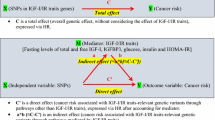
Abstract
Background
An insulin-related pathway to breast cancer has been hypothesized.
Methods
We examine the 19 CA repeat of the IGF1 gene, the -202 C > A IGFBP3, the G972R IRS, and the G1057D IRS2 polymorphisms among 1,175 non-Hispanic white (NHW) and 576 Hispanic newly diagnosed breast cancer cases and 1,330 NHW and 727 Hispanic controls living in Arizona, Colorado, New Mexico, and Utah.
Results
Among post-menopausal women not recently exposed to hormones, not having the 19 CA repeat of IGF1 gene was associated with breast cancer among NHW women [odds ratio (OR) 2.14, 95% confidence interval (CI) 1.21–3.79] and having an R allele of G972R IRS1 increased breast cancer risk among Hispanic women (OR 2.70, 95% CI 1.13–6.46). Among post-menopausal Hispanic women recently exposed to hormones the A allele of the -202 C > A IGFBP3 polymorphism increased risk of breast cancer (OR 1.57, 95% CI 1.06–2.33). The IGF1 19 CA repeat polymorphism interacted with hormone replacement therapy (HRT) among NHW post-menopausal women; women who had the 19/19 IGF1 genotype were at reduced risk of breast cancer (OR 0.64, 95% CI 0.47–0.88) if they did not use HRT. We also observed interaction between body mass index and IGF1 19 CA repeat (p=0.06) and between weight gain and the -202 C > A IGFBP3 polymorphism (p=0.05) in NHW post-menopausal women not recently exposed to hormones.
Conclusions
Our data suggest that associations between insulin-related genes and breast cancer risk among women living in the Southwestern United States may be dependent on estrogen exposure and may differ by ethnicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uncontrolled cell growth is central to the carcinogenic process. There is a growing body of evidence that suggests that insulin-like growth factors (IGF), insulin-like growth factor binding proteins (IGFBPs), especially IGFBP3, insulin, and insulin-receptor substrate (IRS) play a significant role in the initiation of cell growth and proliferation of cancer [1–5]. Insulin has been regarded as primarily a metabolic signal while IGF-1 has been implicated as an important mitogen and cell differentiation factor [6, 7]. The IRS protein family contains several members, of which IRS-1 and IRS-2 are expressed in almost all cells and tissues [8–10]. In addition to their role in insulin signaling, IRS-1 and IRS-2 are also substrates for the IGF receptor and thus act in IGF signal transductions. While IRS-1 controls body growth and peripheral insulin action, IRS-2 regulates body weight control and glucose homeostasis [11]. Within tumors, IRS-1 may be a marker of an active IGF signal transduction pathway [4, 12]. Although IRS is involved in insulin and IGF signaling, it also appears to be important in regulating estrogen signaling [13].
Many factors are involved in the regulation of insulin and IGFs, including diet, lifestyle, hormonal, and genetic factors. Studies have shown that diet, physical activity, body size, and sex steroids are involved in the regulation of these hormones [14–21]; some data suggest that serum levels of IGF-1, IGFBP-3, and IRS also may be affected by polymorphisms in these genes [22–25]. We have previously published data on associations between IGF-1 and IGFBP-3 serum levels and genetic variants of these genes in non-Hispanic white (NHW) and Hispanic women living in the Southwest [14]. Data from that study suggest different allele frequencies between NHW women and Hispanic women for the IGFBP3 polymorphism at nucleotide -202. In that study, we showed that serum levels of IGFBP-3 are correlated with genotype in a dose response fashion, i.e., AA > AC > CC. Furthermore, we observed that Hispanic women had higher IGFBP-3 serum levels even after adjusting for genotype. In a separate analysis [26], two associations were observed that were consistent in both Hispanics and NHW women: IGF1 CA repeat alleles of length other than 19 were associated with higher mean waist-to-hip ratios (WHR), p=0.01, and women who carried an IGFBP3 A allele, compared with women with the CC genotype, more often reported high birth weight [odds ratio (OR) 1.9, 95% confidence interval (CI) 1.1–3.2]. Additionally, we observed that having the A allele of IGFBP3 was associated with height and that the R allele of IRS1 G972R polymorphism was associated with smaller WHR. These observations add support for the functionality of these polymorphisms.
