Abstract
Purpose
To measure the effectiveness of a tailored decision aid (DA) designed to help women make informed decisions about genetic testing for breast/ovarian cancer risk.
Methods
A total of 145 women were randomized to receive the DA or a control pamphlet at the end of their first genetic counseling consultation. Of these, 120 (82.8%) completed two questionnaires, 1 week and 6 months post-consultation.
Results
While the DA had no effect on informed choice, post-decisional regret or actual genetic testing decision, the trial showed that women who received the DA had higher knowledge levels and felt more informed about genetic testing than women who received the control pamphlet (χ2(2) = 6.82; P = 0.033; χ2(1) = 4.86; P = 0.028 respectively). The DA also helped women who did not have blood drawn at their first consultation to clarify their values with regards to genetic testing (χ2(1) = 5.27; P = 0.022). Women who received the DA were less likely to share the information with other family members than women in the control condition (χ2(1) = 8.78; P = 0.003).
Conclusions
Decision aids are an effective decision-support strategy for women considering genetic testing for breast/ovarian cancer risk, and are most effective before the patient has made a decision, which is generally at the point of having blood drawn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Genetic testing for cancer risk can empower individuals to make informed choices about their health management for the future. In many countries, women with a strong family history of breast and/or ovarian cancer can be tested for a mutation in one of two breast and ovarian cancer-related genes (BRCA1 and BRCA2). This knowledge enables women identified as carriers of a breast/ovarian cancer-related mutation to significantly reduce their risk of developing cancer through increased use of screening and preventative measures [1–5]. Conversely, proven non-carriers of BRCA mutations, and their offspring, are only at population risk of developing cancer and do not need to consider the increased surveillance and preventative options offered to carriers, saving the costs, concerns and inconvenience of unnecessary procedures [6].
There are generally two stages involved in cancer genetic testing. The first involves the collection of blood from an affected individual for a ‘mutation search’, whereby the BRCA1 and BRCA2 genes are examined for pathogenic mutations. Genetic testing in individuals already affected by cancer can help to clarify a woman’s future risk of developing further breast and/or ovarian cancers. Mutation search however, is often inconclusive because a causative mutation cannot always be found despite a strong family history. If a causative mutation is identified in an affected family member, then other adult at-risk family members can be offered a ‘predictive’ test to find out if they carry the family-specific mutation. Predictive testing in unaffected women is significant because it predicts her future risk of developing breast and ovarian cancer. Women who carry a germline mutation in a BRCA1 or BRCA2 gene have a lifetime risk of developing breast cancer of 50–85% and a lifetime risk of developing ovarian cancer of 15–65% [5, 7, 8], with her offspring having a 50% chance of carrying the same mutation.
One problem of genetic testing for cancer risk is that it is not a failsafe means of determining women’s chances of developing cancer. Inconclusive results or results of uncertain significance are common, and the psychological impact of going through the genetic testing process and receiving an inconclusive result is not yet well understood [9–11]. It is also crucial for women who receive a positive test result to understand that it is not possible to predict when, where and indeed if they will ever develop cancer. For non-carriers, it is similarly important for them to understand that it is still possible for them to develop breast and/or ovarian cancer, despite a negative predictive genetic testing result.
A decision aid for women considering genetic testing
A decision about genetic testing for cancer risk is a ‘preference-sensitive’ decision, and the best choice for each patient is usually made by weighing up how they value the benefits of genetic testing compared to its potentially harmful implications [12, 13]. Given the complexity of the potential benefits and limitations of genetic testing for breast/ovarian cancer risk, a decision aid (DA) for women considering genetic testing was developed. Decision aids are specifically aimed at facilitating decision-making, and are designed to improve patients’ understanding of the potential benefits and risks of their different options, as well as assist patients to consider the personal importance they place on each of these options [14]. The development and evaluation of the DA was theoretically guided by the frameworks developed by O’Connor and colleagues, which provide guidance on the DA development process, selection of study measures, specification of hypotheses, and suggested study outcomes for the evaluation of the effectiveness of DAs [15–17].
