Abstract
In situ synthesis of estrogens is believed to be of great importance for the progression of breast cancer. In postmenopausal women most estrogens are synthesized in peripheral hormone-target tissues from circulating precursor steroids, by the enzymes involved in formation of active estrogens. One of the enzymes involved in this process is 17β-hydroxysteroid dehydrogenase (17β-HSD) type 1. This enzyme catalyzes the interconversion of estrone (E1) to the biologically more potent estradiol (E2). The gene coding for 17β-HSD type 1 (HSD17B1) is located at 17q12-21. The aim of this study was to investigate altered gene copy number of HSD17B1 in breast cancer. We used real-time PCR and examined 387 postmenopausal breast tumors for amplification of HSD17B1, and if an increased mRNA level of this enzyme is associated with amplification of the gene. We also investigated whether amplification of HSD17B1 has a prognostic value. There was a significant correlation between gene copy number of HSD17B1 and mRNA expression level (P = 0.00002). ER-positive patients with amplification of HSD17B1 showed lower breast cancer survival than patients without amplification (P = 0.025). Among ER-negative patients there was no significant correlation between increased gene copy number of HSD17B1 and prognosis. Furthermore, we found that amplification of the gene had prognostic significance in multivariate analysis adjusting for other clinicopathological variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrogens play an important role in the development of hormone-dependent breast cancer. In postmenopausal women estrogens in breast carcinoma tissue originate through two main pathways, one involving aromatase, which converts androgens to estrogens, and the other utilizing sulfatase, which converts estrone sulfate into estrone. Earlier studies have suggested that estradiol can be produced in the same organ where it exerts its biological response, which is also in agreement with the fact that breast cancer tissue possesses all the enzymes necessary for the bioformation of estradiol [1, 2]. Among these enzymes are 17β-hydroxysteroid dehydrogenases (17β-HSD), which control the final balance between more or less potent androgens and estrogens [3].
An earlier study showed that the ratio of bioactive estradiol to estrone (E2/E1) in breast tumors as well as the expression level of 17β-HSD type 1 mRNA, was higher among postmenopausal than premenopausal patients [4]. A few immunohistochemical studies of 17β-HSD type 1 in human breast cancer have been reported, and these suggest that 17β-HSD type 1 may play an important role in the in situ regulation of estradiol production in hormone dependent breast carcinomas [5–7]. A predominance of 17β-HSD type 1 in malignant breast tissue may lead to increased estrogen dependent proliferation and progression of breast cancer. In earlier studies we found that the expression of both 17β-HSD type 1 and 2 differs in breast cancers with or without late relapse in the disease. Patients with late relapse more often had a high expression of 17β- HSD type 1 or loss of 17β-HSD type 2 [8, 9].
In another breast cancer study, patients with tumors expressing 17β-HSD type 1 mRNA or protein had significantly shorter overall and disease free survival than other patients and the presence of 17β-HSD type 1 mRNA was an independent prognostic marker [10].
The gene encoding 17β-HSD type 1 (HSD17B1) is located at 17q12-21, a region that is often rearranged in breast cancer [11, 12]. This region also harbors HER2, which is described as the most amplified gene in breast cancer. In 20–30% of all breast cancer HER2 is overexpressed, and a high expression is frequently seen together with ER-negative status and a worse prognosis [13]. In a previous study we hypothesized that HSD17B1 might be amplified in breast cancer and we found an increased gene copy number in 14% of the tumors [14].
The aim of this study was to investigate the occurrence of HSD17B1 amplification in an extended series of breast cancer and to analyze if gene copy number correlates to the mRNA expression level of 17β-HSD type 1. In addition, the prognostic significance of HSD17B1 was evaluated.
Material and methods
We analyzed frozen tissue from excised primary breast tumors of 387 women. Of these women, 158 were treated in the health care region of southeast Sweden between 1985 and 1991. These patients were participants in a randomized multicentric trial where 2 and 5 years of adjuvant postoperative tamoxifen treatment was compared for postmenopausal patients less than 75 years of age (Swedish Breast Cancer Cooperative group). All patients had primary breast cancer, stage II (UICC), without distant metastasis at the time of diagnosis. The other subset of patients comprised 229 postmenopausal (less than 70 years of age) women who had either histologically verified lymph node metastases or a tumor diameter exceeding 30 mm. The patients did not receive any preoperative treatment and were participants of a randomized clinical trial with a 2 × 2 design, comparing both adjuvant CMF chemotherapy with postoperative radiotherapy and tamoxifen with no endocrine treatment [15]. The current study included a subset of patients from whom frozen tumor tissue was still available after hormone receptor assays. We also investigated different breast cancer cell lines (SKBR3, MCF10A, T47D, MCF7, BT474 and BT 483) regarding their gene copy number of HSD17B1. The study was approved by the regional ethics committee at Linköping University.