These genes also have been examined with cancer and other conditions thought to be important contributors to cancer risk. The Gly972Arg (G972R) polymorphism in IRS1 has been associated with insulin resistance and type 2 diabetes; the IRS1 R allele has been associated with increased risk of colon cancer [27]. The IRS2 G1057D polymorphism has been associated with obesity [28]. Variation in serum IGF-1 levels has been associated with the 19 CA repeat polymorphism in the IGF1 gene 1 kb upstream of the transcription start site [28]. The most common allele containing 19 CA repeats is sometimes denoted “192” for the size of the PCR product [29–32]. This 19 CA repeat variant of the IGF1 gene has been evaluated with prostate and colon cancer, where associations are generally more consistent for prostate cancer than for colon cancer [27, 29–32].
In this paper, we evaluated the associations of genetic polymorphisms in the IGF1, IGFBP3, IRS1, and IRS2 genes with breast cancer. We evaluated interactions between insulin-related genes and hormone replacement therapy (HRT) as well as the body mass index (BMI) of kg/m2, and weight gain. Since the association between obesity and breast cancer risk differs for pre- and post-menopausal women, we examined these groups separately. Associations were determined separately for NHW women and for Hispanic/American Indian (AI) women living in the Southwestern states of Arizona, Colorado, New Mexico, and Utah. Hispanics and AI have markedly higher prevalence of obesity, but lower breast cancer incidence, than NHWs, and the roles of insulin and IGF pathways in breast cancer in these ethnic groups may be different.
Methods
Study participants were women living in Cochise, Coconino, Maricopa, Pima, Pinal, Santa Cruz, and Yuma Counties in Arizona, or the states of Colorado, New Mexico, or Utah at the time of diagnosis or selection, excluding AI women living on reservations. Study hypotheses focused specifically on breast cancer in Hispanic women, therefore sampling was stratified on ethnicity to select Hispanic women in larger proportion than their representation in the population. All Hispanic women diagnosed with breast cancer during the study period were selected for the study. An age-matched sample of NHW women were randomly selected on a 1 to 1 ratio to the distribution of Hispanic cases in Arizona and Colorado; at a 4 to 1 ratio to the distribution of Hispanic cases in Utah; all Hispanic and non-Hispanic cases age 50 and under in New Mexico; and a 1 to 1 ratio for women over 50 in New Mexico. The GUESS program (Generally Useful Ethnic Search System) was used to identify women who were Hispanic [33].
Cases were histologically confirmed in situ and invasive breast cancer (ICDO sites C50.0–C50.6 and C50.8–C50.9) diagnosed between October 1999 and May 2004. State tumor registries were used to initially identify and subsequently confirm case eligibility. An electronic rapid case ascertainment system was used in Utah to identify cases while in the other states cases were identified through normal registry operations. The Utah and New Mexico state cancer registries are NCI funded Surveillance Epidemiology and End Results registries; Arizona and Colorado registries are part of the Center for Disease Control National Program of Cancer Registries. Cases were identified as Hispanic or Native American from registry abstract data where available.
Controls were selected from the target populations to match ethnicity and 5-year age distribution of cases. In Arizona and Colorado, participants under 65 were randomly selected from a commercial mailing list; in New Mexico and Utah controls less than 65 years were randomly selected from driver’s license lists. In all states, women 65 years and older were randomly selected from Center for Medicare Services lists.
All women identified were screened for eligibility prior to study enrollment. As part of the screening, women were asked to self-identify their race and ethnicity. Women who reported their race as only African American or Asian were excluded from the study. Women initially identified as being Hispanic by the GUESS program who were determined not to be Hispanic or AI were ineligible for the study. All participants signed informed written consent prior to participation; the study was approved by the Institutional Review Board for Human Subjects at each institution.
Diet and lifestyle data were collected by trained and certified interviewers using an interviewer-administered computerized questionnaire. These methods have been described in detail [34, 35]. The questionnaire was translated into Spanish by two individuals with an arbitrator resolving differences in translation between the two original translators. The referent period was the year prior to diagnosis for cases or selection for controls. Respondents were given the option of having the interview administered in either English or Spanish.
Respondents were asked to self-identify their ethnicity and race as part of the study questionnaire. If a respondent described herself as belonging to more than one race or ethnic group, both were recorded. The questionnaire included information about medical history, reproductive history, family history, diet, physical activity, use of tobacco, medication use, diabetes history, and weight history, birth weight, and weight at ages 15, 30, 50 and during the referent year. Women were asked to “best describe your menstrual status on (referent date)” by selecting response from a card; this information was used to define individual menopausal status. Weight was measured at the time of interview to the nearest 0.50 lb and height was measured to the nearest 0.25 in. BMI was calculated using the formula of weight in kilograms (kg)/height in meters (m2). Recalled weight at ages 15, 30, 50 (if over 50 years of age), and referent year were used to calculate BMI for each age and the referent year. We evaluated adult BMI using international cutpoints of <25 as normal weight, 25–29.9 as overweight, and 30+ as obese. Weight gain was calculated as difference in weight between recalled weight at age 15 and weight recalled during the referent year.
