Abstract
In postmenopausal women, levels of estrogens, androgens, and perhaps prolactin have been related to risk of breast and other hormonal cancers in women. However, the determinants of these hormone concentrations have not been firmly established. Associations among various demographic, menstrual, and reproductive factors, medication use and endogenous sex hormone concentrations (estradiol, free estradiol, estrone, estrone sulfate, testosterone, free testosterone, sex hormone binding globulin, androstenedione, dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), dihydrotestosterone, and prolactin) were evaluated in a cross-sectional analysis from a simple random sample of 274 postmenopausal women selected from the Women’s Health Initiative Dietary Modification Trial. In multiple regression analyses on log-transformed hormones, the concentrations of DHEA, and DHEAS were negatively and statistically significantly associated with age (both β = −0.03, P < 0.001, respectively). Estradiol, estrone, DHEA, and free testosterone concentrations were higher in African-American than in non-Hispanic White women, but after multivariate adjustment the associations were statistically significant only for free testosterone (β = 0.38, P = 0.01). Women who had a history of bilateral oophorectomy had a mean 35% lower testosterone concentration compared with women with at least one ovary remaining (β = −0.43, P = 0.002), and lower free testosterone (β = −0.42, P = 0.04) after multivariate adjustment. Women who reported regular use of NSAIDs had higher DHEA concentrations (β = 0.20, P = 0.04) and lower prolactin concentrations (β = −0.18, P = 0.02) compared with non-users. These results suggest that while age, oophorectomy status, and NSAID use may be associated with selected sex hormone concentrations, few menstrual or reproductive factors affect endogenous sex hormones in the postmenopausal period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
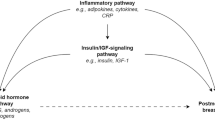
Several demographic, menstrual, and reproductive factors are associated with risk for breast, endometrial, and other hormone-related cancers [1, 2], and one mechanism that may explain these associations with risk later in life may be through their effects on endogenous sex hormone concentrations [3, 4]. Some [5–7], but not other [8–10] studies have found an association between use of nonsteroidal anti-inflammatory drugs (NSAIDs) and reduced risk for breast cancer, but if an association exists there is little information regarding possible explanatory mechanisms.
A recent combined analysis of nested case-control data from nine cohort studies, with data from 663 breast cancer cases and 1,765 women without breast cancer, showed that postmenopausal women with elevated estrogen or androgen levels have increased risk for developing breast cancer [11]. Women with blood hormone concentrations in the top quintile of distribution for estradiol, free estradiol, testosterone, androstenedione, dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) were approximately twice as likely to develop breast cancer compared to women with serum hormones in the bottom quintile, and the trends were highly statistically significant. Some [12, 13], but not other [14] studies have found that prolactin concentrations were associated with increased breast cancer risk. Elevated sex hormone concentrations have also been linked to increased endometrial cancer risk [15].
The determinants of elevated estrogens, androgens, and prolactin in postmenopausal women are not known, although several reports have found an association of elevated adiposity and lower physical activity with increased concentrations of estrone, estradiol, free estradiol, testosterone, and free testosterone [16–19].
After the menopause, the ovarian production of estrogen and progesterone ceases, while the production of various androgens continues. Most women continue to have detectable concentrations of circulating estrogen after the menopause, the most prevalent being estrone which is produced predominantly through peripheral conversion of adrenal androstenedione in adipose tissue [20, 21]. Estradiol, the most metabolically active of the estrogens, is produced in postmenopausal women through the reduction of estrone and through the aromatization of testosterone [22]. Testosterone is produced in postmenopausal women in the ovaries and adrenals; women who have had bilateral oophorectomies have lower concentrations of testosterone compared with women with intact ovaries [22]. Only a small fraction of circulating estradiol exists in the “free” or unbound (thought to be biologically and metabolically active) state (2–3% of total); the remainder is bound to either sex-hormone binding globulin (SHBG) (55%) or albumin (45%) [23]. The free and albumin-bound fraction is termed “bioavailable” estradiol.
Several menstrual and reproductive factors are associated with increased risk for breast cancer, including early menarche (before age 12), late menopause (after age 55), nulliparity, delayed childbirth (after age 30), and lack of lactation [2]. Evidence suggests that the associations between these risk factors and breast cancer risk may in part be mediated by lifetime exposure to endogenous sex hormones. It is not clear if variability in these factors is associated with postmenopausal hormone concentrations, however. In addition, the role of these variables has not been defined separately for Non-Hispanic White and African-American women.
