Abstract
An early age at first full-term birth is associated with a reduction in the subsequent development of breast cancer among women in the general population. A similar effect has not yet been reported among women who carry an inherited BRCA1 or BRCA2 mutation. We conducted a matched case–control study on 1816 pairs of women with a BRCA1 (n = 1405) or BRCA2 (n = 411) mutation in an attempt to elucidate the relationship between age at first full-term pregnancy and the risk of developing breast cancer. Information about the age at first childbirth and other pregnancy-related variables was derived from a questionnaire administered to women during the course of genetic counselling. There was no difference in the mean age at first full-term birth in the cases and controls (24.9 years vs. 24.8 years; P = 0.81, respectively). Compared to women whose first child was born at or before 18 years of age, a later age at first full-term birth did not influence the risk of developing breast cancer (OR = 1.00 per year; 95% CI 0.98–1.03; P-trend = 0.67). Stratification by mutation status did not affect the results. These findings suggest that an early first full-term birth does not confer protection against breast cancer in BRCA mutation carriers. Nonetheless, BRCA mutation carriers opting for a prophylactic oophorectomy as a breast and/or ovarian cancer risk-reducing strategy should complete childbearing prior to age 40 when this prevention modality is most effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A woman’s reproductive history is an important determinant of her breast cancer risk [1]. Factors that protect against breast cancer in the general population include a late age of onset of menarche, multiparity, breastfeeding, and an early age at menopause [2, 3]. Of particular importance is the age at which a woman completes her first full-term pregnancy [4, 5]. An early age at first childbirth (i.e., before the age of 20) has been reported to reduce a women’s risk of developing breast cancer by up to one-half [4]; whereas, a late age at first childbirth (e.g., at age 30 or older) increases her risk. Various studies have shown that the risk associated with a late first-term pregnancy may be as great as, or higher, than the risk associated with nulliparity [6, 7].
Women who inherit a deleterious mutation in either of the two breast cancer susceptibility genes, BRCA1 or BRCA2, face a lifetime risk of breast cancer of approximately 80% [8, 9]. The incomplete penetrance of BRCA1 and BRCA2 suggests that there may be the potential to modify risk [10–12]. To date, various hormonal and reproductive factors have been found to influence a woman’s susceptibility to BRCA-associated breast cancer; these implicate an important role of estrogen-signaling (reviewed in [13]). Various groups have investigated the roles of reproductive and menstrual factors on the risk of developing breast cancer in BRCA mutation carriers, with inconsistent results (reviewed in [13]). However, the role of age at first-full term birth in the etiology of hereditary breast cancer has not yet been elucidated [14–19]. We conducted a case–control study on a sample of BRCA1 and BRCA2 mutation carriers in an attempt to clarify the relationship between age at first full-term pregnancy and the subsequent risk of developing breast cancer.
Materials and methods
Study population and design
Eligible study subjects included living women who were identified at any one of 54 participating centers in 11 countries. These women were participants in ongoing research or clinical research protocols at the host institutions. All study subjects (with the exception of those from the University of Utah and the University of California Irvine) received counselling and provided written informed consent for genetic testing.
The institutional review boards of the host institutions approved the study. In most cases, testing was initially offered to women who had been affected with breast or ovarian cancer. When a BRCA1 or BRCA2 mutation was identified in a proband or her relative, genetic testing was offered to other at-risk individuals in the family. Mutation detection was performed using a range of techniques, but all nucleotide sequences were confirmed by direct sequencing of DNA. A woman was eligible for the current study when the molecular analysis established that she was a carrier of a deleterious mutation in the BRCA1 or BRCA2 gene. Most (>95%) of the mutations identified in the study subjects were either nonsense mutations, deletions, insertions, or small frameshifts resulting in a premature termination of protein translation.