The breast/ovarian cancer DA provides balanced information about three options: doing nothing (i.e. not undergoing genetic testing), undergoing genetic testing or deciding to consider the issue at a later date. The topics in the 40-page DA include background information about cancer and cancer-related genes, a description of the genetic testing process, possible test results and a discussion of the potential impact of genetic testing on the individual and their family. The DA explains the evidence available on cancer risks and genetic testing, as well as explaining the differences between mutation search and predictive testing and the potential benefits, risks and limitations of testing. The DA includes information on the chances of receiving different types of test results (true and false, and positive and negative results, as well as inconclusive results), and describes the next steps for the patient based on their result. Where possible, visual diagrams are used in conjunction with words and numbers to describe probabilities, and diagrams allow clinicians to tailor the DA to the patient by circling the risk levels appropriate for their age group. The DA concludes with two patient stories, and a blank personal worksheet (values clarification exercise) is provided for individuals to list the benefits and risks of genetic testing in their situation. The worksheet also asks individuals to rate the importance of each risk and benefit as a ‘leaning’ towards or against having the genetic test by allocating 0–5 stars to each listed item. A more detailed description of the DA is provided in Wakefield et al. [18].
Aims and hypotheses
This study aimed to evaluate a DA for women considering genetic testing for breast and ovarian cancer risk in a randomized controlled trial. It was hypothesized that compared to women receiving the standard best practice (a general educational pamphlet), those who received the purpose designed DA would have:
-
(a)
decreased decisional conflict about genetic testing (primary outcome);
-
(b)
increased knowledge about genetic testing; and
-
(c)
an increased rate of informed choice.
Materials and methods
Sample
The research sample included women (both affected and unaffected by cancer) who approached one of five Australian familial cancer clinics participating in this study. Women are referred to Australian familial cancer clinics by general practitioners, oncologists and surgeons who become aware of, or concerned about, a woman’s family history of cancer. While some procedures vary across clinics, most clinics conduct a brief telephone interview with potential patients prior to their appointment in order to ascertain a brief verbal family history, and provide the patient with information about further details they need to bring to their appointment.
To be eligible to participate in the study, women were:
-
(i)
eligible for genetic testing in Australia, that is, women with a family history consistent with a dominantly inherited hereditary breast/ovarian cancer syndrome who have an affected, living relative willing to provide a blood sample [19, 20];
-
(ii)
able to give informed consent;
-
(iii)
able to read English; and
-
(iv)
aged 18 years or older.
Males were excluded due to the low numbers of men currently attending familial cancer clinics, making meaningful statistical analysis of responses difficult. Affected women considering predictive testing were also excluded due to differing informational needs compared to unaffected women considering predictive testing.
Procedure
The human research ethics committee of each clinic approved the study and informed consent was obtained from all participants. A randomized controlled trial was used to compare the efficacy of the DA with an educational pamphlet currently used in many clinics in Australia [21]. The 4-page pamphlet is comparable to the DA in describing hereditary breast and ovarian cancer, its pattern of inheritance, and the general benefits and risks of genetic tests. The pamphlet does not however include characteristic DA features, such as balanced information describing different decision options, patient stories and a values clarification exercise [22].
Recruitment of participants
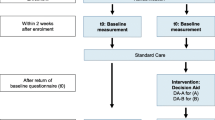
Figure 1 presents a flowchart of the recruitment process. Consent to participate in the study was sought by clinic staff following each eligible patient’s initial consultation. Those who agreed were given a pre-randomized envelope containing the DA or the control pamphlet, a consent form, the first questionnaire and a reply-paid envelope. They were asked to complete and return the questionnaire within 7 days after counseling whenever possible. The questionnaire was clearly marked “Please read your genetic testing information booklet before completing your questionnaire” and this message was reinforced by clinicians at the time of recruitment.
Randomization at the end of genetic counseling minimized the potential impact of the knowledge of randomization status on the course of the consultation. Randomization after the patient had left the consultation (which would have ensured blindness of the clinician to the intervention assignment) was not possible because some clinicians expressed a preference for being able to tailor the DA to the patient by entering personalized information (such as risk estimations based on family history) into the DA. Indeed, previous research has shown that patients at high risk of developing cancer prefer tailored over stand-alone-tools [23, 24].
Participants were randomized according to family-wise randomization. That is, all patients who were the first of their family to attend the clinic were randomly allocated to the control or DA condition. Subsequent members of the same family attending the same clinic were then assigned to the same condition as their other family members, in order to prevent potential contamination across groups.
Six months post-consultation, a second questionnaire was mailed to assess any longer-term effect of the DA. Reminder letters and phone calls were made as appropriate.
Measures
Staff at familial cancer clinics provided the following data.
-
(1)
Type of genetic test: Clinicians indicated which type of genetic test the woman was considering (mutation search or predictive testing). For predictive testing, the clinician indicated whether a family-specific mutation had already been identified, and if not, whether mutation search had commenced in the family.
-
(2)
Participant’s disease status: Women were classified as either affected or unaffected by breast/ovarian cancer, and if unaffected, their mutation carrier risk was given.