In a sub-sample of postmenopausal women randomly selected from the Women’s Health Initiative Dietary Modification clinical trial, we assayed several sex hormones including: estradiol, free estradiol, bioavailable estradiol, estrone, estrone sulfate, testosterone, free testosterone, SHBG, androstenedione, DHEA, DHEAS, dihydrotestosterone (DHT), and prolactin. The purpose of this study was to investigate the associations among various demographic, menstrual, reproductive factors, and NSAIDs use, and endogenous sex hormone concentrations in a cross-sectional analysis.
Materials and methods
Study population
The Women’s Health Initiative (WHI) Dietary Modification Trial from which study subjects were selected, was a multi-center, multi-ethnic clinical trial testing the effect of a low-fat dietary pattern on risk of breast cancer and other chronic diseases in postmenopausal women [24–26]. Women were enrolled into the study between October 1993 and December 1998 through 40 clinical centers across the United States. Women were eligible for the WHI Dietary Modification Trial if they were aged 50–79 years, planned to live in the clinical center area for at least three years, and were consuming a diet that consisted of at least 32% of calories from fat, as measured by a 120-item food frequency questionnaire [27]. Exclusion criteria for the Dietary Modification Trial included eating 10 or more meals per week outside the home, previous diagnosis of breast or colon cancer, type 1 diabetes, gastrointestinal conditions that prohibited a high-fiber diet, or any serious health conditions that might reduce survival over three years (e.g. class IV congestive heart failure). Details of the scientific rationale, design, eligibility requirements, and baseline characteristics of the WHI cohort have been published elsewhere [25, 26, 28]. The Institutional Review Boards at all participating institutions including the coordinating center, subcontractors, and clinical centers approved study protocols and procedures. All participants signed informed consent forms.
Sample selection
A subset of 300 women was selected to assess sex hormones. The simple random sample was chosen from Diet Modification trial participants who were (1) not in the WHI Hormone trials, (2) not part of another blood specimen subsample, and (3) not taking menopausal hormone therapy at study enrollment. The sampling probability was stratified by clinical center and age. Women were also excluded from the present analyses if they reported using menopausal hormone therapy within three months prior to blood draw, or were missing data for the specific blood hormone being assessed.
Exposure assessment
All exposure information in this analysis was collected when women entered the study. A standardized written protocol, centralized training of clinic staff, and periodic quality assurance visits by the coordinating center were used to assure uniform administration of data collection instruments.
At a required baseline screening clinic visit, participants completed several self-administered questionnaires which addressed medical history, reproductive and menstrual history, health behavior including physical activity and diet, and family history of select diseases including breast cancer. By self-report, women identified their ethnicity/race, selecting from six offered categories: American Indian/Alaskan Native; Asian/Pacific Islander; African-American; Hispanic; Non-Hispanic White; and unknown. Staff interviewed participants regarding lifetime use of menopausal hormone therapy. Participants were asked to bring all prescription and regularly used (two or more times per week) over-the-counter medications to their clinic visit, which were then entered into a medications database. In addition, participants were queried about use of NSAIDs specifically, including type and dose (but not number of doses per day), and duration of use. Reproducibility of WHI questionnaire data has been evaluated in a random sample of 536 women who had health-related information collected a second time, approximately ten weeks after baseline [28]. The test-retest reliability (weighted kappas) for the various demographic, reproductive, and menstrual variables included in the present analyses ranged from 0.77 to 0.99.
Diet, physical activity, and anthropometric variables were included as covariates, as they have been reported to be associated with sex hormone concentrations [16–18]; more detailed analyses of these data have been reported elsewhere [19]. Staff performed anthropometric measures (height, weight, waist circumference). Body Mass Index (BMI) was calculated as weight (kg)/height (m) [2].
Participants provided a blood sample after a 12-h fast on the same clinic visit as questionnaires were returned. Participants were asked not to take aspirin or NSAIDs for 48 h before the blood draw, to refrain from smoking for at least one hour before the draw, and not to perform any vigorous physical activity for at least 12 h prior to blood draw. Blood was processed within two hours and serum was aliquoted and stored at −70°C.