Information was available on cancer and reproductive histories for a total of 7,243 women who carried a BRCA1 or BRCA2 mutation. Case subjects were study subjects with a diagnosis of invasive breast cancer. Control subjects were women who never had breast cancer and who were also carriers of a mutation in BRCA1 or BRCA2. Potential subjects were excluded if they had been diagnosed with ovarian (62 women) or other cancer (450 women) prior to the year of breast cancer diagnosis of the case, or if information about pregnancy-related information was incomplete (362 women). Three potential case subjects were excluded due to missing information regarding the year of breast cancer diagnosis. After exclusions, there were a total of 6,366 eligible women, including 2,904 women with breast cancer (potential case subjects) and 3,462 women without breast cancer (potential controls).
Data collection
Case and controls subjects completed a questionnaire that asked for information regarding family history, reproductive and medical histories, and selected lifestyle factors including smoking and the use of oral contraceptives. Questionnaires were administered at the individual centers at the time of a clinic appointment or at their home at a later date. The participants were asked if they had ever been pregnant and were asked to consider all pregnancies, in order, from first to last and provide the year and outcome of each pregnancy. Additional variables of interest included information on residence and ethnic group.
Statistical analyses
A matched case–control analysis was performed to evaluate the association between the age at first full-term pregnancy and risk of breast cancer. A single control subject was selected for each case subject, matched according to mutation in the same gene (BRCA1 or BRCA2), year of birth (within 1 year), country of residence and a diagnosis of ovarian cancer (the ovarian cancer diagnosis had to have occurred after the year of breast cancer diagnosis of the matched case subject). In addition, bilateral mastectomy had to have occurred after the year of breast cancer diagnosis of the matched case subject. A total of 1,816 matched case–control pairs was generated, including 1,405 pairs with BRCA1 mutations and 411 pairs with BRCA2 mutations. The mean age at first full-term pregnancy for parous woman was compared between the case subjects and control subjects using the Student’s t-test. This test statistic was also used for all other continuous variables. The χ 2-test was used to test for differences in categorical variables and to compare the proportion of case and control subjects in each age at first full-term birth category. The multivariate odds ratios (OR), 95% confidence intervals (CI) and tests for linear trend were estimated by use of conditional logistic regression. Women with a first birth at or before the age of 18 were used as the referent group. A multivariate analysis was carried out to control for the potential confounding effects of age at menarche, oral contraceptive use (ever/never), prophylactic oophorectomy (yes/no), parity (one, two, three, four or more births), breastfeeding (ever/never) and ethnicity (Caucasian, French–Canadian, Jewish, and other). Parity included both live- and still-born births, and twin or triplet pregnancies were coded as one birth. All statistical tests were two-sided. All analyses were performed using the SAS statistical package, version 8.1 (SAS Institute, Cary, NC).
Results
Case and control subjects were similar with respect to age, oral contraceptive use, and parity (Table 1). The average age at menarche of the case subjects was significantly earlier than that of the control subjects (12.9 vs. 13.0 years; P = 0.005) and cases were less likely to have had an oophorectomy than controls (2.7% vs. 4.7%; P = 0.001). Breastfeeding was less common among the case subjects (57.4% vs. 60.4%), although this difference did not reach statistical significance (P = 0.08).
Among parous women, there was no difference in the mean age at first full-term birth in the entire study population (24.9 vs. 24.8; P = 0.81 for cases and controls, respectively). The mean age at first full-term birth did not differ between BRCA1 case and control subjects (24.7 vs. 24.6; P = 0.49), nor between BRCA2 case and control subjects (25.3 vs. 25.7; P = 0.37). The proportions of cases and controls with a first-full term birth in each age category were similar (P = 0.13; frequency test) (Table 2).
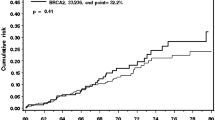
When compared to women whose first child was born at or before 18 years of age, an increasing age at first full-term birth did not influence the risk of developing breast cancer (OR = 1.00 per year; 95% CI 0.98–1.02; P-trend = 0.82) (Table 3). Adjusting for the covariates in the multivariate model did not modify the results (Table 3 and Fig. 1). We also examined the association with age at first full-term pregnancy and breast cancer risk following stratification by mutation status (BRCA1 vs. BRCA2), with similar results (data not shown). The univariate and multivariate OR’s associated with each first-full term birth age category are depicted graphically in Fig. 1.
