Abstract
To explore the changes of brain structure and function in attention-deficit/hyperactivity disorder (ADHD), fifteen ADHD patients (inattention subtype) and 15 normal control participants were recruited, the brain structure and function of these subjects were investigated by combining structural magnetic resonance imaging (MRI), diffusion tensor imaging and resting-state functional MRI. The results showed that ADHD patients had a significant decrease in the volume of the white matter (P = 0.04), and a trend toward decreased volume of brain structures except for the putamen and globus pallidus. The visualization of statistical difference maps of the cortical thickness showed that ADHD patients had focal thinning in bilateral frontal regions and the right cingulate cortex (P < 0.05 uncorrected, except for a cluster threshold of 10 voxels). Statistical analysis of the FA maps revealed that ADHD patients had significantly decreased FA in the forceps minor, the internal capsule, the corona radiata, the splenium of the corpus callosum, and the bilateral basal ganglia (P < 0.05 uncorrected as above). ADHD patients had significantly decreased functional connectivity in the anterior cingulate cortex, posterior cingulate cortex, lateral prefrontal cortex, left precuneus and thalamus, but increased functional connectivity in bilateral posterior medial frontal cortex in the default mode network (P < 0.05 uncorrected as above). Our results provide new insights into the changes of the brain structure and function in ADHD, which suggests that alterations in the brain structural and functional connectivity might implicate the pathophysiology of ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common childhood disorders and can continue through adolescence and adulthood. The symptoms of ADHD include difficulty in staying focused, paying attention, controlling behavior, and hyperactivity (Garrett et al. 2008; Krain and Castellanos 2006). Previous studies showed that the anatomical abnormalities associated with ADHD can be visualized using high-resolution magnetic resonance imaging (MRI) (Berquin et al. 1998; Castellanos et al. 1996; Mataro et al. 1997). However, there are important discrepancies among various studies, probably due to very small sample sizes or the use of different techniques (Table 1), and these MR studies could not identify microstructural properties of white matter. Diffusion tensor imaging (DTI) is a valuable tool in studying the microstructure of the brain in vivo, and has the ability to reveal microstructural properties of white matter (Silk et al. 2008). Therefore, among the in vivo MR imaging techniques, DTI in particular is a useful tool for examining white matter integrity in ADHD (Ashtari et al. 2005; Makris et al. 2008a; Pavuluri et al. 2009).
Blood oxygenation level-dependent (BOLD) fMRI is also a valuable technique for ADHD pathology analysis (Hale et al. 2007; Roy et al. 2009; Rubia 2002; Solanto et al. 2009; Suskauer et al. 2008; Tian et al. 2008). Up to the present time, almost all former BOLD fMRI studies on ADHD are task-based (Makris et al. 2008a). As compared to the task-based fMRI studies, studies conducted during resting state are easy to carry out (without experiment design and subject training) and the results are comparable across different patient groups (Greicius and Menon 2004; Greicius et al. 2009; Long et al. 2008; Raichle et al. 2001; Thomason et al. 2008). Recently, resting state has been applied to the pathology analyses of neuropsychiatric disorders such as depression, Alzheimer’s disease and multiple sclerosis (Greicius et al. 2007; He et al. 2007; Lowe et al. 2008). The most prominent network in the clinical neuroscience literature on spontaneous intrinsic brain activity is the default mode network (DMN). The DMN comprises medial (medial prefrontal cortex, posterior cingulate/precuneus) and lateral (posterior parietal) brain regions that routinely exhibit coherent decreases in activity during attention-demanding cognitive tasks (Greicius et al. 2003, 2007; Raichle et al. 2001). Tian et al. (2008) found that the ADHD patients exhibited more significant resting-state brain activities in basic sensory and sensory-related cortices, and that dACC had more significant resting-state functional connectivity with several other brain regions in the ADHD patients as compared to the controls (Tian et al. 2006). However, other studies found reduced functional connectivity between the anterior cingulate cortex and various nodes of the DMN (Castellanos et al. 2008; Uddin et al. 2008). In a recent review, DMN interference during task performance was suggested to be a potential underlying cause of performance variability in ADHD (Sonuga-Barke and Castellanos 2007). Although there are numerous theoretical reasons for suspecting DMN dysfunction in ADHD, the findings of the previous studies in ADHD are variable and their interpretations regarding the functional abnormalities are controversial (Table 1). If a more detailed examination of DMN integrity in ADHD is warranted, a study carried out during resting state will probably provide us a new perspective on ADHD pathology.