Age at menopause was determined as the youngest age at which the participant experienced any of the following: last menstrual bleeding (all participants were 12 or more months post last menstrual period), removal of both ovaries, or starting menopausal hormone therapy. Age at first birth was calculated as the age at first pregnancy of six months duration or greater. Women were classified as NSAID users if they reported using any of aspirin, ibuprofen, or naprosyn at least twice per week for at least two weeks at study entry. Total daily kilocalorie intake and percentage of kilocalories from fat (covariates) were assessed from the food frequency questionnaire. Total physical activity (covariate) was determined from self-reported frequency, duration, and intensity of walking and recreational activity [29].
Hormone assays
The following assays were used to quantify estrogens and androgens. Assays were performed at Esoterix Laboratory Services Inc. (Calabasas Hills, CA) between January of 2001 and January of 2003. Placement of samples into batches was randomly ordered. In addition, each batch included split duplicates and pooled quality control samples.
SHBG was measured using an immunoradiometric (IRMA) assay using plastic beads coated with monoclonal antibody against human SHBG and a I125-labeled soluble antibody against humans SHBG. The assay sensitivity was 0.1 μg/dl. Intra- and inter-batch percent coefficients of variation (%CV) were 5.7 and 17.7, respectively.
Estradiol and estrone were measured by radioimmunoassay after organic extraction with hexane:ethyl acetate and LH20 column chromatography. Minimum reportable level was 0.5 ng/dl for both estrogens. Intra- and inter-batch %CV were 7.6 and 18.9 for estradiol, and 7.3 and 9.9 for estrone, respectively. For estrone sulfate, serum samples were extracted with hexane:ethyl acetate to remove unconjugated estrone, and digested overnight with sulfatase to remove the sulphate group. The resulting estrone was then extracted with hexane: ethyl acetate, chromatographed on LH-20 columns and quantitated by an RIA using an antiserum against estrone-6-oxime albumin conjugate. The minimal detectable level was 10.0 ng/dl. Intra- and inter-batch %CV were 27.2 and 36.8, respectively.
Testosterone was measured by radioimmunoassay after extraction with hexane:ethyl acetate and aluminum oxide column chromatography. Sensitivity of the method was 3 ng/dl. Intra- and inter-batch %CV were 8.9 and 19.1, respectively. Dihydrotestosterone (DHT) was measured by radioimmunoassay after extraction and column chromatography. Sensitivity of the method was 2 ng/dl. Intra- and inter-batch coefficients of variability were 20.7 % and 51.5 %, respectively. Androstenedione and DHEA were measured by radioimmunoassay after extraction with hexane:ethyl acetate. Sensitivity of the method was 10 ng/dl and 20 ng/dl, respectively. Intra- and inter-batch %CV were 6.2 and 11.1 for androstenedione and 7.5 and 20.1 for DHEA respectively. DHEAS was measured as DHEA by radioimmunoassay after enzymolysis of the DHEAS. The assay sensitivity was 10 ug/d. Intra- and inter-batch %CV were 6.6 and 25.2, respectively.
Prolactin was measured by a double-antibody chemiluminescent sandwich method. All samples were analyzed at two doses to avoid false negatives caused by the high dose hook effect. The assay sensitivity was 0.2 ng/ml. Intra- and inter-batch %CV were 7.7 and 18.9, respectively.
Free estradiol and free testosterone were calculated using the measured estradiol, testosterone, albumin, and SHBG concentrations [30]. This method has been found to have high validity compared with direct measurement [31].
Statistical analyses
The present analyses included baseline data from study entry only (collected prior to randomization). Analyses were limited to women for whom data for all variables of interest were available (complete-case analysis). The analysis was performed using SAS version 9 (SAS institute, Cary, NC). Hormone concentrations were log-tranformed to reduce the positive skewness of the distributions. Associations between hormone concentrations and other variables were assessed using Pearson and partial correlation coefficients. Geometric means and 95% confidence intervals (back transformed to the original scale) were calculated. Multiple regression models were used to evaluate the relationship between hormone assays and demographic, menstrual, reproductive and NSAIDs variables including age, race/ethnicity, smoking, age at menarche, years since menopause, number of term pregnancies, age at first pregnancy, age at last menstrual bleeding, NSAID use, hysterectomy at randomization, prior bilateral oophorectomy, and, as covariates, BMI, daily kilocalorie intake, percent calories from fat, and physical activity (total energy expenditure). All explanatory variables were evaluated simultaneously in the multiple regression models, and models were run with all hormones included. All P-values presented are two-sided. Due to the number of comparisons in these analyses, some results will be significant by chance.
Due to the small numbers in individual groups, the only possible comparisons between racial/ethnic groups was between African-American and Non-Hispanic White women.