Discussion
Our findings suggest that an early full-term birth does not confer protection against breast cancer in BRCA mutation carriers. We found that the risk of breast cancer did not increase with an increasing age of first pregnancy (OR = 1.00 per year; 95% CI 0.98–1.03; P-trend = 0.67). It appears that the protective role of an early age at first childbirth, which is seen in the general population, is not an important risk factor for genetically predisposed women. Our findings are in agreement with other smaller studies that have reported that an earlier age at first full-term pregnancy does not confer protection against BRCA-associated breast cancers [14, 15, 17, 18]. However, two studies have shown that the age at first full-term pregnancy does influence BRCA-associated breast cancer. Rebbeck et al. observed a threefold increase in BRCA-associated breast cancer risk among nulliparous mutation carriers or whose age at first live birth occurred after 30 (OR = 3.06; 95% CI 1.52–6.16) [16]. It should be mentioned that approximately one third of the participants in the Rebbeck et al. study were also included in this study. In a recent international study of 1601 of BRCA carriers, the authors reported a significant effect in the opposite direction [19]. Compared with those who had a first birth before the age of 20, a late age at first childbirth (at or after the age of 30) among BRCA1 mutation carriers was associated with a hazard ratio of 0.58 (95% CI 0.36–0.94; P-trend = 0.03).
In summary, it appears that the age of a first full-term birth does not affect a woman’s subsequent risk of developing breast cancer. This observation suggests that factors that predict risk in the general population may not be directly applicable to genetically predisposed women. Table 4 summarizes the reproductive risk factors and their respective odds ratios in BRCA mutation carriers from previous studies derived from this cohort of women. It is perhaps surprising that a later age at menarche and oophorectomy confer protection, but that an early age at first full-term birth does not. However, the role of pregnancy in the etiology of BRCA-breast cancer is not clear. A recent study by Cullinane et al. showed that a protective effect of multiparity only present among BRCA1 mutation carriers with four or more children, but that increasing parity was a risk factor for BRCA2-associated breast cancer [20]. Other reproductive factors (i.e. age at menarche and breastfeeding) appear to play a less important role in the etiology of BRCA2- than BRCA1-associated carcinogenesis [21, 22].
Studies suggest an important role of the BRCA1 and BRCA2 proteins in mammary cell differentiation and proliferation [23, 24]. Rajan et al. have shown that mRNA expression of both BRCA1 and BRCA2 are at their maximum in proliferating cells in mice undergoing puberty- or pregnancy-induced differentiation and that the spatial and temporal patterns of expressions are similar [24]. However, in response to the ovarian hormones 17β-estradiol and progesterone, there was a significantly greater up-regulation of BRCA1 than BRCA2 mRNA in the breast, demonstrating different responses of the two genes to sex hormones [24]. Levels of BRCA1 and BRCA2 mRNA are also elevated in the mammary glands of parous mice indicating their role as markers of breast differentiation [24, 25]. The BRCA proteins interact with Stat5a, a mammary gland transcription factor that is stimulated by prolactin at the end of pregnancy, and are involved in the growth and terminal differentiation of breast epithelial cells [26]. BRCA1 has also been shown to suppress estrogen-mediated breast cell proliferation, in vitro [27].
The breasts of nulliparous women are predominantly composed of undifferentiated (type 1) lobules, which have a high proliferative index and a high concentration of steroid hormone receptors [28]. They are believed to be more susceptible to carcinogenic insult and are considered to be the sites of origin of breast carcinomas. The parity-induced protective effect is believed to be due to the hormone-induced differentiation that occurs in the lobules of mammary epithelial cells turning them into type 2 and predominantly, type 3 lobules, making them less susceptible to carcinogens [28–30]. Thus, an early age at first childbirth should result in a differentiated state and reduced risk of developing breast cancer.