The aim of the study is to investigate the changes of brain structure and function in ADHD patients by combining high resolution structural MRI with diffusion tensor imaging, and to explore the differences of the resting-state default mode network between ADHD patients and normal control subjects by resting fMRI. We hypothesize that there are structural changes in the white matter integrity and gray matter, and abnormal default mode network in the ADHD patients when compared to the normal control subjects.
Methods
Subjects
Fifteen ADHD patients (age range 10.6–14.8 years, mean 12.65 ± 1.82 years) and 15 normal control participants (age range 10.5–15.0 years, mean 13.21 ± 1.73 years) were recruited. Inclusion criteria for all subjects were within the age range of 10–15 years, male, right-handedness, and IQ >80 (as assessed with Wechsler Intelligence Scale for Children-revised (WISC-R)). Exclusion criteria for all subjects were any contraindications for MRI and neuropsychiatric disorder such as childhood schizophrenia, mental retardation and epilepsy. Group matching was based on age, gender, and education. All the fifteen ADHD patients met the DSM-IV criteria for ADHD (as assessed with Clinical Diagnostic Interview Scale, CDIS). All of ADHD patients were of the inattention subtype, and medication-free for at least half a year. The parents of all participants gave written informed consent after receiving a complete description of the study.
Image Acquisition
The resting-state fMRI data were acquired by a Siemens 3T scanner using the following parameters: TR/TE/FA = 2000 ms/30 ms/90o, 30 slices, thickness = 4.0 mm, FOV = 220 mm × 220 mm. Each session lasted for 320 s. During the resting state, the subjects were told not to concentrate on any particular subject, but just to relax with their eyes closed. Then 12 direction diffusion tensor images were acquired with a spin-echo echo-planar imaging pulse sequence on each subject for six times on average (b = 1000 s/mm2, matrix = 128 × 128, 34 axial 2.5 mm slices with zero gap, TR/TE/FA = 6500 ms/90 ms/90o). High-resolution T1-weighted images were also acquired with 3D MPRAGE sequence (TR/TE/FA = 2600 ms/3.93 ms/8o, matrix = 256 × 240, with 1 mm slice thickness).
Image Processing and Analysis
The volume of brain structures and the cortical thickness were analyzed by Freesurfer (http://surfer.nmr.mgh.harvard.edu/). Automated surface reconstruction yielded measurements of the cortical thickness for each subject’s entire brain and computed cross-subject statistics based on the cortical anatomy. Statistical analysis of the volume of brain structures was analyzed by SPSS13.0. Statistical thickness difference maps were generated by performing t-tests between ADHD patients and normal control subjects, significance was placed at P < 0.05, uncorrected for multiple comparisons, except for a cluster extent threshold of 10 voxels.
Post-processing of the DTI data included eddy current correction and the computations of the diffusion tensor elements. Then FA maps were generated in FSL (www.fmrib.ox.ac.uk/fsl), on which the white matter integrity was investigated using the SPM5 package (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). The non-diffusion-weighted (b0) images were normalized to the Montreal Neurological Institute (MNI) EPI template that is supplied by the SPM5 package. The FA maps were then spatially transformed according to the normalized b0 images. Normalized images were re-formatted into 69 slices with 2 × 2 × 2 mm3 voxels. The FA maps were smoothed with Gaussian kernels with full width half maximum (FWHM) of 6 mm. An intergroup two-sample t-test comparison was performed using an absolute threshold of FA >0.15. The contrasts used P < 0.05, uncorrected for multiple comparisons, except for a cluster extent threshold of 10 voxels.