-
(3)
Blood drawn: Clinic staff noted whether or not blood was drawn on the day of the first consultation. This was a critical variable, as it determined whether the participant was able to read the DA or control pamphlet before having blood drawn for testing. In most Australian clinics it is standard practice to allow a ‘cooling off’ period after the patient’s first consultation before drawing blood for a predictive genetic test (usually at a separate appointment two weeks later). In some clinics however, and in other individual cases (for example, if the patient has traveled a long distance or the patient has a good background knowledge before clinic attendance), blood may be drawn immediately after the first consultation at the patient’s request.
-
(4)
Participant’s decision: Clinicians reported the genetic testing decision they felt was the best choice for the woman at the end of the consultation (response options included: ‘In my opinion the best choice for this patient would be to: ‘undergo genetic testing’, ‘not undergo genetic testing’, or ‘defer their decision to a later date’). The concordance between clinician opinion and the patient’s decision after the first consultation was rated as a dichotomous variable (‘patient and clinician agreed’ and ‘patient and clinician disagreed’ on best decision).
The initial questionnaire for participants elicited:
-
(1)
Demographic characteristics: Including age, educational level, previous medical or health training and marital status.
-
(2)
Reading the materials: Participants were asked to rate how thoroughly they read the information materials they received (response options included: ‘from cover to cover’, ‘thoroughly’, ‘briefly’, ‘just the relevant parts’ and ‘not at all’).
-
(3)
Decisional conflict: The Decisional Conflict Scale (DCS) was used to assess uncertainty about choosing among alternatives [25]. It has four subscales which assess modifiable factors contributing to decisional conflict, including feeling informed, being clear about values, feeling supported in decision-making and feeling certain about the decision [15].
-
(4)
Knowledge of genetic testing: Eight true–false items were purposively designed for this study and assessed knowledge about the genetic testing process, and the benefits, risks and limitations of genetic testing.
-
(5)
Multidimensional Measure of Informed Choice (MMIC): This scale classifies respondents as having made an informed or uninformed choice. An informed choice is one based on relevant knowledge, consistent with the decision maker’s values and behaviorally implemented [26]. The measure combines the knowledge test described above, a 4-item attitudes scale, and genetic testing decision. As described in the scale’s validated scoring instructions, two groups were classified as having made an informed choice: those who scored above the sample median on the knowledge scale, had a positive attitude towards testing and decided to undergo testing, and those who had a good knowledge score, a negative attitude towards testing and did not undergo testing. All other women were categorized as having made an uninformed choice.
-
(6)
Family involvement: Two ‘yes/no’ items assessed whether any other family member read the information materials given to the patient and whether other family members contributed to their decision-making process. Those who responded ‘yes’ were asked to indicate, using Likert-type scales separately for each family member, the extent to which they had read the materials (‘from cover to cover’ through to ‘briefly’), and their level of involvement in the decision-making process (‘extremely involved’ to ‘a little involved’).
-
(7)
Impact of Event Scale (IES): The 15-item IES was used to measure the frequency and severity of intrusive and avoidant thoughts about being at risk of developing breast and/or ovarian cancer [27]. The scale has good psychometric properties in women at increased risk of breast cancer [28].
-
(8)
Hospital Anxiety and Depression Scale (HADS): The HADS is a 14-item self-report scale which requires respondents to choose between 4 responses that most closely describe how they have been feeling in the past week [29]. The scale has been validated in cancer patients [30–32].
-
(9)
Genetic testing decision: One item asked participants about their decision about genetic testing at this point in time (i.e. 1 week after their first consultation). In order to reduce the demand for potentially sensitive information from participants, they were not asked about any genetic testing results they had received at either time point.
The second questionnaire collected data on measures 2–9 described above, as well as the:
Decision Regret Scale (DRS): The 5-item DRS assesses level of healthcare decision regret, and has good internal consistency and validity [33].
Data analysis
Data were analyzed using SPSS 14.0 [34] and STATA 9.2 [35]. Descriptive statistics were used to describe the socio-demographic and other characteristics of the sample. This was followed by an ‘intention to treat’ analysis on the effects of the randomized trial of DA provision. As DCS and regret scores were highly skewed, these variables were recoded into binary dichotomous variables, with participants who received the minimum score for these scales (1.5 for DCS; 0 for regret) being grouped into the ‘low DCS’ and ‘low regret’ groups respectively. Similarly, knowledge was recoded into a categorical variable with three levels (low, medium and high knowledge).