Dietary intake data were collected using an extensive diet history questionnaire that was modified to incorporate foods commonly eaten in the Southwestern United States [36]. An extensive physical activity questionnaire that was modified from the Cross Cultural Activity Participation Survey [37] and was used to collect information on activities performed at home, at work, and during leisure and included intensity of the activity and frequency at which activities were performed during the referent year, at ages 15, 30, and 50. Total metabolic equivalents or MET hours of activity during the referent year was calculated based on the compendium of MET values for physical activities [38].
Sixty-eight percent (68%) of women contacted participated in the study. Of these cases, 798 Hispanic and AI, and 1,527 NHW women were diagnosed with first primary breast cancer and are included in these analyses. Of controls identified, 945 Hispanic and AI and 1,671 NHW women participated (42% of participants contacted). Blood was collected and DNA extracted for 76.6% of participants in Arizona, 74.8% of participants in Colorado, 75% of participants in New Mexico, and 93.6% of participants in Utah.
Genotyping
IRS1
The G972R polymorphism was detected using PCR amplification with primers 5′-CTT CTG TCA GGT GTC CAT CC and 5′-TGG CGA GGT GTC CAC GTA GC. PCR cycling consisted of an initial denaturation at 94°C for 2 min, 10 cycles at 94°C 10 s, 60°C 10 s, and 72°C 10 s followed by 30 cycles at 94°C 10 s, 55°C 10 s, and 72°C 10 s. BstNI was used to digest the PCR products following manufacturer’s instructions. Alleles were scored as either G for glycine or R for arginine (absence or presence of the restriction site, respectively).
IRS2
The G1057D polymorphism was detected using a TaqMan assay. Primer sequences were IRS2-F 5′-GGA GCT GTA CCG CCT GCC and IRS2-R 5′-ACC AAA AGC CAT CTC GGT GT. The probes were 5′-FAM-CCG GGC GCC GCC TCA T-Tamra and 5′-VIC-CGG ACG CCG CCT CAT CGT T-Tamra [39]. Each 17 μl PCR reaction contained 20 ng genomic DNA, 900 nM of each primer, 130 nM of each TaqMan probe, and 8.5 μl TaqMan Universal PCR Master Mix (contains AmpErase UNG and AmpliTaq Gold enzymes, dNTPs, and reaction buffer). PCR was carried out under the following conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 62°C for 1 min using the BIO-RAD IQ detection system. The fluorescence of each sample was collected and analyzed version 3.0 of the iCycler IQ Real-Time detection software.
IGF1
IGF1 CA repeat genotypes were determined by PCR amplification using primers IGF1-F 5′-FAM-GCT AGC CAG CTG GTG TTA TT-3′ and IGF1-R 5′-ACC ACT CTG GGC GAA GGG TA-3′. PCR conditions consisted of a 2-min denaturation at 94°C followed by 30 cycles at 94°C 10 s, 57°C 10 s, and 72°C 15 s. The products were then sized using an ABI 3700 fluorescent sequencer. Alleles were assigned by the size of the fragment in base pairs and classified as “192” or not “192.” “192” is the PCR product size of the most common allele which contains 19 CA repeats.
IGFBP3
The -202 A > C polymorphism was amplified using primers F 5′-CCA CGA GGT ACA CAC GAA TG and R3 5′-TGA GCA GCC GGG GCC GAG. A 0.5 units of Amplitaq gold and 5% DMSO were used to increase efficiency of amplification. PCR conditions were 9 min initial denaturation at 95°C followed by 40 cycles at 95°C 10 s, and 66°C 20 s. The resulting PCR product was digested with 4 units of Alw21I at 37°C overnight. Digested products were separated on a 2% Nusieve gel stained with ethidium-bromide and visualized with ultraviolet light. Alleles were scored as either A or C (presence or absence of the restriction site, respectively).
Statistical methods
SAS statistical package, version 9 was used to conduct the analyses. IRS1 genotypes were GG, GR, and RR, with the R allele being less common. Because of the rarity of the RR genotype (Table 1), associations with IRS1 were only conducted on the dominant model. IRS2 genotypes were GG, GD, and DD, with the GG genotype being most common. IGF1 genotypes analyzed were 19/19 CA repeats, heterozygous, or no 19 CA repeat alleles; the absence of the 19 CA repeat allele was less common than the presence of the 19 CA repeat allele. IGFBP3 genotypes were CC, CA, and AA. The dominant model was used to assess interaction.