HER2 and ERα expression
Overexpression of the HER2 protein was quantified with an immunocytochemical method using flow cytometry [16]. Briefly, a cell suspension was prepared from frozen tissue and fixed in 1% paraformaldehyde (PFA) for 3 min. The cells were incubated with a monoclonal antibody (c-neu, Ab-2, Oncogene Research Products, Cambridge, MA), or an irrelevant isotype control antibody, followed by incubation with a secondary FITC-conjugated antibody. A measure of HER2 expression was calculated from the paired samples. The cutoff value for overexpression was the same as used in previous studies [17]. ER content was measured in cytosols with isoelectric focusing or with immunoassays (Abbott Laboratories, Chicago, IL, USA) at the time of diagnosis. Tumors with an ER content >0.1 fmol/μg DNA were classified as positive.
DNA extraction
Tumor tissue was disintegrated with scissors and then digested with 50 μl proteinase K (10 mg/ml, Boehringer Mannheim) in 500 μl TEN buffer (10 mM Tris, 1 mM EDTA, 100 mM NaCl) containing 100 μl SDS (20%) and incubated at 55°C. DNA was separated from proteins by repeated extractions with phenol, phenol/chloroform (1:1) and chloroform. Nucleic acids were precipitated by addition of two volumes of ice-cold 95% ethanol and 1/10 volume 3 M sodium acetate incubated over night at −20°C. DNA was pelleted by centrifugation at 15,000g for 1 h, after which salt residues and RNA were removed by washing with 70% ethanol. Nucleic acids were repelleted as above for 10 min and then vacuum-dried and suspended in sterile Milli-Q water.
RNA extraction
RNA extraction was performed in 210 tumors, as previously described [8, 9]. Thirty milligram frozen breast tumor tissue was homogenized in a microdismembrator (B. Brown, Melsungen, Germany) and total RNA was extracted using the SV Total RNA Isolation System (Promega, SDS, Sweden). The purified RNA was stored at −70°C and the concentration of RNA content was determined by spectrophotometry. RNA from normal mammary gland was used as a reference (Clontech, Becton Dickinson, Sweden)
cDNA synthesis
Four hundred nanogram of total RNA was reverse transcribed in a final volume of 20 μl using the Gibko BRL kit (Invitrogen, Sweden) with the following concentrations: 1× first strand buffer, 0.5 mM dNTP, 2.5 μM Random hexamers, 10 mM DTT, 0.5 μl Superscript II reverse transcriptase (Invitrogen, Sweden). The thermal conditions used were: 20°C for 10 min, 42°C for 50 min, 99°C for 5 min, and 5°C for 5 min. The samples were stored at 4°C until analyzed.
Primers and probes
Primers and probes were designed as previously described [14] to recognize human HSD17B1 and APP (amyloid precursor protein), which was used as a reference gene (Table 1). The location of this gene at 21q21 is not known to show chromosomal alterations in breast cancer. Both primers and probes were purchased from Applied Biosystems AB, Sweden.
Primers and probe recognizing human 17β-HSD type 1 and β-actin cDNA sequences were chosen as described earlier [8, 9].
Real-time polymerase chain reaction
All reactions were performed in the ABI Prism 7700 Sequence Detection System (Applied Biosystems AB, Sweden) using the TaqMan methodology. The design of the TaqMan probes, combined with the 5′–3′nuclease activity of AmpliTaq Gold DNA polymerase (Applied Biosystems AB, Sweden), allows the direct detection of the PCR product by the release of a fluorescent reporter during the PCR. We examined the DNA content of HSD17B1 and APP in 387 tumors. The breast cancer cell line MDA-MB-231, purchased from ATCC (American type culture collection), was used to construct standard curves. MDA-MB-231 cells show normal gene copy levels at 21q21 and 17q12-21 (http://www.nhgri.nih.gov/DIR/CGB/CR2000/). The target content in unknown samples was quantified by using the standard curves to determine a relative measure of the starting amount. Each sample was then normalized on the basis of its APP content.