In order to maximize the usefulness of all data collected and take account of the family-wise randomization, observations from the same family were treated as clusters using the method described by Williams [36]. This approach improves the accuracy of the analysis by taking into account the fact that observations from the same cluster (or family) are likely to be more correlated than data collected from independent observations (or individuals). A Bonferroni adjustment for multiple tests was not employed, as we wanted to control for Type 1 error at a 0.05 level of significance for each dependent variable separately, rather than over all dependent variables considered together. Extensive data analysis revealed no significant differences between scores for women considering mutation search compared to predictive testing on any of the dependent variables. As well, analysis of all two-way interactions revealed that the DA did not have a significantly different effect on the two groups of women for any dependent variable, so the two groups were combined for the purposes of the analyses.
The analyses used binary logistic regression for dichotomous dependent variables (DCS, informed choice, family involvement and regret) and multinomial logistic regression for knowledge (a test of proportional odds showed that ordinal regression was not appropriate because the effect of the independent variable was not uniform over the three levels of the dependent variable). Multinomial logistic regression essentially combines two binary logistic regression analyses, each based on a comparison of two of the levels of the outcome variable with the third level, or reference category. Results are reported as Wald’s tests of parameters, which allow significance testing using χ2. All regression models were run separately for each dependent variable, and always included time as a covariate and the clinic the woman attended as a fixed factor in the models. Thus, each model included dummy codes for clinic, group, time and the interaction between group and time. This strategy allowed us to incorporate the available data from both time points in the same analysis, as well as account for any potential differences between clinics.
Results
Response rates and sample
Figure 2 presents the composition of the study participants. Briefly, 155 women were invited to participate in the study, of whom 145 (93.5%) agreed to participate. Of these, 119 returned the first questionnaire (82.1%) and 120 returned the second questionnaire (82.8%). Table 1 presents the participants’ demographic characteristics. There were no significant group or clinic differences in demographic characteristics nor in any of the clinician reported data, indicating that randomization was successful in spreading potential confounding variables equally between the groups and across clinics.
Clinician report
Type of genetic test
Sixty-one percent of participants were considering mutation search and 39% were considering predictive genetic testing (see Table 1). Of those considering predictive testing, 66.0% were considering predictive testing after a mutation had been identified in their family, and 34.0% comprised of other types of predictive testing situations, such as women considering screening for founder mutations and women considering future predictive testing in families where a mutation had not yet been identified. There were no significant differences between women considering different types of predictive testing on any of the dependent variables, so they were combined for further analysis.
Participant disease status
Of the 73 participants (60.8%) who had had a previous diagnosis of cancer, 67 (91.8%) were affected with breast cancer only, 5 (6.8%) with ovarian cancer only and 1 (1.4%) with both breast and ovarian cancer. Of the 52 women who were unaffected by cancer, 46 (88.5%) had a 50% mutation carrier risk status (MCR), 4 (7.7%) had a 25% MCR, 1 (1.9%) woman had a 12.5% MCR and one (1.9%) had a 100% MCR as she was an intervening relative.
Blood drawn
Blood was drawn immediately after the first consultation in 43 (35.8%) cases. Having blood drawn was equally distributed in the study groups, with 32.9% of women in the DA group having blood drawn after the consultation compared to 34.7% of women in the control group (χ2(1) = 0.06; P = 0.814). Giving blood at the first appointment was more common in women considering mutation search, with 29 (39.7%) of these women having blood drawn on the day of the consultation, compared to 14 (29.8%) of women considering predictive testing, although this difference was not statistically significant (χ2(1) = 1.23; P = 0.268).
Participant’s decision
In 113 (94.2%) cases, the clinician’s opinion on the best decision for the patient agreed with the participant’s decision one week after the consultation, with only 7 (5.8%) cases in which the clinician’s opinion did not agree with that of the participant. Of these cases, three women remained undecided after the consultation, while their clinician recommended genetic testing and four women decided to undergo testing while the clinician felt it was best to either defer the decision or to not undergo testing.
Questionnaire 1
Reading the materials
Both the DA and the control pamphlet were read thoroughly before completing questionnaire 1, with 69.6% of the DA group and 76.2% of the control group reporting having read the DA or the control pamphlet ‘from cover to cover’ or ‘quite thoroughly’. Women who had blood drawn on the day of their consultation were less likely to thoroughly read the educational materials they received than those who did not have blood drawn on the day (χ2(1) = 9.51; P = 0.050).
Decisional conflict
All dependent variable mean scores and proportions are presented in Table 2.