Analyses included evaluating the distribution of the genotypes in the population, the independent associations of genetic polymorphisms with breast cancer risk, and the joint effect of genotypes and HRT and body size on breast cancer risk. Multivariable logistic regression models were used to estimate relative risk. Adjustment variables in these models included age, center, race/ethnicity (if not stratified analysis), parity, BMI, long-term physical activity, and energy intake. Center was used as an adjustment variable to help control for different proportion of cases and controls interviewed at each center. Data were analyzed by ethnicity and by menopausal status. Additionally, among post-menopausal women we evaluated differences in association between those women who had become post-menopausal within the past 2 years or were using HRT and those who have not been exposed to hormones during the past 2 years. Statistical interaction between polymorphisms and HRT, BMI, and weight change were assessed using multiplicative interaction models.
Results
IRS1, IRS2, IGF1, and IGFBP3 were in HWE for both NHW women and Hispanic/AI women. Hispanic/AI women were significantly less likely to have an R allele of the G972R IRS1 polymorphism than NHW women, and less likely to have an A allele of the -202 A > C IGFBP3 polymorphism (Table 1). There were no significant differences in genotype frequency between Hispanic/AI and NHW women for either the G1057D IRS2 polymorphism or the number of 19 CA repeats in IGF1. The majority of women were post-menopausal and had used HRT.
There were few significant associations in the polymorphisms assessed among NHW and Hispanic/AI women for pre-menopausal women or for post-menopausal women overall (Table 2). However, among post-menopausal women who had not recently been exposed to hormones (Table 3), we observed a significant increased risk of breast cancer among NHW women without the more common 19 CA repeat allele of the IGF1 gene. Having an R allele of the G972R IRS1 polymorphism significantly increased risk of breast cancer among post-menopausal Hispanic/AI women who had not recently been exposed to hormones (OR 2.70, 95% CI 1.13–6.46). Having an A allele of the IGFBP3 polymorphism was associated with increased breast cancer risk among Hispanic/AI women. Although the AA genotype was not statistically significantly associated, there were few Hispanic women with that genotype. Using the dominant model to assess the association between IGFBP3 C > A polymorphism and breast cancer risk resulted in a significant increased risk associated (OR 1.57, 95% CI 1.06–2.33 for CA/AA genotypes relative to CC).
Interactions between the IGF1 19 CA repeat, the -202 C > A IGFBP3 polymorphism and recent HRT use, BMI, and weight gain since age 15 are presented in Table 4 for NHW women and in Table 5 for Hispanic/AI women. There were no significant interactions between pre-menopausal breast cancer and IGF1 and IGFBP3 polymorphisms assessed in either Hispanic or NHW women. Having the 19/19 genotype of the IGF1 gene resulted in reduced breast cancer risk among NHW women not recently using HRT (p interaction <0.01). Because of this significant interaction with HRT, subsequent interactions were presented stratified by recent hormone exposure. Among women not recently exposed to hormones, there was a significant interaction between IGF1 CA repeat and referent year BMI that was borderline significant for both NHW and Hispanic/AI women (p=0.06). The greatest risk associated with obesity was among women who did not have the IGF1 19/19 CA repeat genotype. The IGFBP3 polymorphism interacted with weight gain since age 15 among NHW post-menopausal women recently exposed to hormones. Women with the least weight gain since age 15 were at increased risk when they also had an A allele of the −202 IGFBP3 polymorphism, however, gaining weight increased risk only among women with the CC genotype. This association was stronger among NHW women. There were no significant interactions between IRS1 and IRS2 polymorphisms and BMI, weight gain, and recent use of HRT in either Hispanic or NHW women (data not shown in table).
Discussion
Different allele frequencies for both the G972R IRS1 and −202 IGFBP3 polymorphisms were observed between Hispanic/AI and NHW women living in the Southwestern United States. The R allele of the G972R IRS1 polymorphism was less common among Hispanic/AI women and also was associated with increased risk of breast cancer among Hispanic/AI women not recently exposed to hormones. Likewise, the A allele of the −202 IGFBP3 polymorphism was less common in the Hispanic/AI women and was associated with increased risk of breast cancer among post-menopausal Hispanic/AI women. Our data suggest that the associations between insulin-related genes and breast cancer are influenced by hormone exposure and body size.
