Abstract
Objective
We prospectively evaluated the association between average 10-year use of NSAIDs and invasive breast cancer.
Methods
Between 2000–2002, 35,323 postmenopausal women participating in the Vitamins And Lifestyle (VITAL) study provided detailed information regarding NSAID use, lifestyle and breast cancer risk factors. Using a Cox proportional hazards model, we analyzed associations between NSAID use and incident breast cancer (N = 482) ascertained through linkage to the SEER cancer registry.
Results
Use of low-dose aspirin at 4+ days/week over ten years was associated with a decreased risk of breast cancer (HR 0.65, confidence interval [CI] 0.43–0.97) versus no use, as was moderate use of other types of NSAIDs (HR 0.78, CI 0.61–0.98) for 10-yr average use up to 3 days/week. However, more frequent use of NSAIDs other than low–dose aspirin was associated with an increased risk (HR 1.26, CI 0.96–1.65), particularly frequent use of regular or extra strength aspirin (HR 1.43, CI 1.02–2.00).
Conclusions
We did not find evidence of a global protective effect of NSAID use for the development of breast cancer. However, long-term moderate use (frequent use of low doses or moderate frequency of high doses) was associated with reduced risk, while frequent use of higher dose products was associated with increased risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are thought to interfere with breast carcinogenesis via multiple mechanisms, many of which appear to be related to the inhibition of the cyclooxygenase 1 and 2 enzymes (COX 1 and 2) [1]. COX 1 and 2, which generate inflammatory prostaglandins from arachidonic acid, have been found to be over-expressed in breast cancer tissue when compared to normal tissue [2]. Inhibiting these enzymes may impact breast carcinogenesis via multiple pathways including interference with DNA adduct formation [3], angiogenesis [4] and aromatase production [5] and via increasing apoptosis [6].
Epidemiologic studies and randomized trials support NSAIDs as chemopreventive agents for colorectal cancer and pre-cancerous polyps [7, 8] but studies of the associations between NSAIDs and breast cancer risk are far less consistent. Several cohort studies have found a reduced risk of incident breast cancer associated with aspirin use [9–12] with statistically significant risk reductions ranging from 19% to 30%. Other studies have not confirmed this association [13, 14]. Associations between non-aspirin NSAIDs and breast cancer have been similarly inconsistent, with some cohort studies suggesting reduced risk [10, 12], some reporting no association [11, 13, 15] and still others suggesting the possibility of an increased risk with the use of these medications [14, 15]. A randomized controlled trial of low-dose aspirin administered every other day for an average follow-up of 10.1 years was not associated with a reduced risk of breast cancer (RR = 0.98, confidence interval [CI] 0.87–1.09) [16].
There are stark differences in the quality and completeness of data on NSAID use between cohort studies. Some studies collected only frequency of use (days/week), others only duration of use (years), and still others only use (yes/no) at baseline. Few of the studies differentiated between low-dose aspirin and regular-strength aspirin, or collected data on all types of non-aspirin NSAIDs. It may be that any association between NSAID use and breast cancer varies by NSAID type, dose, frequency and duration of use, which may have been incompletely characterized in many of the previous studies.
We prospectively evaluated the association between NSAID use and breast cancer incidence using detailed data regarding NSAID type (including separate assessment of low-dose versus regular strength aspirin), and frequency and duration of use over the 10 years preceding baseline. We evaluated whether any NSAID-breast cancer associations were modified by characteristics of the participants such as BMI, the presence of inflammatory conditions, or use of hormone replacement therapy (HRT) because few prior studies have assessed such effect modification [12, 15]. We also examined risk differences by tumor subtypes (tumor size, stage or estrogen receptor status) since prior studies have led to conflicting findings on these associations [11, 14, 17]. Identification of a reduction in risk for breast cancer with NSAID use, either across the entire population or within defined sub-groups could present a much-needed avenue for chemoprevention.