Main effects
Overall, there was no significant difference between decisional conflict full-scale scores in women who received the DA compared to those who received the control pamphlet (χ2(1) = 0.01; P = 0.937). However, the DA had a significant effect on scores on the informed subscale, such that women who received the DA were significantly more likely to be in the ‘informed’ group than women who received the control pamphlet (χ2(1) = 4.86; P = 0.028). The DA had no effect on the remaining subscales, including support (χ2(1) = 2.2; P = 0.138), certainty (χ2(1) = 0.70; P = 0.401) and clear values (χ2(1) = 2.52; P = 0.113).
Interaction with having blood drawn
There was no significant interaction between choosing to have blood drawn on the day of the consultation and the effect of the DA on decisional conflict full-scale score, nor on three of the subscale scores (DCS: χ2(1) = 0.21; P = 0.644; Informed: χ2(1) = 1.63; P = 0.201; Support: χ2(1) = 0.26; P = 0.608; Certain: χ2(1) = 0.64; P = 0.422). However, there was a significant interaction between choosing to have blood drawn and group for the clear values subscale (χ2(1) = 4.01; P = 0.045), such that women in the DA group who did not have blood drawn on the day of their consultation had significantly clearer values with regards to genetic testing than women who received the control pamphlet (χ2(1) = 6.67; P = 0.022). In contrast, the DA had no effect on mean clear values scores for women who did have blood drawn on the day of their consultation (χ2(1) = 0.61; P = 0.433). See Fig. 3.
Knowledge scores
Main effects
The main effects model showed that the DA significantly improved knowledge at both time points (χ2(2) = 6.82; P = 0.033). More specifically, the odds of women who received a DA being in the high knowledge group (relative to the low knowledge group) were more than twice as high as those for women in the control group (RRR = 2.73; P = 0.015) (see Fig. 4).
Interaction with having blood drawn
There was no significant interaction between choosing to have blood drawn on the day of the consultation and the effect of the DA on knowledge score (χ2(2) = 5.51; P = 0.064).
Informed choice
The effect of the DA on the measure of informed choice was not significant (χ2(1) = 1.06; P = 0.304), nor was there any significant interaction effect of having blood drawn on the day of the consultation (χ2(1) = 0.20; P = 0.657).
Family involvement
The majority of women reported sharing the information materials they received with other family members, particularly by 6 months after their consultation (see Table 2). Logistic regression with the dichotomous variable (‘information shared with any family member’ versus ‘not shared with any family member’) showed that women who received a DA were less likely to share the materials they received, relative to women who received the control pamphlet (χ2(1) = 8.78; P = 0.003). There was no significant group difference in the level of perceived family involvement in decision-making (χ2(1) = 0.81; P = 0.368).
Participants named their spouse or partner most often as the family member who had read the information materials they received and had contributed to their decision-making process. By time 2, 40.3% of women in the DA group and 46.0% of women in the control group reported that their spouse or partner had read the information materials they received at their consultation. As well, 38.6% of women in the DA group and 28.6% of women in the control group reported that their spouse or partner had contributed to their decision-making about genetic testing.
Psychological variables
The DA did not appear to affect women’s reported levels of psychological distress, with no significant group differences in intrusive and avoidant thoughts, anxiety or depression.
Regret
Logistic regression revealed no significant group differences in women’s reported regret about their genetic testing decision six months after their consultation (χ2(1) = 2.70; P = 0.100).
Genetic testing uptake
Genetic testing uptake was high, with 105 out of 114 (92.1%) participants eligible for genetic testing after their first consultation deciding to undergo testing. Of the six women who were awaiting the results of a relative’s genetic test, four were still waiting for the results 6 months post-consultation, and two had become unable to undergo genetic testing as a mutation was not identified in their affected family member. Of the remaining nine women, 6 (5.0%) reported being undecided about genetic testing and 3 (2.5%) women reported that they did not want to undergo genetic testing. Apart from the two women whose family member’s results became available, all participants’ genetic testing decisions were stable over the time period 6 months post-consultation. Receipt of the DA had no effect on genetic testing decision (χ2(1) = 1.04; P = 0.793).
Discussion
This randomized trial revealed three significant main effects of a DA for women considering genetic testing for breast/ovarian cancer risk: women who received the DA had significantly higher knowledge scores, felt more informed about genetic testing and were less likely to report that other family members had read the information materials they received, compared to those in the control condition. Moreover, for women who did not have blood drawn on the day of their consultation, receiving the DA also helped them to clarify their values with regards genetic testing. Consistent with previous research, the DA did not appear to affect women’s psychological functioning, their actual genetic testing decision, nor their scores on the certainty and feeling supported subscales of the decisional conflict scale [22, 37].