The resting-state fMRI data were spatially normalized to the standard MNI (Montreal Neurological Institute) template and resampled to 3 mm × 3 mm × 3 mm. Subsequently, the functional scans were spatially smoothed with a 4 mm × 4 mm × 4 mm full width half maximum Gaussian kernel to decrease spatial noise. All these processes were also conducted using SPM5. For each subject, the smoothed, normalized fMRI images were concatenated across time to form a single four-dimensional image. This image was then analyzed with FSL’s melodic ICA software (www.fmrib.ox.ac.uk/fsl/melodic2). ICA is a statistical technique that separates a set of signals into independent—uncorrelated and non-Gaussian-spatiotemporal components. In order to overcome the difficulty of sorting ICA components, Greicius et al. have developed a template-matching procedure which identifies a network of interest by its goodness-of-fit to a pre-specified template mask (Greicius et al. 2007; Greicius and Menon 2004). The automated, two-step process developed in previous studies (Greicius et al. 2003, 2007; Greicius and Menon 2004) was then employed to select the components in each subject that most closely matched the default-mode network. Using SPM, two-sample t-tests were calculated to compare the default-mode network between the two groups, the best-fit components from both groups were entered into the analysis, and the z-scores (assigned by the ICA) at each voxel were averaged within each group and then compared across groups. The contrasts used P < 0.05, uncorrected for multiple comparisons, except for a cluster extent threshold of 10 voxels. Statistical maps were superimposed on the mean normalized high resolution T1-weighted images of all the subjects.
The two most widely used spaces in the neuroscience community are the Talairach space and the Montreal Neurological Institute (MNI) space. The International Consortium of Brain Mapping (ICBM) has adopted the ICBM152 template as an international standard, this ICBM152 template is used by several functional imaging analysis packages, such as SPM5 and FSL. The Talairach space is based on a stereotaxic atlas of the human brain published by Talairach and Tournoux, this template is used by FREESURFER software. The Talairach coordinate system has become the standard reference for reporting the brain locations. For reporting the brain locations in a common reference space, we obtained the Talairach coordinates of the center-of-gravity of focal thinning regions in FREESURFER using the Talairach space. As to SPM and FSL, we obtained the Talairach coordinates of the center-of-gravity of each cluster with the mni2tal script (http://eeg.sourceforge.net/mridoc/mri_toolbox/mni2tal.html), a tool commonly used to map the MNI coordinates to the Talairach coordinates.
Results
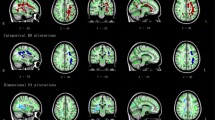
The volume of the white matter decreased significantly in ADHD patients compared with normal control subjects (P = 0.04), and the volume of the brain structures was generally smaller in ADHD patients than in control subjects except for the putamen and globus pallidus (Table 2). ADHD patients had a decrease of 9% in the volume of cerebral cortex and of 8% in the volume of caudate nucleus, though there is no significant difference with P-value 0.12 and 0.27 respectively. The visualization of statistical difference maps of the cortical thickness showed focal thinning in bilateral frontal regions and the right cingulate cortex in ADHD patients (P < 0.05, uncorrected) (Fig. 1; Table 3).
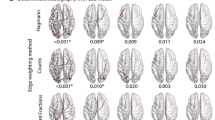
Statistical analysis of the FA maps revealed a significant decrease of FA in ADHD patients compared to normal controls (P < 0.05, uncorrected), and decreased FA was observed in the forceps minor, the internal capsule, the corona radiata, the splenium of the corpus callosum, and the bilateral basal ganglia in ADHD subjects (Fig. 2; Table 3).
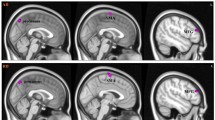
Comparing the resting-state default mode network between ADHD patients and normal control subjects, we found the ADHD patients had significantly decreased functional connectivity in brain regions such as the anterior cingulate cortex, posterior cingulate cortex, lateral prefrontal cortex, left precuneus and thalamus, but had increased functional connectivity in brain regions such as bilateral medial frontal lobe(P < 0.05, uncorrected) (Fig. 3; Table 3).