Materials and methods
The VITAL cohort
Study participants are members of the VITamins And Lifestyle (VITAL) cohort, a group of men and women followed prospectively for incident cancer. The goal of the VITAL Study is to examine the relationship between nutrient supplements and other over-the-counter medications with cancer risk. Further details of the VITAL study design are provided in White et al. [18]. Women aged 50–76 were eligible to participate if they lived in the thirteen county area of western Washington State covered by the Surveillance, Epidemiology and End Results (SEER) cancer registry. Recruitment, which was conducted from October 2000 to December 2002, was initiated via a mass mailing using names from a commercial mailing list. We mailed 168,953 baseline questionnaires to women, followed by a post-card reminder 2 weeks later. Among eligible women, the response rate was 25.6%, for a total of 40,339 participants.
We excluded subjects with a self-reported history of breast cancer at baseline (3078), those who failed to answer the question regarding breast cancer history (82) or who did not complete the questionnaire page pertaining to NSAID use (368), pre-menopausal women (1,356), four women who were subsequently diagnosed with breast cancer histology of sarcoma, phyllodes and lymphoma, and cases with in situ disease (128). After exclusions, our cohort was comprised of 35,323 women.
Baseline data collection
Detailed information regarding the use of non-steroidal anti-inflammatory drugs (NSAIDs), breast cancer risk factors, personal and family medical history and diet and supplement use was elicited through a 24-page self-administered questionnaire at baseline. Subjects were asked whether they had taken any of the following medications at least once per week for a year over the previous ten years: low-dose aspirin (81 mg), regular or extra-strength aspirin, ibuprofen, naproxen, celecoxib or rofecoxib, other pain relievers (piroxicam or indomethacin) and acetaminophen. We included acetaminophen to examine possible biases in our study (such as confounding by indication and medical surveillance biases) because acetaminophen has no systemic anti-inflammatory effect and there is no biological basis to expect an association with breast cancer. For each type of drug, the most common brand names were given as examples, including both over-the-counter and prescription brands. We also asked participants about both the frequency (days/week) and duration (years) of use within the ten years preceding baseline for each medication.
We used data from the baseline questionnaire to develop three types of exposure variables for each NSAID medication. First, we defined “any use” as at least once a week for a year during the last ten years. Second, we examined duration of use, defined in the questionnaire as 1–3 years, 4–8 years and 9–10 years. Third, as a measure of cumulative use, we computed average days per week of use during the past ten years as follows: we multiplied the reported days per week of use (1–3, 4–6, 7) by years of use and divided this product by ten. Subjects could only fall into the highest category of ten-year average use if they reported long-term use. In addition to analyzing each NSAID medication separately, we also developed two variables measuring use of combined NSAIDs. The first combines all NSAIDs except low-dose aspirin and the second combines all non-aspirin NSAIDs.
Subjects also identified the presence of health conditions that may be indications for the use of NSAIDs including chronic neck, back or joint pain, within the year prior to baseline and physician-diagnosed arthritis, rheumatoid arthritis or coronary artery disease (defined as history of heart attack, coronary bypass surgery, angioplasty and/or physician-diagnosed angina).
Other information collected at baseline and considered in this analysis included factors known or suspected to be associated with breast cancer, such as: race, education, age at menarche, age at first birth, age at menopause, history of surgical menopause, family history of breast cancer, history of mammography within the two years preceding baseline, history of breast biopsy, years of combined estrogen and progesterone use and multi-vitamin use. Body mass index (BMI) was computed as weight in kg/height in meters2 and physical activity over the preceding ten years was computed as Met-hours per week based on a one-page questionnaire previously described [19]. Alcohol use over the preceding year was calculated from separate questions from the baseline food frequency questionnaire on intake of white wine, red wine, beer, liquor and mixed drinks.
Case ascertainment
Incident breast cancer cases were ascertained through annual linkage of the VITAL cohort database to the SEER cancer registry, which is maintained by the Fred Hutchinson Cancer Research Center. All cases of cancer (except non-melanoma skin cancer) diagnosed within the 13-county area of western Washington State covered by the SEER cancer registry are reported to SEER along with data regarding stage and other tumor characteristics. Cases are ascertained through all area hospitals, offices of pathologists, oncologists, and radiotherapists, and from state death certificates, with extensive quality control procedures to ensure that registry data are accurate and complete. Linkage to SEER is based on ranking of the agreement between variables common to VITAL and SEER (name, social security number, date of birth, address, etc) with automated matching for those with high concordance and visual review of matches that are concordant on some items.