Estrogen has been shown to regulate IRS−1 expression [40]. IRS-1 has been shown to be the predominant signaling molecule activated by IGF-1 and insulin [41]. Thus our finding that exposure to hormones mediates the association with insulin-related genes is reasonable given the close relationship of the estrogen and insulin pathways. Our data suggest that in the absence of estrogen, post-menopausal women not recently exposed to hormones, variants of genes associated with increased IGF-1 levels were associated with increased risk of breast cancer. These results reinforce the close relationship between the estrogen and insulin pathways and support their influence on each other in influencing breast cancer risk.
Studies evaluating associations with serum levels of IGF-1 and breast cancer specifically report mixed results, although some studies suggest stronger associations among pre-menopausal women [42–49]. Our studies of genetic variants that may influence serum levels is undertaken as a means to explore these associations. The IGF1 19 CA repeat has been the most commonly studied polymorphism. Although it was not associated with serum IGF-1 levels in postmenopausal women in the Multiethnic Cohort [31], as study of predominately African American women showed a direct association between plasma levels of IGF-1 and the 19 CA repeat polymorphism in the IGF1 gene [50]. In the Nurses’ Health Study, women without a 19 CA allele had lower IGF-1 levels [51]. A study of 807 breast cancer patients and 1,588 matched controls from the EPIC Study examined 23 common SNPS in IGF1, IGFBP1, IGFBP3 and the IGF acid-labile subunit. While they observed associations between SNPs of these genes and respective serum levels, the associations between the SNPs and breast cancer were weaker and limited to younger women [52]. In an earlier report based on the same 4-Corners study population as presented here, we observed higher IGF-1 levels among NHW women without the 19 CA repeat in the IGF1 gene, while among Hispanic women, those with the 19 CA repeat had the highest IGF-1 levels [14] One of the few studies that evaluated polymorphisms of the IGF1 gene and breast cancer showed an association with the C allele of the IGF1 maker rs1520220 (not examined in this study). In that study of 4,647 breast cancer cases and 4,564 controls conducted in East Anglian region of the United Kingdom an increased risk of breast cancer was associated with the C allele of the IGF1 gene rs1520220 (OR 1.41, 95% CI 1.11–1.79).
IGFBP-3 binds IGF-1 and thus it has been hypothesized that higher IGFBP-3 would reduce risk of breast cancer [53]. Some studies show no association between breast cancer and IGFBP-3 serum levels [54] and others observe that higher IGFBP-3 levels lower breast cancer risk [55]. We observed significantly higher levels of serum IGFBP-3 among both Hispanic and NHW women who had the AA genotype, as has been reported by others [14, 56]. However, the literature is limited on the association between polymorphisms of the IGFBP3 gene, IGFBP-3 serum levels, and breast cancer [54]. The -202 polymorphism of the IGFBP3 gene was not associated with breast cancer in the EPIC study [52], nor was there an association with breast cancer in a study by Schernhammer et al. [56]. However, a modest inverse association with the A allele of the -202 polymorphism of IGFBP3 (0.87, 95% CI 0.77–0.99) was reported in the UK study population. We find evidence of a slight increased risk of breast cancer associated with the A allele, this risk was strongest among Hispanic women, but only among post-menopausal women who had been recently exposed to hormones.
Only one study to our knowledge has examined polymorphisms of either IRS1 or IRS2 and breast cancer and did not observe a significant association between variants of either gene and breast cancer risk [57]. In this study having an R allele of the G972R IRS1 polymorphism significantly increased risk of breast cancer among post-menopausal Hispanic women not recently exposed to hormones. IRS1 is regulated by estrogen [13], and the increased risk is only observed in the absence of estrogen.
Among NHW post-menopausal women, the 19 CA repeat IGF1 polymorphism interacted with BMI among women not recently exposed to hormones (p=0.06). Our data also suggest that IGFPB3 may regulate the association with weight gain among these women. In our previous work [35], BMI increased risk of breast cancer among NHW post-menopausal women not recently exposed to hormones, but not among Hispanic women. Others have reported similar associations between breast cancer and BMI [58]. Weight change and weight fluctuation have been shown in other studies to be directly associated with breast cancer risk [59–61], with the greatest effect among post-menopausal women who did not use HRT [58, 62]. We believe that the differences in association with body size by genetic polymorphism may indicate that obesity and weight gain operate differently in their association with breast cancer and that underlying metabolic or unidentified factors. Obesity can be representing a lifetime condition, with obesity at an early age having a potentially different metabolic consequence than obesity when older. The metabolic effects of gaining weight may influence insulin levels differently depending on age at onset of obesity or other factors.