Results
A total of 274 women were not using menopausal hormone therapy at the time of blood draw, and had data available for analyses. Seven women had estradiol concentrations greater than 30 pg/ml. Excluding these women did not change the results, and therefore their data are included in all analyses.
Baseline characteristics for the study group are presented in Table 1. Participants were a mean 65 years old. Their mean age at menopause was 49.2 years and on average they were 16 years post-menopause. Approximately 79% were non-Hispanic White and 15% were African-American. They were highly educated (77% had more than a high school degree). Approximately half had never smoked cigarettes, and only 3% were current smokers. Approximately two-thirds had never used oral contraceptives and most were parous. Twenty-four percent reported having had a hysterectomy, and 9% had a history of bilateral oophorectomy. Eighty-one percent had never used menopausal hormone therapy. Almost one third regularly used some form of NSAIDs, including aspirin, ibuprofen, naprosyn, and prescription NSAIDs.
As expected, the androgens and estrogens were highly inter-correlated, and all but one of the correlations were statistically significant (Table 2). Prolactin concentrations were statistically significantly (P < 0.05) and positively correlated with androstenedione, DHEAS, testosterone, free testosterone, estradiol, and estrone. SHBG concentrations were negatively and statistically significantly associated with DHEAS, free testosterone, estrone sulfate, and free estradiol, and positively associated with DHT. (Note that the association between SHBG and both free estradiol and free testosterone is expected, since we used SHBG concentration to calculate the free hormone levels).
Unadjusted geometric mean hormone concentrations are presented for categories of the variables of interest in Tables 3–6. African-American women had statistically significantly higher concentrations of estradiol, estrone, free estradiol, and free testosterone, compared with non-Hispanic White women. There were statistically significantly higher concentrations of estradiol and free estradiol in women whose first menstrual period was at age 12 or less compared with those with first menstrual period at an older age. Women with a history of bilateral oophorectomy had a statistically significantly 25%–33% lower concentrations of androgens compared with women with at least one intact ovary, but estrogen and prolactin concentrations did not vary markedly by ovarian status. SHBG concentrations were statistically significantly higher in women who were aged 70–79 years old compared with younger women. Results for DHT paralleled those for testosterone, and therefore are not shown. In an analysis classifying women according to both history of hysterectomy and of bilaterally oophorectomy (Table 7), women with a history of bilateral oophorectomy had lower concentrations of both testosterone and free testosterone compared with women with at least some ovary remaining, but hysterectomy history was not associated with concentrations of either of these hormones (p for interaction of hystertectomy vs. bilateral oophorectomy on hormone level 0.002 and 0.02, respectively).
Table 8 presents the results of multiple regression analyses of the associations between the demographic, reproductive, hormone use, and hormone assay variables. The concentrations of DHEA, and DHEAS were negatively and statistically significantly associated with age (both β = −0.03, P < 0.001). After multivariate adjustment, only free testosterone was statistically significantly higher in African-American compared with white women (β = 0.38, P = 0.01). Age at menarche and age at first term pregnancy were not consistently related to any hormone values. Compared to women whose age at last menstrual bleeding was 55 years or older, women whose age at last menstrual bleeding was 44 years or younger, had significantly lower concentrations of free testosterone. Prolactin levels increased with lower age at last menstrual bleeding, although only the comparison of women with last menstrual bleeding at age 55 or over vs. women with last menstrual between ages 45–54 years was statistically significant.
Women who had a history of bilateral oophorectomy had a mean 35% lower testosterone concentration compared with women with at least one ovary remaining (β = −0.43, P = 0.002), and lower free testosterone (β = −0.42, P = 0.04) after multivariate adjustment. Prior use of menopausal hormone therapy and oral contraceptives were not consistently associated with hormone concentrations. Women who reported regular use of NSAIDs had higher DHEA concentrations (β = 0.20, P = 0.04) and lower prolactin concentrations (β = −0.18, P = 0.02) compared with non-users.
Discussion
This cross-sectional analysis of a subsample of women from the WHI Dietary Modification trial showed that DHEA and DHEAS were lower with increased age, but testosterone and other androgens did not change with age. Inconsistent associations were observed between reproductive and menstrual variables and hormone concentrations, although there was a clear decrease in testosterone and free testosterone concentrations in women with a history of bilateral oophorectomy compared with women without such a history. We found no associations of testosterone or free testosterone with history of hysterectomy without bilateral oophorectomy, however. Prior use of oral contraceptives or menopausal hormone therapy was not associated with any of the hormones assayed. Other risk factors for breast cancer, such as Causcasian race, age, parity and later menopause, did not show significant correlations with hormone concentrations.