Among our cohort, 482 eligible cases of invasive breast cancer were diagnosed between October 2000 and December 2004. Subjects were censored at the earliest of the following events: the date of death (2.0%), the date the participant moved out of the SEER catchment area (3.6%), the date they requested removal from the study (0.04%) or December 31, 2004, the most recent date that endpoints were ascertained (93% non-cases). Deaths were ascertained by linkage to the Washington State death files, using similar procedures to the SEER linkage. Moves out of the area were identified by linkage to the National Change of Address System and by follow-up of possible moves with letters and phone calls to participants or a contact person identified by participants at baseline.
Statistical analyses
We used unconditional logistic regression to estimate the odds ratio for the association between medical/lifestyle characteristics and use of any NSAID (excluding low-dose aspirin), adjusted for age as a continuous variable. We examined the association between NSAID exposure variables and incident invasive primary breast cancer using a Cox proportional hazards model with age as the timeline. Multivariate models included adjustment for race, body mass index (BMI), family history of breast cancer, history of breast biopsy, history of mammogram in the two years prior to baseline, age at menarche, age at first birth, age at menopause, history of surgical menopause, years of combined estrogen and progesterone hormone therapy, multivitamin use and alcohol use. In the analysis of each NSAID drug or group, we also adjusted for the use of the other NSAID medications. A test for trend across levels of NSAID use was computed as the significance of a single linear “trend” variable.
We also examined the association between NSAID use and breast cancer incidence in models stratified by the following factors: by BMI (<25, 25 ≤ 30, 30 + kg/m2), presence of inflammatory conditions (defined as any of the following: arthritis, rheumatoid arthritis, chronic pain and coronary artery disease) or use of combined hormone replacement therapy (<1, 1–9, 10 + years). We evaluated the significance of the interaction between NSAID use and the above factors using the likelihood ratio test comparing Cox models with and without the interaction terms. We evaluated whether there was a differential association between NSAID use and breast cancer incidence among case subgroups defined by various prognostic factors such as stage (local versus regional/distant), tumor size (≤ 2 cm vs. > 2 cm) and estrogen receptor (ER) status (positive versus negative) using the P-value of the NSAID variable in a model comparing the subgroups. All statistical analyses were performed using STATA SE version 9.0.
Results
The average age of participants was 62 years. The majority of participants had less than a college degree (65%), were Caucasian (91%) and had no first degree relative with breast cancer (84%). Most participants were overweight or obese (55%), had never smoked (55%) and consumed less than one alcoholic beverage per day (75%). The majority had had a mammogram within the two years preceding baseline (90%) and had used combined hormone replacement therapy for less than a year or not at all (59%).
Compared to women who did not use NSAIDs, women who used NSAIDs were less likely to have a college degree and to be black or Asian (Table 1). NSAID users were more likely to have had a recent mammogram and a breast biopsy before baseline, to have been younger at menarche, first birth and menopause, to have had a surgical menopause and to take hormone replacement therapy. As expected, NSAID use was associated with a history of chronic pain, arthritis, rheumatoid arthritis and coronary artery disease (CAD). NSAID users were also more likely to be overweight or obese, current or former smokers, take multivitamins and drink three or more alcoholic beverages per day.
The associations between NSAID use and incidence of primary invasive breast cancer are summarized in Table 2. Our multivariate analysis includes adjustment for factors known or suspected to be associated with breast cancer including: race, family history of breast cancer, mammography within 2 years prior to baseline, history of breast biopsy, age at menarche, age at first birth, age at menopause, history of surgical menopause, years of combined estrogen and progesterone hormone therapy, BMI, multivitamin use and alcohol use. Age was adjusted for in the timeline and we also adjusted for the use of other NSAIDs categories.