The study has limitations. Although the sample size of Hispanic/AI women was one of the largest reported to date, we were hampered by small numbers when looking at interaction between genetic polymorphisms and HRT, BMI, or weight gain. Our participation was less than desired; however associations with BMI are similar to those reported in other prospective and retrospective studies [35]. Although our response differed by center we had the benefit of identical data collection methods and questionnaires to help assure consistency across centers. We had no reason to believe that allele frequencies of the genes studied would be affected by response rates. Additionally, we evaluated one polymorphism of each gene assessed. These polymorphisms were selected because the literature suggested functionality associated with these variants, although other polymorphisms may be important and should be evaluated in future studies.
Our data provide support for the association between insulin-related factors and breast cancer risk among women living in the Southwestern United States. IGF1, IGFBP3, and IRS1 polymorphisms appear to be most importantly associated with breast cancer in subsets of breast cancer cases. Different associations were observed for Hispanic women, consistent with the idea that the relative importance of various metabolic pathways in influencing breast cancer differs between these ethnic groups. Associations may be dependent on estrogen exposure either through endogenous estrogen in pre-menopausal women, exogenous estrogen in post-menopausal women taking HRT, or through estrogen levels in adipose tissue.
References
Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E (2000) Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92(19):1592–1600
Singh P, Rubin N (1993) Insulin like growth factors and binding proteins in colon cancer. Gastroenterology 105(4):1218–1237
Rui L, Fisher TL, Thomas J, White MF (2001) Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem 276(43):40362–40367
Werner H, Le Roith D (1997) The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog 8(1):71–92
Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG (1996) Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol 28(6):619–637
Burks D, White M (2001) IRS proteins and beta-cell function. Diabetes 50(90001):S140–S145
White MF (2003) Insulin signaling in health and disease. Science 302(5651):1710–1711
Shpakov AO, Pertseva MN (2000) Structural and functional characterization of insulin receptor substrate proteins and the molecular mechanisms of their interaction with insulin superfamily tyrosine kinase receptors and effector proteins. Membr Cell Biol 13(4):455–484
Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF (1999) Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23(1):32–40
Waters SB, Pessin JE (1996) Insulin receptor substrate 1 and 2 (IRS1 and IRS2): what a tangled web we weave. Trends Cell Biol 6(1):1–4
Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, et al (2003) Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci 23(18):7084–7092
Van Obberghen E, Baron V, Scimeca JC, Kaliman P (1993) Insulin receptor: receptor activation and signal transduction. Adv Second Messenger Phosphoprotein Res 28:195–201
Vendrell JA, Magnino F, Danis E, Duchesne MJ, Pinloche S, Pons M, Birnbaum D, Nguyen C, Theillet C, Cohen PA (2004) Estrogen regulation in human breast cancer cells of new downstream gene targets involved in estrogen metabolism, cell proliferation and cell transformation. J Mol Endocrinol 32(2):397–414
Slattery ML, Baumgartner KB, Byers T, Guiliano A, Sweeney C, Herrick J, Curtin K, Murtaugh M, Wolff R (2005) Genetic, anthropometric, and lifestyle factors associated with IGF-1 and IGFBP-3 levels in Hispanic and non-Hispanic white women. Cancer Causes Control 16(10):1147–1157
Sandhu MS, Dunger DB, Giovannucci EL (2002) Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 94(13):972–980
Ma J, Giovannucci E, Pollak M, Chan JM, Gaziano JM, Willett W, Stampfer MJ (2001) Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst 93(17):1330–1336
McCarty MF (1997) Up-regulation of IGF binding protein-1 as an anticarcinogenic strategy: relevance to caloric restriction, exercise, and insulin sensitivity. Med Hypotheses 48(4):297–308
Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC (2003) Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev 12(2):84–89
Probst-Hensch NM, Wang H, Goh VHH, Seow A, Lee H-P, Yu MC (2003) Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev 12(8):739–746
Giovannucci E (2001) Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 131(Suppl. 11):3109S–3120S