Discussion
The results show that ADHD children had a significant decrease in the volume of white matter (P = 0.04) and had a decrease of 9% in the volume of cerebral cortex and of 8% in the volume of caudate nucleus (though there is no significant difference with their P-values of 0.12 and 0.27 respectively), which are similar with the previous studies (Carmona et al. 2005; Castellanos et al. 1996; Wolosin et al. 2009; Ahrendts et al. 2010). On the other hand, our results show that there is no significant difference of the volume of putamen and globus pallidus between ADHD patients and control subjects (with their P-values of 0.36 and 0.91 respectively), which is similar with the previous study by Wellington et al. (2006). Wellington et al. (2006) reported that no differences were found in the total, left, and right putamen volumes across the ADHD patients or control group, but a significant reversal of asymmetry across groups was found: children with ADHD tended to have a smaller left putamen than right, while the control group tended to have a smaller right putamen than left.
The statistical tests of FA maps showed that ADHD patients had a significant decrease of FA in the forceps minor, the internal capsule, the corona radiata, the splenium of the corpus callosum, and the bilateral basal ganglia, which is similar to the previous studies (Hamilton et al. 2008; Pavuluri et al. 2009). Hamilton et al. (2008) reported that ADHD patients had significantly lower FA in the corticospinal tract and the superior longitudinal fasciculus than controls, supporting that disruptions in motor and attentional networks may contribute to ADHD pathophysiology. Rusch et al. (2007a, b, 2010) found increased mean diffusivity in inferior frontal white matter, and decreased interhemispheric structural connectivity between both ACCs in fiber tracts that pass through the anterior corpus callosum and connect dorsal areas of the ACCs among women with BPD and comorbid ADHD. Pavuluri et al. (2009) also observed significantly lower FA in anterior corona radiata, anterior limb of the internal capsule, superior region of the internal capsule, posterior limb of the internal capsule, superior longitudinal fasciculus, inferior longitudinal fasciculus, cingulum, and splenium in ADHD patients than that in NC subjects. These affected fibers (the forceps minor, the internal capsule, the corona radiata) anatomically connected with the bilateral frontal regions and the cingulate cortex, which have focal thinning showing by the statistical difference maps of the cortical thickness.
The thickness of cerebral cortex is an additional indicator of integrity of cytoarchitecture in the cortex (Luders et al. 2009; Makris et al. 2007; Shaw et al. 2006). The visualization of statistical difference maps of the cortical thickness showed focal thinning in the bilateral frontal regions and the right cingulate cortex in ADHD, which is similar to some previous studies (Makris et al. 2007; Shaw et al. 2006). Makris et al. (2007) found there was a significant cortical thinning in ADHD in a distinct cortical network supporting attention especially in the right hemisphere involving the inferior parietal lobule, the dorsolateral prefrontal, and the anterior cingulate cortices. Shaw et al. (2006) also found that children with ADHD had global thinning of the cortex, most prominently in the medial and superior prefrontal and precentral regions; children with worse clinical outcome had a thinner left medial prefrontal cortex at baseline than the better outcome group and controls. However, Wolosin et al. (2009) found only a significant decrease in cortical folding bilaterally, but no significant differences in cortical thickness were detected. And Rusch et al. (2007) found Women with BPD and comorbid ADHD had a thinner isthmus of the corpus callosum, compared with healthy women. Our results provide new insights into the difference of the cortical thickness in ADHD, revealing the selective thinning of cerebral cortex that subserves attention and executive function. Because of some limitations, the particular thinning cortical regions identified in this study should be interpreted cautiously and subjected to replication in larger samples. An important limitation of this study is the small sample size, and another is the fully automated method used in FreeSurfer software. The technique of three-D parametric surface modeling of FreeSurfer overcomes the limitations of volumetric segmentation and represents complex sulcal structures. However, errors in the cortical surface model still exist in folded regions because of the limit of the resolution in volume images, which may occasionally obscure subtle neuroanatomic effects (Devlin and Poldrack 2007; Makris et al. 2008b). On the other hand, Cortical thickness measurements suggested differences in rather small spots in the cingulate cortex and dorsolateral frontal lobe, the termination fields of decreased FA in forceps minor, internal capsule, and