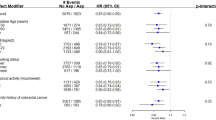
Overall, we found no consistent protective effect of NSAIDs on breast cancer risk. We did, however, observe a statistically significant decreased risk of breast cancer associated with low-dose aspirin at an average of 4 or more days/week over 10 years (HR 0.65, CI 0.43–0.97). Use of all NSAIDs (excluding low-dose aspirin) at a moderate level (≤ 1–3 days/week over 10 years) was also associated with a statistically significant decreased risk of breast cancer (HR 0.78, CI 0.61–0.98), whereas the highest category of use (4 or more days/week over 10 years) was associated with a borderline significant increased risk (HR 1.26, CI 0.96–1.65). This U-shaped pattern held for both regular/extra-strength aspirin, where moderate use was associated with a decreased risk (HR 0.76, CI 0.55–1.05), and higher use of least 4 days per week was associated with an increased risk (HR 1.43, CI 1.02–2.00) and for non-aspirin NSAID use, although not statistically significant, where moderate use was associated with a decreased risk (HR 0.85, CI 0.67–1.07) and higher use an increased risk (HR 1.28, CI 0.91–1.80). Among the individual non-aspirin NSAIDs, the pattern held for naproxen (moderate use HR 0.67, CI 0.44–1.02 and higher use HR 1.72, CI 0.93–3.16) but not ibuprofen. We found no significant associations between acetaminophen, the Cox-2 inhibitors or other NSAIDs (piroxicam and indomethacin) and breast cancer risk (data not shown).
We evaluated whether any association between NSAID use and breast cancer varied by BMI, history of inflammatory conditions or use of combined hormone therapy. There were no significant interactions between BMI and use of baby aspirin, regular/extra-strength aspirin or non-aspirin NSAIDs (P for interaction 0.69, 0.59, 0.33, respectively). Nor did we observe an interaction between the same NSAID categories and a history of inflammatory conditions (P for interaction 0.37, 0.84, 0.69) (data not shown). In evaluating effect modification by combined HRT use (Table 3), we found the increased risk of breast cancer with higher use of regular/extra-strength aspirin use confined to those who used HRT for 10 or more years (HR 2.38, CI 1.30–4.34, P for trend < 0.01). The overall interaction between HRT use and regular aspirin was also statistically significant (P = 0.01). We evaluated whether any association between NSAIDs and breast cancer incidence varied by tumor size, stage of disease or estrogen receptor status. Overall, the P for difference comparing these case groups failed to reach significance for any exposure (data not shown).
Discussion
We found no support for a global protective effect of NSAIDs on breast cancer risk. Instead, we found some evidence for a U shaped dose–response. We observed two independent, statistically significant results for a reduced risk associated with moderate use of NSAIDs (for frequent use of low-dose aspirin and moderate use of other types of NSAIDs). We also observed two independent results that suggest that high use of NSAIDs is associated with increased risk (for frequent use of regular or extra strength aspirin and of non-aspirin NSAIDs), although only one was statistically significant.
In regard to our finding that non-aspirin NSAID use at an average of 4 or more times weekly over the previous 10 years was associated with a non-statistically significant increased risk of breast cancer (HR 1.28, CI 0.91–1.80), past studies on this topic are not consistent, in part because some only characterized NSAID use by frequency irrespective of years of use and some only by duration of use. Across our NSAID categories, we found associations only with our 10-year average use variables, which combine information on both frequency and duration, and it may be that the combination of both is important. Using ten-year average exposure, in which subjects could only fall into the highest category of ten-year average use if they reported long-term use, avoids the problem of some cohort studies in which only recent use (which is not likely to reflect a biologically plausible association) was ascertained. Two previous studies with detailed data on frequency and duration of NSAID use comparable to ours found an increased risk of breast cancer among long-term frequent users of ibuprofen, the most commonly used non-aspirin NSAID in the US [14, 15]. In the California Teachers study, Marshall and colleagues found a significant increase in breast cancer risk among long-term daily users of ibuprofen (HR 1.51, CI 1.17–1.95), while Jacobs and colleagues found a non-significant increased risk among regular users (≥ 30 pills/month for at least 5 years) of ibuprofen (HR 1.29, CI 0.92–1.82). Interestingly, we found no increased risk with ibuprofen specifically, but did observe this association with each of the following categories: all NSAIDs excluding low-dose aspirin (1.26, CI 0.96–1.65), all non-aspirin NSAIDs (as above) and naproxen (HR 1.72, CI 0.93–3.16).