Rosen CJ, Glowacki J, Craig W (1998) Sex steroids, the insulin-like growth factor regulatory system, and aging: implications for the management of older postmenopausal women. J Nutr Health Aging 2(1):39–44
Kido Y, Nakae J, Hribal ML, Xuan S, Efstratiadis A, Accili D (2002) Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J Biol Chem 277(39):36740–36747
Deal C, Ma J, Wilkin F, Paquette J, Rozen F, Ge B, Hudson T, Stampfer M, Pollak M (2001) Novel promoter polymorphism in insulin-like growth factor-binding protein-3: correlation with serum levels and interaction with known regulators. J Clin Endocrinol Metab 86(3):1274–1280
Jernstrom H, Deal C, Wilkin F, Chu W, Tao Y, Majeed N, Hudson T, Narod SA, Pollak M (2001) Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 10(4):377–384
Zou T, Fleisher AS, Kong D, Yin J, Souza RF, Wang S, Smolinski KN, Abraham JM, Meltzer SJ (1998) Sequence alterations of insulin-like growth factor binding protein 3 in neoplastic and normal gastrointestinal tissues. Cancer Res 58(21):4802–4804
Sweeney C, Murtaugh MA, Baumgartner KB, Byers T, Giuliano AR, Herrick JS, Wolff R, Caan BJ, Slattery ML (2005) Insulin-like growth factor pathway polymorphisms associated with body size in Hispanic and non-Hispanic white women. Cancer Epidemiol Biomarkers Prev 14(7):1802–1809
Slattery ML, Samowitz W, Hoffman M, Ma KN, Levin TR, Neuhausen S (2004) Aspirin, NSAIDs, and colorectal cancer: possible involvement in an insulin-related pathway. Cancer Epidemiol Biomarkers Prev 13(4):538–545
Rosen CJ, Kurland ES, Vereault D, Adler RA, Rackoff PJ, Craig WY, Witte S, Rogers J, Bilezikian JP (1998) Association between serum insulin growth factor-I (IGF-I) and a simple sequence repeat in IGF-I gene: implications for genetic studies of bone mineral density. J Clin Endocrinol Metab 83(7):2286–2290
Chokkalingam AP, McGlynn KA, Gao Y-T, Pollak M, Deng J, Sesterhenn IA, Mostofi FK, Fraumeni JF Jr, Hsing AW (2001) Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res 61(11):4333–4336
Cussenot O, Valeri A (2001) Heterogeneity in genetic susceptibility to prostate cancer. 12(1):11–16
DeLellis K, Ingles S, Kolonel L, McKean-Cowdin R, Henderson B, Stanczyk F, Probst-Hensch NM (2003) IGF1 genotype, mean plasma level and breast cancer risk in the Hawaii/Los Angeles multiethnic cohort. Br J Cancer 88(2):277–282
Jernstrom H, Chu W, Vesprini D, Tao Y, Majeed N, Deal C, Pollak M, Narod SA (2001) Genetic factors related to racial variation in plasma levels of insulin-like growth factor-1: implications for premenopausal breast cancer risk. Mol Genet Metab 72(2):144–154
Howard CA, Samet JM, Buechley RW, Schrag SD, Key CR (1983) Survey research in New Mexico Hispanics: some methodological issues. Am J Epidemiol 117(1):27–34
Slattery ML, Caan BJ, Duncan D, Berry TD, Coates A, Kerber R (1994) A computerized diet history questionnaire for epidemiologic studies. J Am Diet Assoc 94(7):761–766
Slattery MLS, Edwards S, Herrieck J, Baumgartner K, Wolff R, Murtaugh M, Baumgartner R, Giuliano A, Byers T (2006) Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat
Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM (1999) Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med 8(6):805–813
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(Suppl. 9):S498–S504
Almind K, Bjorbaek C, Vestergaard H, Hansen T, Echwald S, Pedersen O (1993) Amino acid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet 342(8875):828–832
Ehrmann DA, Tang X, Yoshiuchi I, Cox NJ, Bell GI (2002) Relationship of insulin receptor substrate-1 and -2 genotypes to phenotypic features of polycystic ovary syndrome. J Clin Endocrinol Metab 87(9):4297–4300
Lee S, Mohsin SK, Mao S, Hilsenbeck SG, Medina D, Allred DC (2006) Hormones, receptors, and growth in hyperplastic enlarged lobular units: early potential precursors of breast cancer. Breast Cancer Res 8(1):R6
Jackson JG, White MF, Yee D (1998) Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem 273(16):9994–10003
Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ (1998) Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat 47(2):111–120
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M (1998) Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351(9113):1393–1396
Bohlke K, Cramer DW, Trichopoulos D, Mantzoros CS (1998) Insulin-like growth factor-I in relation to premenopausal ductal carcinoma in situ of the breast. Epidemiology 9(5):570–573
Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, et al (2002) Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 11(11):1361–1368
Krajcik RA, Borofsky ND, Massardo S, Orentreich N (2002) Insulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev 11(12):1566–1573