PCR conditions
DNA or cDNA were added to the reaction mixture which had a total volume of 25 μl. Using TaqMan PCR core Reagent kit (Applied Biosystems AB, Sweden), the concentrations used were: 1× TaqMan buffer A, 5.0 mM MgCl, 0.1 mM dNTP, 0.1 μM each of forward and reverse primer, 0.1 μM probe and 0.025 U/μl AmpliTaq Gold DNA polymerase. The thermal conditions used were 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. Step two and three were repeated for 50 cycles. The different targets were amplified independently in separate reaction wells, in triplicates. On the same plate we included samples for standard curves for the target genes.
Statistical analysis
The relationships between grouped variables were analyzed with the χ2 test, or the χ2 test for trend when required. Survival curves were produced according to the life-table method described by Kaplan and Meier. Differences in breast cancer mortality rates were estimated with the log rank test. Cox hazard regression analysis was performed to estimate breast cancer survival in a multivariate analysis. All the procedures are comprised in the statistical package “Statistica” 7.0 (Statsoft, inc 1999, Tulsa, OK, USA, Statistica for Windows). The criterion for statistical significance was P < 0.05.
Results
Using the APP gene as a reference, the HSD17B1 to APP copy-number ratio was calculated in 387 breast tumors and six cell lines. Samples with ratios higher than 2 were considered amplified.
The mean HSD17B1:APP ratio for the tumors was 1.23 (range 0.2–8.6). In all, 40 tumors (10%) showed a ratio higher than 2. The mRNA for 17β-HSD type 1 enzyme was detected in all of the 210 tumors analyzed with a mean value of 2.9. The 10th and the 90th percentile for 17β-HSD type 1 were 0.1 and 7.2 respectively. To discriminate between low, intermediate and high expression of 17β-HSD type 1, the material was divided into three groups; low (<0.5), intermediate (0.5–2) and high (>2.0). There was a significant correlation between gene copy number of HSD17B1 and mRNA expression level when categorizing the tumors into three groups (P = 0.00002) (Table 2). We also noted a correlation between gene copy ratio and mRNA expression when treating the estimates as continuous variables (P = 0.00005).
Tumors with amplification of HSD17B1 more frequently overexpressed HER2 but this relationship was not statistically significant (P = 0.17) (Table 3). The association between the mRNA levels of 17β-HSD type 1 and HER2 expression was not significant. The gene copy number of HSD17B1 was associated with patient’s age and the number of positive lymph nodes.
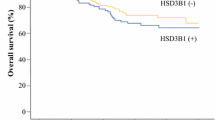
Patients with amplification of HSD17B1 showed lower breast cancer survival than patients without amplification (Fig. 1). This stayed true when restricting the analysis to ER-positive patients, and after adjustment for other characteristics (Table 4). Among ER-negative patients there was no significant correlation between HSD17B1 amplification and prognosis.
Among the breast cancer cell lines analyzed, T47D and BT474 showed increased gene copy number of HSD17B1.
Discussion
Irrespectively of E1 production is a result of aromatase or steroid sulfatase activity, 17β-HSD type 1 and 2 are important for the balance between estrone (E1) and estradiol (E2). The primary function of 17β-HSD type 1 is to catalyze the final step of estradiol biosynthesis, by reducing estrone to the biologically more active estradiol. Husen et al. [18] have recently shown that the estrogenic response is increased by the local action of 17β-HSD type 1 in vivo and they suggest this enzyme as a potential target for pharmacological inhibition of estrogen action. These results suggest that intratumoral regulation of estradiol levels is of importance, and that higher reductive 17β-HSD activity contributes to higher levels of E2 in tumors, thus resulting in tumor growth.
In the present study we found amplification of HSD17B1 in 10% of the breast tumors, in an earlier study we detected amplification in 14.5% of the tumors [14]. In the earlier study we noticed coamplification of HSD17B1 and HER2 in approximately half of the HSD17B1 amplified tumors. In our extended series, we lack amplification data for HER2, however, in tumors with HER2 protein overexpression HSD17B1 amplification was more common. HSD17B1 amplification was more common in tumors from postmenopausal patients under the age of 60 than from older patients. One may speculate that breast cancer diagnosed shortly after menopause, when the estrogen serum levels dramatically fall, are more likely carcinomas with a high capability of local synthesis of estradiol than other carcinomas, but this needs to be investigated further.