Russo et al. have reported that the developmental pattern of breast tissue from parous women with a family history of breast cancer or a BRCA1 mutation was similar to that of nulliparous women, suggesting a functional role of the BRCA1 gene in the branching pattern of the breast during lobular development associated with pregnancy [31]. Lack of BRCA1 results in no, or poor, differentiation and development of the breast. Others have also demonstrated abnormal mammary development in response to pregnancy using a mouse model with a tissue-specific BRCA1 mutation [32]. As suggested by Russo et al., it may be that pregnancy does not induce the expected terminal differentiation and reduction of the at-risk population of breast cells in BRCA1 mutation carriers [29] and thus, does not confer protection against the development BRCA1-associated breast cancer. This hypothesis is consistent with our observation. The effect of pregnancy on the lobular architecture of BRCA2 mutation carriers has not yet been reported.
Ours was a case–control study and we used self-reported historical data. However, personal recall of age at first birth is without a doubt accurate for most women. Strengths of our study included a large sample of known BRCA mutation carriers. We included 1,816 matched pairs and our matching criteria ensured that the case and control subjects were similar with respect to year of birth, mutation, and country of residence. All potential confounding factors were accounted for in the multivariate model. Previous studies have been limited by their small sample sizes as well as the inclusion of specific subgroups of women (i.e., women of Ashkenazi Jewish ethnicity or women with the Icelandic BRCA2 founder mutation).
Here we report no effect of age at first full-term pregnancy on the risk of breast cancer in BRCA1 and BRCA2 mutation carriers as a whole. There appears to be no compelling reason for BRCA1 or BRCA2 mutation carriers to start their childbearing early with respect to breast cancer; however, women who have a birth should be encouraged to breastfeed [21]. Nevertheless, those women considering a prophylactic oophorectomy as a means to protect against the development ovarian cancer should aim to complete their childbearing prior to the age of 40 when the risk for ovarian cancer starts to rise [33]. These data offer valuable information for use in the counselling and clinical management of BRCA mutation carriers of childbearing age.
References
Pike MC, Krailo MD, Henderson BE et al (1983) ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature 303(5920):767–770
Kelsey JL, Gammon MD, John EM (1993) Reproductive factors and breast cancer. Epidemiol Rev 15(1):36–47
Key TJ, Verkasalo PK, Banks E (2001) Epidemiology of breast cancer. Lancet Oncol 2(3):133–140
MacMahon B, Cole P, Lin TM et al (1970) Age at first birth and breast cancer risk. Bull World Health Organ 43(2):209–221
Chie WC, Hsieh C Newcomb PA et al (2000) Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol 151(7):715–722
Brinton LA, Hoover R, Fraumeni JF Jr (1983) Reproductive factors in the aetiology of breast cancer. Br J Cancer 47(6):757–762
Layde PM, Webster LA Baughman AL et al (1989) The independent associations of parity, age at first full term pregnancy, and duration of breastfeeding with the risk of breast cancer. Cancer and Steroid Hormone Study Group. J Clin Epidemiol 42(10):963–973
Ford D, Easton DF, Stratton M et al (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62(3):676–689
Antoniou A, Pharoah PD, Narod S et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
Narod S, Lynch H, Conway T et al (1993) Increasing incidence of breast cancer in family with BRCA1 mutation. Lancet 341(8852):1101–1102
Wooster R, Neuhausen SL, Mangion J et al (1994) Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science 265(5181):2088–2090
Easton DF, Ford D, Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56(1):265–271
Narod SA (2002) Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer 2(2):113–123
Narod SA, Goldgar D, Cannon-Albright L et al (1995) Risk modifiers in carriers of BRCA1 mutations. Int J Cancer 64(6):394–398
Chang-Claude J, Becher H, Eby N et al (1997) Modifying effect of reproductive risk factors on the age at onset of breast cancer for German BRCA1 mutation carriers. J Cancer Res Clin Oncol 123(5):272–279