Intriguingly, prolactin concentrations were lower in current NSAID users compared with non-users. The association of NSAID use with some hormones suggests one possible mechanism for the association between use of NSAIDS and reduced risk for breast cancer [6], as elevated prolactin concentrations have been associated with increased risk for breast cancer [12–14]. One possible mechanism for the negative association of regular NSAID use with prolactin concentrations is through blunting of pituitary response to stress since prostaglandins modulate the hypothalamus-pituitary axis [32]. Although there is biological rationale for NSAIDS to be associated with estrogen levels, e.g. upregulation of aromatase [33, 34], we found no association between estrogen concentrations and regular use of NSAIDS.
The results of this analysis are for the most part consistent with other studies that have reported on a more limited array of sex hormones in relation to menstrual and reproductive variables. In a study that included 456 postmenopausal women with intact uteri and ovaries, no associations were observed between estradiol and SHBG and age at menarche, parity, age at menopause, or previous use of oral contraceptives [18]. In a study of 441 postmenopasual Chinese women, menstrual and reproductive factors (age at menarche, age at menopause, parity, and age at first live birth) were unrelated to plasma estradiol, estrone, estrone sulfate, testosterone, DHEAS, and SHBG [16]. A study in 170 postmenopausal, overweight, sedentary women from the greater Seattle area found that time since menopause was negatively associated with estradiol and free estradiol, and that among parous women, parity was positively associated with SHBG and negatively associated with free estradiol concentrations [3]. To our knowledge, no epidemiologic studies have reported on the association between NSAID use and sex hormones.
There are several strengths of the study. First, the sample was drawn from a large multi-ethnic/racial cohort of postmenopausal women from a large cross-section of U.S. geographic areas, which adds new information since except for one study in China [16], most studies of this type have been largely limited to non-Hispanic White women. We also assayed multiple hormones, unlike most previous studies that concentrated on just a few hormones.
The study also has also several weaknesses. The cross-sectional design does not allow for determination of cause and effect. The sample size was small, resulting in small cell size for some comparisons. Because of this, therefore, we were not able to separately assess associations in women of race or ethnic groups other than Non-Hispanic Whites and African-Americans. In order to be eligible for the WHI Dietary Modification Trial, women had to have diets consisting of 32% or more of calories from fat as measured by the food frequency questionnaire. This resulted in a population of women with relatively high dietary fat intake, which could limit the generalizability to women with lower intake of dietary fat. Since we did not know participants’ premenopausal hormone concentrations, we could not examine whether a woman’s underlying hormonal profile influenced both her reproductive capabilities and her postmenopausal sex hormone concentrations. Only one blood specimen was assayed per woman. However, a single measure of many of these estrogens and androgens has predicted risk of breast cancer [11] and has been shown to reliably reflect usual hormone levels for several estrogens [35]. Women were asked to refrain from use of NSAIDs for 48 h prior to blood draw, thus any association observed between NSAID use and hormone concentrations would likely reflect chronic rather than acute effects. Furthermore, we did not have information about dose of NSAID use, nor on use prior to enrollment in the study. Finally, we made multiple comparisons, which could have resulted in some associations occurring by chance.
In summary, we found inconsistent evidence for associations between demographic and reproductive factor effects and circulating postmenopausal hormone concentrations, but did observe variation in some hormones by age, race, bilateral oophorectomy, and NSAID use. Our results suggest that a woman’s previous reproductive history, other than the presence or absence of both ovaries, is unlikely to affect her hormone milieu after menopause.