In general, women were knowledgeable and felt confident about their genetic testing decision, regardless of whether or not they received the DA. This can be seen in the highly skewed knowledge, decisional conflict and regret scores reported in Table 2. Reflecting this, we report a high genetic testing uptake rate of 92.1%, confirming previous research showing that women who approach familial cancer clinics tend to be more interested in genetic testing than women with a family history of breast/ovarian cancer in the general population and in genetics research settings [38–41].
In Australia, where, as a general rule, genetic testing decisions are made only after at least one consultation with a genetic counselor and/or clinical geneticist or oncologist, it is perhaps not surprising that knowledge and certainty levels are high in this population. Despite this high level of face-to-face education and support, the DA was still able to add value over and above the consultation. Further research on the effectiveness of the DA in women who decide not to attend a familial cancer clinic after hearing about their eligibility for genetic testing (either through referral from another medical service or in the public arena) would provide valuable information about the effect of the DA on women who may have lower knowledge and certainty levels than the group who do currently attend the clinics. Indeed, recent research suggests that women who do not attend familial cancer clinics after receiving a referral from their breast cancer treatment team list more cons of genetic testing than those who do attend after their referral [42].
The finding that women who did not give blood on the day of their consultation were more likely to benefit from the DA in terms of having clearer values is consistent with the data showing that they were more likely to read the information materials they received than women who did have blood drawn on the day of their consultation. Indeed, a recent evaluation of a French genetic testing information booklet showed a significant dose–response relationship, such that women who read the booklet most thoroughly experienced the most benefits from the tool [43].
Our data raise an important question about when the decision about genetic testing is made by most women. It is possible that a patient who changes their mind about genetic testing after having blood drawn may choose not to receive their test results, implying that the theoretical point of decision-making is actually at the receipt of test results [12]. In practice, however, none of the women in our study changed her genetic testing decision in the 6 months post-consultation, suggesting that the actual decision-making point for the individual is at, or even before, the drawing of blood. This raises important considerations for DA developers, who generally seek to expose the patient to DAs before they feel they have made their decision. A second randomized controlled trial of this DA is currently underway, using the DA as a communication aid during genetic counseling. Data from this trial will speak to the impact of using a DA during counseling to ensure that all participants have the opportunity to use the tool before having blood drawn.
The family involvement data warrants further discussion. Firstly, the data revealed a novel finding in that women who received the tailored DA were less likely to share the tool with their family members. We did not ask about reasons for sharing or not sharing the information, but it is possible that women did not want to disclose the unique information contained in it, including the personalized risk information entered by their clinician and the personal worksheet, which was completed by more than 70% of the women who received the DA. Alternatively, the DA may have been less likely to be shared with other family members due to its length.
Secondly, of those women who did share the DA or control pamphlet with their family, the family member most likely to be named as having read the materials, and having contributed to the decision-making process, was the participant’s spouse or partner. This data supports recent research emphasizing the importance of considering the spouse within the familial cancer setting [44, 45]. Traditionally, given that they are not blood relatives of the patient and hence not at genetic risk, the role of the spouse has received little attention in the familial cancer clinic process, but they clearly play a critical role in patients’ decision-making about genetic testing. Given that the DA was less likely to be shared with family members than the control pamphlet, clinicians should consider providing additional, specific information for family members.
Limitations
It was not possible to collect a baseline (i.e. pre-counseling) assessment of participants because; (i) it is often not possible to establish a patient’s eligibility for genetic testing until a full family history has been collected and verified during the first consultation; and (ii) the lead time between patients’ contacting the clinic and their first appointment is often very short (i.e. 2–3 days), indicating that many baseline questionnaires would have not been completed before the consultation. However, given that the participants were randomized to receive the DA or the control pamphlet, any pre-counseling characteristics in patients should have been evenly distributed across both groups.
It was also not possible for clinicians to be blinded to the randomization status of the participants because the majority of clinicians expressed a preference for entering personalized risk information into the DA. However, we attempted to minimize the impact of this by instructing clinicians to open the recruitment package at the end of the consultation. It was also not possible to control the content of the consultation for each patient, or the additional information materials they received from their clinic or from other external sources such as the world-wide web. However, any additional sources of information should have been evenly distributed across both groups. As well, it is possible that some participants completed their questionnaires before reading their allocated information materials, despite clear written and verbal requests not to do so. The data provided on reading the materials however, showed that the large majority of participants reported that they did read the materials before completing the questionnaire.