Similar to our results for non-aspirin NSAIDs, we also found regular or extra-strength aspirin use at an average of 4 or more time a week over 10 years was associated with an increased risk of breast cancer (HR 1.43, CI 1.02–2.00). This is in contrast to previous cohort studies that have reported either a reduced risk of breast cancer among aspirin users or no association. None of the cohort studies that reported reduced breast cancer risk with aspirin use, however, collected detailed data on NSAID use reflecting long-term cumulative exposure (both frequency and duration of use). For example, one study categorized participants as “regular users” if they had used the medication twice in the two weeks preceding baseline and then looked only at years of use [12] while the two others only collected data on current use at baseline [9, 11]. Of the studies that found no association between aspirin use and breast cancer risk, two did not differentiate between low versus high dose aspirin [13, 14] and one combined the doses by calculating a low-dose aspirin as a 1/4 regular aspirin [15], leaving the possibility that different doses of aspirin may confer different effects, which is what we found in our study.
We found a reduced risk of breast cancer associated with low-dose aspirin use of an average of 4 or more times per week over ten years (HR 0.65, CI 0.43–0.97). It is unclear whether this may simply be due to a healthy user effect. To our knowledge, we are the first to report this association between low-dose aspirin and breast cancer risk reduction. However, a randomized controlled trial found no effect of low-dose aspirin every other day on breast cancer risk compared to placebo (RR 0.98, CI 0.87–1.09) [16]. This level of usage over an average follow-up time of 10.1 years (with compliance defined as taking two-thirds of the medication and an average compliance rate of 67% at ten years) would correspond to our category of use of ≤ 1–3 days/week over ten years. We also found no risk reduction at this level of usage (RR1.23, CI 0.96–1.59). It is possible that the lack of effect in this clinical trial was due to the low frequency of use.
Consistent with our finding of a protective effect of low-dose aspirin, we found that moderate use (an average of 3 days /week or less over ten years) of NSAIDs (for all NSAIDs, regular/extra-strength aspirin, non-aspirin NSAIDs and naproxen) was also associated with a reduced risk of breast cancer. It is not clear why high doses of aspirin or other NSAIDs may confer an increased risk of breast cancer and lower doses a decreased risk. One possible explanation is that those who use high levels of these medications are treating medical conditions that respond to NSAIDs, and that these conditions may be associated with breast cancer risk. However, we found no association between a history of inflammatory conditions and breast cancer risk in this cohort (data not shown).
Another explanation is that NSAID dose may be important in relation to cancer risk. Support for this comes from a randomized controlled trial comparing low-dose aspirin and regular strength aspirin to placebo, which found a protective effect only in the low-dose group for the development of recurrent adenomatous polyps (RR 0.81, CI 0.69–0.96) and advanced colorectal neoplasms (RR 0.59, CI 0.38–0.92), while no effect was seen with regular strength aspirin [20].
It has been hypothesized that NSAIDs may have differing effects on breast cancer risk among subgroups with different hormone receptor status. Aspirin and other NSAIDs impair aromatase activity, an enzyme responsible for synthesis of estrogen from androgen precursors within the breast tissue [5]. It is therefore plausible that NSAIDs may be more protective against the development of estrogen responsive (ER+) tumors compared to estrogen non-responsive (ER–) tumors. Although at least one cohort and one case-control study have suggested reduced risk of only ER+ breast cancer among aspirin users [14, 17], we found no evidence for an interaction between use of any NSAID and ER status of the tumors in our cohort.
Long-term combined (estrogen + progestin) HRT use is a well-established risk factor for breast cancer [21] and any interaction between HRT use and NSAID use on breast cancer risk would be of public health importance. To our knowledge only two cohort studies have evaluated the interaction between NSAIDs and HRT use on breast cancer risk, and both have found no interaction [12, 15]. Although we found no significant interaction between low-dose aspirin or non-aspirin NSAIDs and breast cancer by HRT use, we did find that the increased risk of breast cancer among those with the highest level of use of regular or extra-strength aspirin was confined to those who also used combined HRT for 10 or more years and this interaction was statistically significant.
The strengths of this study include its prospective design, large cohort size and detailed information regarding use of NSAIDs including type, frequency of use (days/week) and duration (years of use). Limitations of this study include a lack of data on number of pills per day taken by participants and the fairly small number of cases, which may limit the stability of our statistical analyses. Also, as with any study in which exposures are based on self-report, one must acknowledge the possibility of misclassification of NSAID use, however, this misclassification would likely not differ by case status and would therefore bias the results toward the null.