References
Cook LS, Weiss NS (2000) Endometrial cancer. In: Goldman MB, Hatch MC (eds) Women and health. San Diego, Academic Press, 916–931
Kelsey JL, Gammon MD, John EM (1993) Reproductive factors and breast cancer. Epidemiol Rev 15(1):36–47
Chubak J, Tworoger S, Yasui Y, Ulrich C, Stanczyk F, McTiernan A (2004) Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev 8:1296–1301
Hankinson S, Colditz G, Hunter D, Manson J, Willett W, Stampfer M, Longcope C, Speizer F (1995) Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses’ Health Study (United States). Cancer Causes Control 6:217–224
Garcia Rodriguez L, Gonzalez-Perez A (2004) Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer 91(3):525–529
Harris R, Chlebowski R, Jackson R, Frid D, Ascenseo J, Anderson G, Loar A, Rodabough R, White E, McTiernan A (2003) Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res 63:6096–6101
Terry M, Gammon M, Zhang F, Tawfik H, Teitelbaum S, Britton J, Subbaramaiah K, Dannenberg A, Neugut A (2004) Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA 291(20):2433–2440
Cook N, Lee I, Gaziano J, Gordon D, Ridker P, Manson J, Hennekens C, Buring J (2005) Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294(1):47–55
Jacobs E, Thun M, Connell C, Rodriguez C, Henley S, Feigelson H, Patel A, Flanders W, Calle E (2005) Aspirin and Other Nonsteroidal Anti-inflammatory Drugs and Breast Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol Biomarkers Prev 14:261–264
Marshall S, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross P, Mohrenweiser H, Peel D, Pinder R, Purdie D, Reynolds P, Stram D, West D, Wright W, Ziogas A, Ross R (2005) Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst 97(11):805–812
Endogenous Hormones and Breast Cancer Collaborative Group (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616
Hankinson S, Willett W, Michaud D, Manson J, Colditz G, Longcope C, Rosner B, Speizer F (1999) Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 91(7):629–634
Tworoger S, Eliassen A, Rosner B, Sluss P, Hankinson S (2004) Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res 64(18):6814–6819
Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, Lenner P (2003) Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden). Cancer Causes Control 14(7):599–607
Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore R, Biessy C, Muti P, Riboli E, Koenig K, Levitz M, Stattin P, Berrino F, Hallmans G, Kaaks R, Toniolo P, Zeleniuch-Jacquotte A (2004) Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer 108(3):425–432
Boyapati S, Shu X, Gao Y, Dai Q, Yu H, Cheng J, Jin F, Zheng W (2004) Correlation of blood sex steroid hormones with body size, body fat distribution, and other known risk factors for breast cancer in post-menopausal Chinese women. Cancer Causes Control 15(3):305–311
McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, Gilliland F, Stanczyk FZ, Yasui Y, Ballard-Barbash R (2003) Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol 21(10):1961–1966
Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ (2001) Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 12(1):47–59
McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY (2006) Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 14(9):1662–1677
Meldrum D, Davidson D, Tateryn I, Judd H (1981) Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol 57:624–628
Siiteri PK (1987) Adipose tissue as a source of hormones. Am J Clin Nutr 45(1 Suppl):277–282
Judd HL, Shamonki IM, Frumar AM, Lagasse LD (1982) Origin of serum estradiol in postmenopausal women. Obstet Gynecol 59(6):680–686
Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW (1982) The serum transport of steroid hormones. Recent Prog Horm Res 38:457–510
Ritenbaugh C, Patterson R, Chlebowski R, Caan B, Fels-Tinker L, Howard B, Ockene J (2003) The women’s health initiative dietary modification trial: overview and baseline characteristics of participants. Ann Epidemiol 13:S87-S97
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 19(1):61–109
Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM (2006) Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 295(6):629–642
Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T (1999) Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 9(3):178–187
Langer R, White E, Lewis C, Kotchen J, Hendrix S, Trevisan M (2003) The Women’s Health Initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 13:S107-S121
McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Ockene J (2003) Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA 290(10):1331–1336
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84(10):3666–3672
Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R (2002) Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11(10 Pt 1):1065–1071
DiLuigi L, Guidetti L, Romanelli F, Baldari C, Conte D (2001) Acetylsalicylic acid inhibits the pituitary response to exercise-related stress in humans. Med Sci Sports Exerc 33(12):2029–2035
Brueggemeier R, Quinn A, Parrett M, Joarder F, Harris R, Robertson F (1999) Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett 140:27–35
Zhao Y, Agarwal V, Mendelson C, Simpson E (1996) Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 137:5739–5742
Toniolo P, Koenig K, Pasternack B, Banerjee S, Rosenberg C, Shore R, Strax P, Levitz M (1994) Reliability of measurements of total, protein-bound, and unbound estradiol in serum. Cancer Epidemiol Biomarkers Prev 3(1):47–50
Acknowledgements
The WHI program is funded by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix: Short list of WHI investigators
Appendix: Short list of WHI investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Rights and permissions
About this article
Cite this article
McTiernan, A., Wu, L., Barnabei, V.M. et al. Relation of demographic factors, menstrual history, reproduction and medication use to sex hormone levels in postmenopausal women. Breast Cancer Res Treat 108, 217–231 (2008). https://doi.org/10.1007/s10549-007-9588-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9588-6