in some reports combined with chemoembolization [21], or with inducing a stop-flow of the hepatic artery obtained by the placement of a balloon-catheter [18]. However, until now no significant benefit was proven compared to the intra-hepatic infusion of MMC alone.
Most previous reports of intra-hepatic administration of MMC through bolus infusion in the treatment of LMBC, were published in the late eighties. In the study of Maral et al. [14] 16 patients with breast cancer and liver metastases received a bolus infusion of MMC (10 mg/m2) in a combination regimen with adriamycin and fluoro-uracil. The response rate was about 60%. Overbosch et al. [24] used a bolus infusion of MMC in 37 patients with liver metastases of colorectal or breast cancer. They reported a high response rate of 70% in breast cancer patients. The median DR was 10 months. Arterial infusion therapy with a MMC-containing regimen seemed a useful treatment for controlling LMBC, although Tada et al. [25] could not repeat the spectacular results mentioned above. In their study of 45 Japanese patients with LMBC who had been treated with intra-hepatic infusion of MMC and adriamycin, the response rate was 38% and the median DR was 5 months.
Despite these interesting results, all studies were retrospective and the patient series too small and heterogeneous to conclude that the intra-hepatic administration of dose MMC added further benefit to the conventional treatment of extensive LMBC. In recent years, reports of the use of intra-hepatic MMC were mainly anecdotal and published in the Japanese literature, leaving the place of intra-hepatic MMC infusion unclear [23, 26].
Our recent study analyses the largest group of patients with LMBC treated with intra-hepatic MMC administered through bolus infusion up to date. We found an overall response in 26.6% of the treated patients. Local response at hepatic level was even higher, but progression outside the liver occurred due to the lack of control on micrometastases outside the liver. Accordingly, further studies with combined or sequential use of local and systemic chemotherapy are warranted.
The median OS in our patient group was 7 months. However, the impact of intra-hepatic MMC on survival may be better reflected by PFS since this surrogate is not influenced by the patient status at inclusion and subsequent therapies. The median PFS was 3 months, but the median DR was 4 months, which we judge to be very encouraging given the fact that the majority of our patients were heavily pretreated and because the presence of progressive and life-threatening liver disease was one of the main reasons for intra-hepatic MMC administration. In patients who had no documented metastatic sites outside the liver at inclusion there was a trend of achieving a better response. A possible explanation for this finding is the lower tumour burden in this subset, which was recently suggested by Atalay et al. [1]. However, the number of patients analysed was too small to recommend intra-hepatic administration of MMC specifically in this subgroup. Remarkably, no significant treatment responses were reported in patients who had a liver dysfunction of grade 2 or higher at inclusion. These results are consistent with the statements of Mano et al. that patients with a significant liver dysfunction may not be the best candidates for the intra-hepatic administration of chemotherapy [3], but the limited number of patients and retrospective analysis do not allow generalisation at present. Of note however, the number of previously administered systemic chemotherapeutic regimens seems of lesser importance for the response rate we encountered.
No major complications occurred during the percutaneous procedures to administer MMC, except for allergic reactions, probably due to the iodinated contrast material administered. Although high MMC concentrations may cause severe intra-hepatic vascular damage [19, 28], the administered MMC dose we used seems safe. Possibly the majority of MMC undergoes first pass effects, and the low dose that reaches the systemic circulation might be too low to induce HUS. The main side effects of MMC are thrombocytopenia and leucocytopenia [19, 29]. We reported thrombocytopenia as the most important one, which was the reason to cancel the percutaneous procedure to administer MMC in five patients. In our study, haematologic recovery was checked immediately before each MMC administration, but no nadir haematologic counts were systematically performed between courses, which may have caused an underestimation of the intercurrent haematologic toxicity. However, this seems to be of lesser clinical relevance since no episodes of symptomatic bleeding of febrile neutropenia were documented in our present series. Other toxic effects include nausea, vomiting and anorexia [19, 29]. In only one patient this was judged severe enough to cancel further MMC administration, but this patient already received a high cumulative dose of seven cycles of MMC. Thus, in our experience, the intra-hepatic infusion of MMC was well tolerated. We encountered none of the rare but severe side effects such as HUS, interstitial pneumonitis and cardiac failure [19, 29].
To conclude, this is the largest retrospective study investigating intra-hepatic bolus infusion of MMC in patients with predominant LMBC. This treatment seems to add further benefit with minimal additional toxicity to the conventional treatment of extensive LMBC, especially in patients with no documented metastatic sites outside the liver and no severe liver dysfunction. The main limitation is the retrospective nature of our study, which might underreport toxicity, while a bias in the response rate reported here is very unlikely. Prospective, randomized and multicentre trials are warranted to confirm our results. Furthermore, combined or sequential use of regional and systemic chemotherapy to control tumour progression also deserve consideration in order to define the place of intra-hepatic MMC administration for treating patients with LMBC.