The current DA did not cover the needs of all familial cancer clinic patients. Men considering genetic testing for breast cancer risk for example, need to consider a unique set of issues in their decision-making process about genetic testing, including the implications of their test result for their own health as well as the risks that may be passed on to their daughters. Similarly, women affected with breast cancer considering predictive testing may have different informational needs with regards to issues such as their risk of developing further cancers, either of the breast or ovaries. Indeed, it is worthwhile considering the information needs of each patient as an individual, as sometimes a ‘one size fits all’ approach may not fully utilize the benefits of a decision support tool such as this. It may be more beneficial, for example, for clinicians to use DAs during, or even before (when eligibility for testing has been confirmed and there is a reasonable time available before the appointment), their consultations for some patients in order ensure that they are exposed to decision support tools before making their decision about testing [46].
Finally, the women in this study tended to have a higher education level than the general population, with 63% having a post-school qualification, compared to 52% of the general population [47]. Although this phenomenon has been identified in previous studies in the familial cancer setting, the discrepancy between the general and clinical population appears to be narrowing [39]. For example, earlier Australian data reported post-school qualifications in over 70% of women attending familial cancer clinics [48, 49], suggesting that the numbers of women with lower educational levels is increasing in familial cancer clinics.
Practice implications
Use of a genetic testing DA in conjunction with genetic counseling can increase women’s actual knowledge and feelings of being informed about genetic testing for breast/ovarian cancer risk. Family members who attend a familial cancer clinic might benefit from each receiving their own information materials, as patients may be less likely to share information tools about genetic testing if they are personalized. Finally, it is important to consider the needs and concerns of the spouse or partner in the familial cancer setting.
References
International Breast Cancer Intervention Study Investigators (2002) First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360:817–824
Hartmann LC, Schaid DJ, Woods JE et al (1999) Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 340:77–84
Scheuer L, Kauff N, Robson M et al (2002) Outcome and preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol 20:1260–1268
Meijers-Heijboer H, Van Geel B, Van Putten W et al (2001) Breast cancer after prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 345:159–164
Levy-Lahad E, Friedman E (2007) Cancer risks among BRCA1 and BRCA2 mutation carriers. BJC 96:11–15
Lodder LN, Frets PG, Trijsburg RW et al (2002) One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 73:97–112
Ford D, Easton DF, Stratton M et al (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 62:676–689
National Health and Medical Research Council (1999) Familial aspects of cancer: A guide to clinical practice. National Health and Medical Research Council, Canberra, November 1999
Meiser B (2005) Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology 14:1060–1074
van Dijk S, Otten W, Timmermans DR et al (2005) What’s the message? Interpretation of an uninformative BRCA1/2 test result for women at risk of familial breast cancer. Genet Med 7:239–245
van Dijk S, Timmermans DRM, Meijers-Heijboer H et al (2006) Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. J Clin Oncol 24:3672–3677
Ropka M, Wenzel J, Phillips E et al (2006) Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev 15:840–855
O’Connor AM, Mulley AG Jr, Wennberg JE (2003) Standard consultations are not enough to ensure decision quality regarding preference-sensitive options. J Natl Cancer Inst 95:570–571
O’Connor AM, Rostom A, Fiset V et al (1999) Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ 319:731–734
O’Connor A, Tugwell P, Welles GA et al (1998) Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventative hormone replacement therapy. Med Decis Making 18:295–303
O’Connor AM (1999) Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. J Natl Cancer Inst 25:67–80
Elwyn G, O’Connor A, Stacey D et al (2006) Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 333:417
Wakefield CE, Meiser B, Homewood J et al (in press) Development and pilot testing of two decision aids for individuals considering genetic testing for cancer risk. J Genet Couns
Australian Cancer Network (1999) Guidelines on familial aspects of cancer. Australian Cancer Network, Sydney, November 1999
National Breast Cancer Centre (2000) Current best advice about familial aspects of breast/ovarian cancer. NHMRC National Breast Cancer Centre, Sydney, 2000
Centre for Genetics Education (2007) Centre for Genetics Education. http://www.genetics.com.au/. Cited 19 Jan 2007
O’Connor AM, Stacey D, Entwistle V et al (2006) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 1:1