Keinan-Boker L, Bueno De Mesquita HB, Kaaks R, Van Gils CH, Van Noord PA, Rinaldi S, Riboli E, Seidell JC, Grobbee DE, Peeters PH (2003) Circulating levels of insulin-like growth factor I, its binding proteins -1, -2, -3, C-peptide and risk of postmenopausal breast cancer. Int J Cancer 106(1):90–95
Li BD, Khosravi MJ, Berkel HJ, Diamandi A, Dayton MA, Smith M, Yu H (2001) Free insulin-like growth factor-I and breast cancer risk. Int J Cancer 91(5):736–739
Malin A, Dai Q, Yu H, Shu XO, Jin F, Gao YT, Zheng W (2004) Evaluation of the synergistic effect of insulin resistance and insulin-like growth factors on the risk of breast carcinoma. Cancer 100(4):694–700
Daws MR, Westley BR, May FE (1996) Paradoxical effects of overexpression of the type I insulin-like growth factor (IGF) receptor on the responsiveness of human breast cancer cells to IGFs and estradiol. Endocrinology 137(4):1177–1186
Missmer SA, Haiman CA, Hunter DJ, Willett WC, Colditz GA, Speizer FE, Pollak MN, Hankinson SE (2002) A sequence repeat in the insulin-like growth factor-1 gene and risk of breast cancer. Int J Cancer 100(3):332–336
Canzian F, McKay JD, Cleveland RJ, Dossus L, Biessy C, Boillot C, Rinaldi S, Llewellyn M, Chajes V, Clavel-Chapelon F, et al (2005) Genetic variation in the growth hormone synthesis pathway in relation to circulating insulin-like growth factor-I, insulin-like growth factor binding protein-3, and breast cancer risk: results from the European prospective investigation into cancer and nutrition study. Cancer Epidemiol Biomarkers Prev 14(10):2316–2325
Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK (2005) Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer 41(11):1515–1527
Rinaldi S, Toniolo P, Muti P, Lundin E, Zeleniuch-Jacquotte A, Arslan A, Micheli A, Lenner P, Dossus L, Krogh V, et al (2005) IGF-I, IGFBP-3 and breast cancer in young women: a pooled re-analysis of three prospective studies. Eur J Cancer Prev 14(6):493–496
Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA, Luccarini C, Munday H, Perkins B, Smith P, et al (2006) IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet 15(1):1–10
Schernhammer ES, Hankinson SE, Hunter DJ, Blouin MJ, Pollak MN (2003) Polymorphic variation at the -202 locus in IGFBP3: influence on serum levels of insulin-like growth factors, interaction with plasma retinol and vitamin D and breast cancer risk. Int J Cancer 107(1):60–64
Wagner K, Hemminki K, Grzybowska E, Klaes R, Butkiewicz D, Pamula J, Pekala W, Zientek H, Mielzynska D, Siwinska E, et al (2004) The insulin-like growth factor-1 pathway mediator genes: SHC1 Met300Val shows a protective effect in breast cancer. Carcinogenesis 25(12):2473–2478
Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, Berrino F, Tjonneland A, Bigaard J, Olsen A, et al (2004) Body size and breast cancer risk: findings from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 111(5):762–771
Jernstrom H, Barrett-Connor E (1999) Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J Womens Health Gend Based Med 8(10):1265–1272
Baumgartner KB, Hunt WC, Baumgartner RN, Crumley DD, Gilliland FD, McTiernan A, Bernstein L, Ballard-Barbash R (2004) Association of body composition and weight history with breast cancer prognostic markers: divergent pattern for Hispanic and non-Hispanic White women. Am J Epidemiol 160(11):1087–1097
Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjonneland A, Olsen A, Overvad K, Key TJ, Allen NE, Khaw KT, et al (2005) Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC). Br J Cancer 93(5):582–589
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC (1997) Dual effects of weight and weight gain on breast cancer risk. JAMA 278(17):1407–1411
Acknowledgments
This study was funded by grants: CA 078682, CA 078762, CA 078552, CA 078802. This research also was supported by the Utah Cancer Registry, which is funded by Contract #N01-PC-67000 from the National Cancer Institute, with additional support from the State of Utah Department of Health, the New Mexico Tumor Registry, funded by contract #, and the Arizona and Colorado cancer registries, funded by the Centers for Disease Control and Prevention National Program of Cancer Registries and additional state support. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute. We would like to acknowledge the contributions of Sandra Edwards, Roger Edwards, Leslie Palmer. Betsy Risendal, Karen Curtin, Tara Patton, Jason Witter, and Kelly May to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slattery, M.L., Sweeney, C., Wolff, R. et al. Genetic variation in IGF1, IGFBP3, IRS1, IRS2 and risk of breast cancer in women living in Southwestern United States. Breast Cancer Res Treat 104, 197–209 (2007). https://doi.org/10.1007/s10549-006-9403-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9403-9