Another possible limitation is uncontrolled confounding. Specifically, one explanation for our finding of a reduced risk of breast cancer with low-dose aspirin is that the association may be due to hidden confounding. Users of low-dose aspirin may be more health-conscious than non-users, and though we attempted to account for this possibility by adjusting for BMI, history of recent mammography and multi-vitamin use, it is possible that confounding by other factors remains.
A definitive answer as to the association between NSAIDs and breast cancer risk remains elusive. Our findings of a reduced risk of breast cancer associated with moderate use of NSAIDs (frequent use of low doses or moderate frequency of high doses) and increased risk associated with higher use of NSAIDs would need to be reproduced in other studies with information on dose, frequency and duration of use. It may be that type, dose and frequency of use are all important in any potential effects of NSAIDs on breast cancer risk.
References
Davies GL, Martin LA, Sacks N, Dowsett M (2002) Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol 13(5):669–678
Hwang D, Scollard D, Byrne J, Levine E (1998) Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cells. J Natl Cancer Inst 90(6):455–460
Abbadessa G, Spaccamiglio A, Sartori ML et al (2006) The aspirin metabolite, salicylate, inhibits 7, 12-dimethylbenz(a)anthracene-DNA adduct formation in breast cancer cells. Int J Oncol 28(5):1131–1140
Masferrer JL, Leahy KM, Koki AT et al (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60:1306–1311
Diaz-Cruz ES, Shapiro CL, Bruggemeier RW (2005) Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 90(5):2563–2570
Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL (2002) Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res 62(3):625–631
Asano TK, McLeod RS (2006) Nonsteroidal anti-inflammatory drugs (NSAID) and aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2
Huls G, Koornstra JJ, Kleibeuker JH (2003) Non-steroidal anti-inflammatory drugs and molecular carcinogenesis of colorectal carcinomas. Lancet 362(9379):230–232
Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5(2):133–135
Harris RE, Kasbari S, Farra W (1999) Prospective study of nonsteroidal anti-inflammatory drugs and breast cancer. Oncol Rep 6:71–73
Johnson TW, Anderson KE, Lazovich D, Folsom AR (2002) Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. CEBP 11:1586–1591
Harris RE, Chlebowski RT, Jackson RD et al (2003) Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res 63:6096–6101
Egan KM, Stampfer MJ, Giovannuccci E, Rosner BA, Colditz GA (1996) Prospective study of regular aspirin use and the risk of breast cancer. JNCI 88(14):988–942
Marshall SF, Bernstein L, Anton-Culver H et al (2005) Nonsteroidal anti-inflammatory drugs use and breast cancer risk by stage and hormone receptor status. J NCI 97(11):805–812
Jacobs EJ, Thun MJ, Connell CJ et al (2005) Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. CEBP 14(1):261–264
Cook NR, Lee IM, Gaziano JM et al (2005) Low-dose aspirin in the primary prevention of cancer: The Women’s Health Study: a randomized controlled trial. JAMA 294(1):105–106
Terry MB, Gammon MD, Zhang FF et al (2004) Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA 291(20):2433–2440
White E, Patterson RE, Krystal AR et al (2004) VITamins And Lifestyle Cohort study: study design and characteristics of supplement users. Am J Epidemiol 159(1):83–93
Littman AJ, White E, Kristal AR, Patterson RE, Satia-About a J, Potter JD (2004) Assessment of a one-page questionnaire on long-term recreational physical activity. Epidemiology 15(1):105–113
Baron JA, Cole BF, Sandler RS et al (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348(10):891–899
Roussouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333
Acknowledgements
This publication was made possible by grant CA74846 from the National Cancer Institute and T32-AT00815 from the National Center for Complementary and Alternative Medicine (NCCAM). The authors wish to acknowledge Alyson Littman for her guidance on statistical analysis, Alan Kristal for his useful input on analysis and interpretation and Ilonka Evans for her data management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ready, A., Velicer, C.M., McTiernan, A. et al. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat 109, 533–543 (2008). https://doi.org/10.1007/s10549-007-9665-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9665-x