Rimer BK, Glassman B (1999) Is there a use for tailored print communications in cancer risk communication? J Natl Cancer Inst 25:140–148
Bastani R, Maxwell A, Bradford C et al (1999) Tailored risk notification for women with a family history of breast cancer. Prev Med 29:355–364
O’Connor AM (1995) Validation of a decisional conflict scale. Med Decis Making 15:25–30
Michie S, Dormandy E, Marteau TM (2002) The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns 48:87–91
Horowitz MJ, Wilner N, Alvarez W (1979) Impact of event scale: a measure of subjective stress. Psychosom Med 41:209–218
Thewes B, Meiser B, Hickie IB et al (2001) Psychometric properties of the impact of event scale amongst women at increased risk for hereditary breast cancer. Psychooncology 10:459–468
Zigmond A, Snaith R (1983) The hospital anxiety and depression scale. Psychiatr Scand 67:361
Hall A, A’Hern R, Fallowfield L (1999) Are we using appropriate self-report questionnaires for detecting anxiety and depression in women with early breast cancer? Eur J Cancer 35:79–85
Johnston M, Pollard B, Hennessey P (2000) Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res 48:579–584
Ibbotson T, Maguire P, Selby P et al (1994) Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer 30A:37–40
Brehaut JC, O’Connor AM, Wood TJ et al (2003) Validation of a decision regret scale. Med Decis Making 23:281–292
SPSS Inc. (2005) Statistical Program for the Social Sciences: Release 14.0. SPSS Inc., Chicago, IL
StataCorp (2005) Stata Statistical software: Release 9.0. StataCorp, College Station, TX
Williams RL (2000) A note on robust variance estimation for cluster-correlated data. Biometrics 56:645–646
Bekker HL, Legare F, Stacey D et al (2003) Is anxiety a suitable measure of decision aid effectiveness: a systematic review? Patient Educ Couns 50:255–262
Botkin JR, Smith KR, Croyle RT et al (2003) Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A 118:201–209
Cull A, Anderson EDC, Campbell S et al (1999) The impact of genetic counselling about breast cancer risk on women’s risk perceptions and levels of distress. BJC 79:501–508
Lerman C, Narod S, Schulman K et al (1996) BRCA1 testing in families with hereditary breast-ovarian cancer. JAMA 275:1885–1892
Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT et al (2000) Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 335:2015–2020
O’Neill SM, Peters JA, Vogel VG et al (2006) Referral to cancer genetic counseling: are there stages of readiness? Am J Med Genet C 142:221–231
Mancini J, Nogues C, Adenis C et al (2006) Impact of an information booklet on satisfaction and decision-making about BRCA genetic testing. Eur J Cancer 42:871–881
Mireskandari S, Meiser B, Sherman K et al (2006) Evaluation of the needs and concerns of partners of women at high risk of developing breast/ovarian cancer. Psychooncology 15:96–108
Manne S, Audrain J, Schwartz M et al (2004) Associations between relationship support and psychological reactions of participants and partners to BRCA1 and BRCA2 testing in a clinic-based sample. Ann Behav Med 28:211–225
Schwartz MD, Lerman C, Brogan B et al (2005) Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer Epidemiol Biomarkers Prev 14:1003–1007
Australian Bureau of Statistics (2006) Australian social trends 2006. Australian Bureau of Statistics, Canberra
Meiser B, Butow P, Friedlander M et al (2002) Psychological impact of genetic testing for women for breast cancer susceptibility. Eur J Cancer 38:2025–2033
Tiller K, Meiser B, Gaff C et al (2006) A randomized controlled trial of a decision aid for women at increased risk of ovarian cancer. Med Decis Making 26:360–372
Acknowledgements
We would like to thank our consumer representative, Sandra Tanner, as well as each of the clinicians who reviewed numerous drafts of the DAs and recruited their patients for the study. We would also like to thank the women who completed the questionnaires. The study was funded by a project grant from the Cancer Council of New South Wales (Project Grant 300441). Ms. Wakefield is supported by an Australian Postgraduate Award. Dr. Meiser is supported by a Career Development Award from the National Health and Medical Research Council of Australia (ID 350989). The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
AGenDA Collaborative group
The members of the Australian GENetic testing Decision Aid Collaborative Group are in alphabetical order of group or institution: Centre for Genetics Education, Sydney (K. Barlow-Stewart); Familial Cancer Service, Westmead Hospital, Sydney (G. Fenton, A. Goodwin, P. Zodgekar); Hereditary Cancer Clinic, Prince of Wales Hospital, Sydney (L. Andrews, J. Koeler, A. Overkov, J. Tyler, B. Warner); Hunter Genetics, Newcastle (M. Gleeson, C. Groombridge, S. O’Donnell, A. Spigelman); Macquarie University (C. McMahon); Peter McCallum Cancer Institute, Melbourne (L. Hossack, M. Kentwell); Royal Melbourne Hospital, Melbourne (C. Aragona, R. D’Souza, C. Gaff, L. Hodgkin); St Vincent’s Hospital, Sydney (R. Ward), University of Sydney (P. Butow, H. Davey).
Rights and permissions
About this article
Cite this article
Wakefield, C.E., Meiser, B., Homewood, J. et al. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Res Treat 107, 289–301 (2008). https://doi.org/10.1007/s10549-007-9539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9539-2