In the present study we have found a correlation between 17β-HSD type 1 mRNA and gene copy number of HSD17B1. Day and colleagues [19] showed that mRNA expression of 17β-HSD type 1 correlates well with enzyme activity. Other enzymes involved in estrogen synthesis may have prognostic significance, and steroid sulfatase has been suggested as a useful marker for identification of high-risk breast cancer patients [20]. Mioyshi et al. [4] observed that intratumoral E2 levels are not significantly different between premenopausal and postmenopausal patients and the authors suggested that up-regulation of 17β-HSD type 1 is important in the maintenance of high intratumoral E2 levels in especially postmenopausal patients. In a more recent study Mioyshi et al. [21] demonstrated that the intratumoral sulfatase mRNA levels have prognostic value in ER-positive breast cancer patients. In the same study patients with high levels of 17β-HSD type 1 tended to have a worse prognosis than those with low levels.
In the present study the clinical outcome was significantly worse among patients with HSD17B1 amplification. This result stayed true in multivariate analysis, however, among ER-negative breast cancer patients there was no correlation between HSD17B1 and prognosis. This result consolidates our previous finding that patients with amplified HSD17B1 may have decreased breast cancer survival [14]. Furthermore, we previously found that a high level of 17β-HSD type 1 mRNA level indicated an increased risk of late relapse in breast cancer among postmenopausal breast cancer patients [8]. Oduwole et al. found that breast tumors expressing 17β-HSD type 1 mRNA or protein had significantly shorter overall and disease-free survival than other patients [10]. They also detected that 17β-HSD type 1 mRNA expression had independent prognostic significance, adjusting for ER-status. The regulation of the enzymes involved in steroid synthesis is complex and probably other members in these families are important.
In estrogen receptor positive human breast cancer cell lines, the reductive pathway leading to the formation of E2 from E1 is predominant, whereas in hormone independent cell lines the oxidative pathway predominates [22]. In the present study two of the cell lines (BT474 and T47D) showed increased gene copy number of HSD17B1, and both these cell lines are estrogen receptor positive. In addition BT 474 cells show amplification of HER2 and T47D cells express high mRNA levels of 17β-HSD type 1 [23].
Among the tumors without HSD17B1 amplification in the present study, some did still show a high mRNA expression. Gene amplification is probably not the only reason for overexpression of 17β-HSD type 1, and a lot of knowledge about the regulation of the enzyme is still missing. Speirs and colleagues [24–26] suggested that cytokines can regulate E2 synthesis in breast cancer by stimulating enzyme activity, they especially pointed out IL-6 and IL-8 as possible estrogen modulators. Other studies have also shown that IL-6 can up-regulate HSD17B1 [2] and we have previously reported that the protein levels of 17β-HSD type 1 significantly correlates with those of COX-2 and aromatase in tumor cells [27].
In summary, our results demonstrate increased gene copy number of the gene encoding 17β-HSD type 1 in a subset of breast cancers and this correlates to increased mRNA expression of the enzyme. This event may lead to a higher concentration of estradiol in the tumor. We found that amplification of the gene had prognostic significance, and in particular, for estrogen receptor positive patients, increased gene copy number indicated a decreased breast cancer survival.
References
Yue W, Wang J, Hamilton C, Demers L, Sauten R (1998) In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res 58:927–932
Purohit A, Newman SP, Reed MJ (2002) The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res 4:65–69
Labrie F, Luu-Thee V, Lin S-X, Labrie C, Simard J, Breton R, Belanger A (1997) The key role of 17beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148–158
Miyoshi Y, Ando A, Shiba E, Taguchi T, Tamaki Y, Noguchi S (2001) Involvement of up-regulation of 17 beta-hydroxysteroid dehydrogenase type 1 in maintenance of intratumoral high estradiol levels in postmenopausal breast cancers. Int J Cancer 94:685–689
Poutanen M, Isomaa V, Lehto V-P, Vihko R (1992) Immunological analysis of 17 beta-hydroxysteroid dehydrogenase in benign and malignant human breast tissue. Int J Cancer 50:386–390
Sasano H, Frost AR, Saitoh R, Harada N, Poutanen M, Vihko R, Buhm SE, Silverberg SG, Nagura H (1996) Aromatase and 17 beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 11:4042–4046