References
Atalay G, Biganzoli L, Renard F et al (2003) Clinical outcome of breast cancer patients with liver metastases alone in anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer 39:2439–2449
Pentheroudakis G, Fountzilas G, Bafaloukos D et al (2005) Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat 97:237–244
Mano MS, Cassidy J, Canney P (2005) Liver metastasis from breast cancer: management of patients with significant liver dysfunction. Cancer Treat Rev 31:35–48
Giordano SH, Buzdar AU, Smith TL et al (2004) Is breast cancer survival improving? Trends in survival for patients with recurrent breast cancer diagnosed from 1974 through 2000. Cancer 100:44–52
Marinelli A, van De Velde CJ, Kuppen PJ et al (1990) A comparative study of isolated liver perfusion versus hepatic artery infusion with mitomycin C in rats. Br J Cancer 62:891–896
Marinelli A, Pons DH, Vreeken JA et al (1991) High mitomycin C concentration in tumour tissue can be achieved by isolated liver perfusion in rats. Cancer Chemother Pharmacol 28:109–114
Maksan SM, Lehnert T, Bastert G, Herfarth C (2000) Curative liver resection for metastatic breast cancer. Eur J Surg Oncol 26:209–212
Pocard M, Pouillart P, Asselain B et al (2001) Liver resections from breast cancer metastasis: results and prognosis factors after hepatic resection in 65 cases. Ann Chir 126:413–420
Raab R, Nussbaum KT, Behrend M, Weimann A (1998) Liver metastases of breast cancer: results of liver resection. Anticancer Res 18:2231–2233
Schneebaum S, Walker MJ, Young D et al (1994) The regional treatment of liver metastases from breast cancer. J Surg Oncol 55:26–31
Selzner M, Morse MA, Vredenburgh JJ et al (2000) Liver metastases from breast cancer: long-term survival after curative resection. Surgery 127:383–389
Singletary SE, Walsh G, Vauthey JN et al (2003) A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 8:241–251
Livraghi T, Goldberg SN, Solbiati L et al (2001) Percutaneous radiofrequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology 220:145–149
Maral J, Curet P, Baumer R et al (1985) Intra-arterial chemotherapy for hepatic metastases. Experience of the Pitie-Salpetriere hospital group. Ann Gastroenterol Hepatol 21:99–101
Liu LX, Zhang WH, Jiang HC et al (2002) Arterial chemotherapy of 5-fluorouracil and mitomycin C in the treatment of liver metastases of colorectal cancer. World J Gastroenterol 8:663–667
Walters RS, Frye D, Buzdar AU et al (1992) A randomized trial of two doses schedules of mitomycin C in advanced breast cancer. Cancer 69:476–481
Bradner WT (2001) Mitomycin C: a clinical update. Cancer Treat Rev 27:35–50
Gadaleta C, Catino A, Mattili V (2006) Stop-flow perfusion with mitomycin-C as locoregional approach in the treatment of large-unresectable liver metastases. In Vivo 20:769–771
Rump AF, Woschee U, Theisohn M et al (2002) Pharmacokinetics of intra-arterial mitomycin C in the chemoembolization treatment of liver metastases with polyvinylalcohol or degradable starch microspheres. Eur J Clin Pharmacol 58:459–465
Marinelli A, Dijkstra FR, Van Dierendonck JH et al (1991) Effectiveness of isolated liver perfusion with mitomycin C in the treatment of liver tumours of rat colorectal cancer. Br J Cancer 64:74–78
Giroux MF, Baum RA, Soulen MC (2004) Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 15:289–291
Lorenz M, Hottenrot C, Seufert RM et al (1986) Continuous intravenous or intra-arterial administration via a subcutaneous implantable infusion chamber. Preliminary clinical experiences with particular reference to intra-arterial chemotherapy. Dtsch Med Wochensch 111:772–779
Onogawa S, Ito K, Nishimura K et al (1997) Liver metastases from breast cancer 14 years after radical mastectomy; successful treatment with hepatic arterial infusion chemotherapy and systemic endocrine therapy—a case report. Gan To Kagaku Ryoho 24:1804–1808
Overbosch EH, Kuijpers TJ (1986) Hepatic artery bolus infusion chemotherapy with mitomycin C. Angiographic results and complications. Diagn Imaging Clin Med 55:276–281
Tada A, Ogawa M, Inagaki J et al (1986) Arterial infusion of combination chemotherapy consisting of adriamycin and mitomycin C for liver metastases of breast cancer. Gan To Kagaku Ryoho 13:70–74
Tokura H, Ikeda T, Kitajima M (2002) Intra-arterial infusion chemotherapy for breast cancer. Gan To Kagaku Ryoho 29:176–181
Bangash AK, Atassi B, Kaklamani V et al (2007) 90Y Radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol 18:621–628
Oldhafer KJ, Frerker MK, Lang H et al (1998) High-dose mitomycin C in isolated hyperthermic liver perfusion for unresectable liver metastases. J Invest Surg 11:393–400
Verweij J, Pindeo HM (1990) Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs 1:5–13
Author information
Authors and Affiliations
Corresponding author
Additional information
Toon Maes and Hans Wildiers contributed equally to this work
Rights and permissions
About this article
Cite this article
Maes, T., Wildiers, H., Heye, S. et al. Intra-hepatic Mitomycin C bolus infusion in the treatment of extensive liver metastases of breast cancer. Breast Cancer Res Treat 110, 135–142 (2008). https://doi.org/10.1007/s10549-007-9707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9707-4