Rebbeck TR, Wang Y, Kantoff PW et al (2001) Modification of BRCA1- and BRCA2-associated breast cancer risk by AIB1 genotype and reproductive history. Cancer Res 61(14):5420–5424
Hartge P, Chatterjee N, Wacholder S et al (2002) Breast cancer risk in Ashkenazi BRCA1/2 mutation carriers: effects of reproductive history. Epidemiology 13(3):255–261
Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S et al (2003) BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res 5(5):R121–R128
Andrieu N, Goldgar DE, Easton DF et al (2006) Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer Inst 98(8):535–544
Cullinane CA, Lubinski J, Neuhausen SL et al (2005) Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer 117(6):988–991
Jernstrom H, Lubinski J Lynch HT et al (2004) Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 96(14):1094–1098
Kotsopoulos J, Lubinski J, Lynch HT et al (2005) Age at menarche and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Causes Control 16(6):667–674
Rajan JV, Wang M, Marquis ST et al (1996) Brca2 is coordinately regulated with Brca1 during proliferation and differentiation in mammary epithelial cells. Proc Natl Acad Sci USA 93(23):13078–13083
Rajan JV, Marquis ST, Gardner HP et al (1997) Developmental expression of Brca2 colocalizes with Brca1 and is associated with proliferation and differentiation in multiple tissues. Dev Biol 184(2):385–401
Marquis ST, Rajan JV, Wynshaw-Boris A et al (1995) The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet 11(1):17–26
Vidarsson H, Mikaelsdottir EK, Rafnar T et al (2002) BRCA1 and BRCA2 bind Stat5a and suppress its transcriptional activity. FEBS Lett 532(1–2):247–252
Fan S, Wang J, Yuan R et al (1999) BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284(5418):1354–1356
Russo J, Rivera R, Russo IH (1992) Influence of age and parity on the development of the human breast. Breast Cancer Res Treat 23(3):211–218
Russo J, Russo IH (1994) Toward a physiological approach to breast cancer prevention. Cancer Epidemiol Biomarkers Prev 3(4):353–364
Russo J, Russo IH (1999) Cellular basis of breast cancer susceptibility. Oncol Res 11(4):169–178
Russo J, Lynch H, Russo IH (2001) Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J 7(5):278–291
Xu X, Wagner KU, Larson D et al (1999) Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet 22(1):37–43
Eisen A, Lubinski J, Klijn J et al (2005) Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case–control study. J Clin Oncol 23(30):7491–7496
Acknowledgements
Joanne Kotsopoulos is supported by a fellowship from the Canadian Breast Cancer Foundation, Ontario Chapter. Charis Eng is a recipient of the Doris Duke Distinguished Clinical Scientist Award. Susan L. Neuhausen is supported by NIH CA74415.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Other members of the Hereditary Breast Cancer Clinical Study Group—J. Garber, Dana Farber Cancer Center, D. Gilchrist, University of Alberta, M. Osborne, Strang Cancer Prevention Centre, New York, NY, USA, D. Fishman, Northwestern University, E. Warner, Toronto-Sunnybrook Regional Cancer Center, Toronto, ON, Canada, J. McLennan, University of San Francisco, W. McKinnon, University of Vermont, S. Merajver, University of Michigan Comprehensive Cancer Center, H. Olsson, Jubileum Institute, Department of Oncology, Lund University Hospital, Lund, Sweden, D. Provencher, University of Montreal, B. Pasche, Northwestern Medical Facility, Chicago, IL, USA, G. Evans, Regional Genetics Service, St. Mary’s Hospital, Manchester, UK, WS Meschino, North York General, North York, ON, Canada, E. Lemire, Division of Medical Genetics, Royal University Hospital and the University of Saskatchewan, Canada, A. Chudley, Children’s Hospital, Winnipeg, Manitoba, Canada, D. Rayson, Queen Elizabeth Health Sciences Centre, Halifax, Nova Scotia, Canada and C. Bellati, Dipartimento di Genetica, Biologia e Biochimica, Università di Torino, Italy.
Rights and permissions
About this article
Cite this article
Kotsopoulos, J., Lubinski, J., Lynch, H.T. et al. Age at first birth and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 105, 221–228 (2007). https://doi.org/10.1007/s10549-006-9441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9441-3