Suzuki T, Moriya T, Ariga N, Kanazawa M, Sasano H (2000) 17Beta-hydroxysteroid dehydrogenase type 1 and type 2 in human breast carcinoma: a correlation to clinicopathological parameters. Br J Cancer 82:518–523
Gunnarsson C, Olsson B, Stål O (2001) Abnormal expression of 17β-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res 61:8448–8451
Gunnarsson C, Hellqvist E, Stål O (2005) 17beta-hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br J Cancer 92:547–552
Oduwole O, Li Y, Isomaa V, Mäntyniemi A, Pulkka A, Soini Y, Vihko P (2004) 17beta-hydroxysteroid dehydrogenase type 1 is an independent prognostic marker in breast cancer. Cancer Res 64:7604–7609
Plummer S, Paris M, Myles J, Tubbs R, Crowe J, Casey G (1997) Four regions of allelic imbalance on 17q12-qter associated with high-grade breast tumors. Genes Chromosomes Cancer 20:354–362
Kauraniemi P, Barlund M, Monni O, Kallioniemi A (2001) New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res 61:8235–8240
Révillion F, Bonneterre J, Peyrat JP (1998) ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer 34:791–808
Gunnarsson C, Ahnstrom M, Kirschner K, Olsson B, Nordenskjold B, Rutqvist LE, Skoog L, Stål O (2003) Amplification of HSD17B1 and ERBB2 in primary breast cancer. Oncogene 22:34–40
Rutqvist LE, Johansson H (2006) Long-term follow-up of the Stockholm randomized trials of postoperative radiation therapy versus adjuvant chemotherapy among ‘high risk’ pre- and postmenopausal breast cancer patients. Acta Oncol 45:517–527
Stål O, Sullivan S, Sun X-F, Wingren S, Nordenskjöld B (1994) Simultaneous analysis of c-erbB-2 expression and DNA content in breast cancer using flow cytometry. Cytometry 16:160–168
Stål O, Borg Å, Fernö M, Källström A-C, Malmström P, Nordenskjöld B, members of the South Sweden Breast Cancer Group, the Southeast Sweden Breast Cancer Group (2000) ErbB2 status and the benefit from two or five years of adjuvant tamoxifen in postmenopausal early stage breast cancer. Ann Oncol 11:1545–1550
Husen B, Huhtinen K, Saloniemi T, Messinger J, Thole H, Poutanen M (2006) Human hydroxysteroid (17-beta) dehydrogenase 1 expression enhances estrogen sensitivity of MCF-7 breast cancer cell xenografts. Endocrinology 147:5333–5339
Day JM, Tutill HJ, Newman SP, Purohit A, Lawrence HR, Vicker N, Potter BV, Reed M (2006) 17 Beta-hydroxysteroid dehydrogenase Type 1 and Type 2: association between mRNA expression and activity in cell lines. Mol Cell Endocrinol 248:246–249
Utsumi T, Yoshimura N, Takeuchi S, Ando J, Maruta M, Maeda K, Harada N (1999) Steroid sulfatase expression is an independent predictor of recurrence in human breast cancer. Cancer Res 59:377–381
Miyoshi Y, Ando A, Hasegawa S, Ishitobi M, Taguchi T, Tamaki Y, Noguchi S (2003) High expression of steroid sulfatase mRNA predicts poor prognosis in patients with estrogen receptor-positive breast cancer. Clin Cancer Res 9:2288–2293
Pasqualini JR, Gelly C, Nguyen BL, Vella C (1989) Importance of estrogen sulfates in breast cancer. J Steroid Biochem 34:155–163
Jansson A, Gunnarsson C, Stål O (2006) Proliferative responses to altered 17beta-hydroxysteroid dehydrogenase (17HSD) type 2 expression in human breast cancer cells are dependent on endogenous expression of 17HSD type 1 and the oestradiol receptors. Endocr Relat Cancer 13:875–884
Speirs V, Green AR, Atkin SL (1998) Activity and gene expression of 17beta-hydroxysteroid dehydrogenase type I in primary cultures of epithelial and stromal cells derived from normal and tumourous human breast tissue: the role of IL-8. J Steroid Biochem Mol Biol 67:267–274
Speirs V, Green AR, Walton DS, Kerin MJ, Fox JN, Carleton PJ, Desai SB, Atkin S L (1998) Short-term primary culture of epithelial cells derived from human breast tumours. Br J Cancer 78:1421–1429
Speirs V, Walton DS, Hall M-C, Atkin S-L (1999) In vivo and in vitro expression of steroid-converting enzymes in human breast tumours: associations with interleukin-6. Br J Cancer 81:690–695
Gunnarsson C, Jansson A, Holmlund B, Ferraud L, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O (2006) Expression of COX-2 and steroid converting enzymes in breast cancer. Oncol Rep 16:19–24
Acknowledgments
This work was funded by the Swedish Cancer Society and Östergötland County Council research and development fund. There is no conflict of interest that would prejudice impartiality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunnarsson, C., Jerevall, PL., Hammar, K. et al. Amplification of HSD17B1 has prognostic significance in postmenopausal breast cancer. Breast Cancer Res Treat 108, 35–41 (2008). https://doi.org/10.1007/s10549-007-9579-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9579-7