corona radiata are much larger showed by DTI analysis. The mismatches between the two methods could be caused by: (1) Cortical thickness measurements just focus on the cortex, but the difference of white matter (there are significant decrease of the volume of the white matter in ADHD patients) can’t display on the statistical maps of the cortical thickness. (2) DTI just focuses on the white matter and more sensitive to the FA change of the white matter. Low FA values of the white matter may reflect axonal degeneration, and/or less well-organized tracts, and may be induced by a variety of influences. (3)These affected fibers anatomically connected with the cortical areas (bilateral frontal regions and the cingulate cortex). They can influence each other, but these changes maybe are not in a synchronous manner, and it’s still unknown which is the initiator or the starter. Anyway, future studies are needed to further explore the relationships between the cortex and white matter abnormalities in this disorder.
Comparing the resting-state default mode network between ADHD and NC subjects, we found that the ADHD patients had significantly decreased functional connectivity in the anterior cingulate cortex, posterior cingulate cortex, lateral prefrontal cortex, left precuneus and thalamus, as well as significantly decreased structural connectivity in the forceps minor, and the splenium of the corpus callosum(by DTI analysis). These results showed that resting state fMRI and DTI suggest the same connectivity patterns in ADHD patients. It implies that the structural connectivity and the functional connectivity can influence each other. On the other hand, DTI also showed lower FA in the internal capsule, the corona radiata in ADHD patients, supporting the disruptions in motor and attention networks. The mismatches between the two connectivity types could be caused by: (1) the results of resting fMRI just focus on the decreased functional connected regions in the default mode network, and DTI results showed the whole brain white matter in this paper. (2) The functional connectivity might be linked by multiple segments of fiber tracts, and structurally connected regions might not be functionally connected. Future studies are needed to further explore the relationships between the changes of functional connectivity and structural connectivity in ADHD patients by DTI fiber tracking.
The present resting fMRI study also have confirmed the previously published results and provide further evidence to support that altered resting brain connectivity is involved in the neuropathology of ADHD (Castellanos et al. 2008; Uddin et al. 2008). Uddin et al. examined the default mode network by network homogeneity, and found reduced network homogeneity within the default mode network in ADHD subjects compared to age-matched controls, particularly between the precuneus and other default mode network regions (Castellanos et al. 2008; Uddin et al. 2008). This reduced resting state functional connectivity between the anterior and posterior regions of the DMN may indicate a relationship between working memory deficits and attention lapses in ADHD patients (Castellanos et al. 2008). Zang et al. used amplitude of low-frequency (0.01–0.08 Hz) fluctuation (ALFF) to investigate the baseline brain function of this disorder, and also found that patients with ADHD had decreased ALFF in the right inferior frontal cortex, left sensorimotor cortex, bilateral cerebellum and the vermis, but had increased ALFF in the right anterior cingulate cortex, left sensorimotor cortex, and bilateral brainstem (Zang et al. 2007). Despite differences in methodology, these findings all suggest that decreased functional interactions in the DMN may underlie some of the executive function deficits observed in the ADHD patients. On the other hand, an interesting finding of this study is the increased functional connectivity in bilateral posterior medial frontal cortex (pMFC). A review of primate and human studies, along with a meta-analysis of the human functional neuroimaging literature, suggested that the function of the pMFC is involved in performance monitoring, detection of unfavorable outcomes and response errors (Ridderinkhof et al. 2004a, b). ADHD children are abnormally and easily excitable or exuberant, and have strong emotional reactions or impulsive behavior, which may activate more pMFC to respond in the resting-state. These significant changes in DMN found in the ADHD patients may suggest the abnormalities of autonomic control and performance monitoring functions in them. By virtue of its non-invasiveness, high spatial and temporal resolution, low expense and clinical convenience, resting-state DMN could be an advantageous choice for ADHD pathology analysis. It is commonly assumed that functional brain connectivity reflects structural brain connectivity, and the strength of resting-state functional connectivity is positively correlated with that of structural connectivity (Damoiseaux and Greicius 2009). We also found that ADHD patients had significantly decreased functional connectivity in the anterior cingulate cortex, posterior cingulate cortex, lateral prefrontal cortex, left precuneus and thalamus, and had significantly decreased structural connectivity in the forceps minor, the internal capsule, the corona radiata, the optic radiation, and the splenium of the corpus callosum. However, functional connectivity is also observed between regions where there is little or no structural connectivity, which most likely indicates functional correlations mediated by indirect structural connections (i.e. via a third region) (Damoiseaux and Greicius 2009). For example, emerging evidence points to functional interactions between the pMFC and the lateral prefrontal cortex (LPFC), so that monitoring-related pMFC activity serves as a signal that engages regulatory processes in the LPFC to implement performance adjustments (Ridderinkhof et al. 2004a, b). However, little is known about the structural connectivity between the pMFC and LPFC. Possibly, this functional interplay between the pMFC and LPFC is in part mediated by subcortical structures such as the basal ganglia (Ullsperger and von Cramon 2003) or by the supplementary motor area (SMA) or pre-SMA (Brass and von Cramon 2004). We found that ADHD patients had increased functional connectivity in pMFC but decreased connectivity in lateral prefrontal cortex (LPFC), which suggests that ADHD patients can probably monitor performance by pMFC but cannot implement performance adjustments by LPFC. The problem may be caused by LPFC or the third region such as the basal ganglia.
The approach to combine structural and functional connectivity data in ADHD as derived from resting-state fMRI and DTI is indeed a very interesting and promising method. However, there are still some limitations on these preliminary results such as the small sample size, and also some of the group differences may be chance findings due to the lack of full correction for multiple comparisons. Therefore, these preliminary results should be interpreted with caution. We still have much to learn about the developing human brain in ADHD and more replication will be needed to validate these initial observations. As the methodologies for measuring structural and functional connectivity continue to be improved and their complementary strengths are applied in parallel, we can expect important advances in diagnostic and prognostic capacities in ADHD.
References
Ahrendts JC, Rusch N, Wilke M, Philipsen A, Eickhoff SB, GlaucheV, Perlov E, Hennig J, Tebartz van Elst L (2010) Visual cortex abnormalities in adults with ADHD: a structural MRI study. World J Biol Psychiatry. doi:10.3109/15622975.2010.518624
Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA (2005) Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry 57:448–455
Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX (1998) Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology 50:1087–1093
Brass M, von Cramon DY (2004) Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci 24:8847–8852
Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A (2005) Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett 389:88–93
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL (1996) Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 53:607–616
Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL (2002) Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288:1740–1748
Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP (2008) Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 63:332–337
Damoiseaux JS, Greicius MD (2009) Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct 213:525–533
Devlin JT, Poldrack RA (2007) In praise of tedious anatomy. Neuroimage 37:1033–1041 discussion 1050–1038
Garrett A, Penniman L, Epstein JN, Casey BJ, Hinshaw SP, Glover G, Tonev S, Vitolo A, Davidson M, Spicer J, Greenhill LL, Reiss AL (2008) Neuroanatomical abnormalities in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 47:1321–1328
Greicius MD, Menon V (2004) Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16:1484–1492
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437
Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78
Hale TS, Bookheimer S, McGough JJ, Phillips JM, McCracken JT (2007) Atypical brain activation during simple & complex levels of processing in adult ADHD: an fMRI study. J Atten Disord 11:125–140
Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Phillips OR, Caplan R, Toga AW, McCracken J, Narr KL (2008) Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport 19:1705–1708
He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T (2007) Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage 35:488–500
Krain AL, Castellanos FX (2006) Brain development and ADHD. Clin Psychol Rev 26:433–444
Long XY, Zuo XN, Kiviniemi V, Yang Y, Zou QH, Zhu CZ, Jiang TZ, Yang H, Gong QY, Wang L, Li KC, Xie S, Zang YF (2008) Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Methods 171:349–355
Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, Phillips MD (2008) Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp 29:818–827
Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, Del’Homme M, Strickland T, McCracken JT, Toga AW, Levitt JG (2009) Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry 65:84–88
Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ (2007) Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex 17:1364–1375
Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ (2008a) Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb Cortex 18:1210–1220
Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC (2008b) Cortical thickness abnormalities in cocaine addiction–a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron 60:174–188
Mataro M, Garcia-Sanchez C, Junque C, Estevez-Gonzalez A, Pujol J (1997) Magnetic resonance imaging measurement of the caudate nucleus in adolescents with attention-deficit hyperactivity disorder and its relationship with neuropsychological and behavioral measures. Arch Neurol 54:963–968
Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE (2002) Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry 52:785–794
Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ (2009) Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry 65:586–593
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004a) The role of the medial frontal cortex in cognitive control. Science 306:443–447
Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS (2004b) Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56:129–140
Roy M, Dillo W, Bessling S, Emrich HM, Ohlmeier MD (2009) Effective methylphenidate treatment of an adult Aspergers Syndrome and a comorbid ADHD: a clinical investigation with fMRI. J Atten Disord 12:381–385
Rubia K (2002) The dynamic approach to neurodevelopmental psychiatric disorders: use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and schizophrenia. Behav Brain Res 130:47–56
Rusch N, Luders E, Lieb K, Zahn R, Ebert D, Thompson PM, Toga AW, Tebartz van Elst L (2007a) Corpus callosum abnormalities in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. J Psychiatry Neurosci 32:417–422
Rusch N, Weber M, Il’yasov K, Lieb K, Ebert D, Hennig J, Tebartz van Elst L (2007b) Inferior frontal white matter microstructure and patterns of psychopathology in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. NeuroImage 35:738–747
Rusch N, Bracht T, Kreher BW, Schnell S, Glauche V, Il’yasov KA, Ebert D, Lieb K, Hennig J, Saur D, Tebartz van Elst L (2010) Reduced interhemispheric structural connectivity between anterior cingulate cortices in borderline personality disorder. Psychiatry Res Neuroimaging 181:151–154
Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J (2006) Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63:540–549
Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R(2008) White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp 30:2757–2765
Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH (2009) Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J Neuroimaging 19:205–212
Sonuga-Barke EJ, Castellanos FX (2007) Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 31:977–986
Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH (2008) fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry 47:1141–1150
Thomason ME, Chang CE, Glover GH, Gabrieli JD, Greicius MD, Gotlib IH (2008) Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage 41:1493–1503
Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y (2006) Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400:39–43
Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y (2008) Enhanced resting-state brain activities in ADHD patients: a fMRI study. Brain Dev 30:342–348
Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP (2008) Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 169:249–254
Ullsperger M, von Cramon DY (2003) Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci 23:4308–4314
Wellington TM, Semrud-Clikeman M, Gregory AL, Murphy JM, Lancaster JL (2006) Magnetic resonance imaging volumetric analysis of the putamen in children with ADHD: combined type versus control. J Atten Disord 10:171–180
Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH (2009) Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp 30:175–184
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China [Grant number 30670595, 30870696]. The authors would like to thank Prof. Chen Min, Prof. Liao Rong-xia and Miss Chen Xie-wan (Medical English Department, Third Military Medical University, China) for a critical reading of the manuscript and kindly giving precious advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Mg., Ye, Z., Li, Qy. et al. Changes of Brain Structure and Function in ADHD Children. Brain Topogr 24, 243–252 (2011). https://doi.org/10.1007/s10548-010-0168-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-010-0168-4