Abstract
Lip protrusion requires bilateral symmetrical movements of the facial muscles, but the laterality of the activated sensorimotor cortex corresponding to the area of the face activated during lip protrusion remains under discussion. In this study, blood-oxygenation-level-dependent (BOLD) responses in the sensorimotor cortex during non-verbal lip protrusion were evaluated in a 3T magnetic field in twenty healthy right-handed subjects. The results showed that the activated sensorimotor area on the left side was larger than that on the right side, and there was a statistically significant difference in the number of activated voxels between the left and right sensorimotor cortex in an individual study of the male group, although approximately symmetrical motor action potentials of facial muscles were recorded during lip protrusion. There was a statistically significant difference in interaction between the hemisphere (right and left) and sex (men and women) and multiple comparison test showed statistical significant differences between “men and right” and “men and left”, and between “men and left” and “women and left”. The peak value of the percent changes in BOLD signal responses on the left side was approximately twice as high as that on the right side in the males of the group, though the bilateral sensorimotor cortex was almost equally activated in the females in the group. In addition, the left primary sensory area related to the face area was significantly activated as a region where Male was more active than Female in a general linear model (multi-study, multisubject) analysis. This study revealed the possibility that the left sensorimotor cortex was more closely involved in non-verbal mouth movement in men, suggesting sex-related differences in sensorimotor cortex activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the detection of blood-oxygenation-level-dependent (BOLD) signals in the brain by Ogawa et al. (1990a, b), many functional magnetic resonance imaging (fMRI) studies have been performed worldwide to investigate the functions of the cerebral sensorimotor cortex during symmetrical motor tasks. It was, for example, reported that bimanual alternating movements (pronation and supination of the palms) were associated with bilateral activation of the primary sensorimotor cortex (Tracy et al. 2001). Bilateral activation of the primary sensorimotor cortex can be easily understood because the tasks performed by the subjects involved simultaneous movements of both hands. It is known that lip protrusion usually involves equal activation of the facial muscles of both sides around the mouth. Therefore, it could be surmised that the sensorimotor cortex would be activated bilaterally and equally during such symmetrical movements. Some authors reported that no significant laterality in the activated cerebral hemisphere was observed during lip protrusion (Lotze et al. 2000; Riecker et al. 2000; Salmelin and Sams 2002; Wohlert 1993). However, the mentioned studies involved ten or less subjects, which certainly limited their possibility to evaluate the effect of gender on activity lateralization. An fMRI study showed that the left-sided lateralization for lip motor activity was established in the context of the group analysis in five males and one female (Hesselmann et al. 2004), but based on these small and unbalanced numbers it could be very difficult to interpret any gender difference in the function of the primary motor cortex. In this study, the BOLD signal responses in the bilateral sensorimotor cortex during a lip protrusion task were evaluated with 3T MRI in 20 subjects, comparing male with female subjects.
Materials and Methods
Subjects
Twenty healthy subjects (10 males; age, 22–37 years: 10 females; age, 22–29 years) were enrolled in the present study. All the subjects were right-handed and the laterality quotient was calculated between each other according to the Edinburgh Handedness Inventory (Oldfield 1971). Written informed consent was obtained from all of the subjects prior to their participation in the study, and the study was conducted after obtaining the approval of the relevant ethical committee.
Lip Protrusion Task
Subjects were first provided with verbal instructions on the task, and then given a brief demonstration and a brief practice period prior to the start of the experiment. For the lip protrusion task, the subjects began from a position of fully relaxed facial muscles, with the lips and teeth slightly parted and the tongue resting on the floor of the mouth. The activation task involved protrusion of the lip towards the nose tip at the rate of 2 Hz without sound, words or images. All the subjects practiced the lip symmetrical movement and the symmetry was visually ascertained before MR imaging procedure. The subjects were lying supine in the scanner with their eyes closed. The head was fixated with a foam rubber pad in order to minimize involuntary movements and all subjects were given instructions not to move their head, body, legs, or arms during the scanning procedure. A block-design paradigm was employed for the study. Briefly, the subjects were instructed verbally to start protruding their lips voluntarily every 30 s with no sound and to stop after 10 s. The series involved the first 30-s rest, followed by four cycles of alternating 10-s lip protrusion tasks and 20-s rests, one entire series lasting for 150 s. Therefore, we specified a model with one predictor reflecting a conditioned “facial stimulation”. Each subject was monitored by camera to check whether the subject performed the lip protrusion task as instructed or not. Moreover, in order to check symmetry of lip movement, a muscle action potential was recorded with surface electrodes at the distal marginal mentalis muscle after MR imaging procedure, as in our previous studies (Ishikawa et al. 1996, 1999). To be brief, while the subjects were lying supine on the bed, the active surface electrode was located on the mentalis muscle and the reference surface electrode was placed at the base of the mandible, with a ground electrode under the chin. Unlike with previous studies, measurement of the amplitude of the direct compound muscle action potential was carried using Neuropack μ(MEB-9100, Nihon Kohden, Japan) and ratio of the peak to peak amplitude of the right and the left (R/L amplitude ratio) was calculated on the basis of the data obtained from 80 times of facial movements per subject. To compare R/L amplitude ratio between the subjects, a non-parametric test, the unpaired t-test, was used from statistical software (StatView 5.0, Windows edition). The level of statistical significance was set at P < 0.05.
fMRI Procedure
The fMRI data were acquired using a 3-Tesla Siemens Allegra MRI scanner. Single-shot echo planar images were obtained using the following parameters: echo time = 20 ms, repetition time = 1 s, flip angle = 90°, 64 × 64 matrix, field of view = 200 mm, 21 axial slices, 5 mm thickness with a 0.8 mm gap. For anatomical coregistration, magnetization prepared rapid gradient echo was used (1.0 × 1.0 × 1.0 mm3) to obtain structural three-dimensional volumes.
Data Analysis
The fMRI software package, Brain Voyager 4.9.2 (Brain Innovation, Maastricht, The Netherlands) (Goebel 1996; Goebel et al. 1996), was used for all the image analyses. The software was operated under Microsoft Windows XP. Briefly, for individual data analysis, the two-dimensional (2D) functional data recorded in the same session were aligned with the 3D data sets based on the slice parameters for the MR images, called “2D and 3D position parameters”. The functional images were aligned in a time-series to minimize the effects of head movements, using a 3D motion correction that estimates the three translation and three rotation parameters of rigid body transformation. Data smoothing in the space domain used a gaussian kernel for smoothing which was convoluted with image data and the width of the kernel was controlled with the parameter FWHM (full width at half maximum). A kernel of 4 mm was used for single subject analysis and that of 8 mm was used for multi-subject analysis. The individual anatomical 3D data sets were transformed into the Talairach coordinates (Talairach and Tournoux 1988). The differences between the resting and movement conditions were statistically evaluated using a general linear model (GLM) (single study), in which the predictor was used after the box-car function of the stimulation was convolved with the canonical hemodynamic response function as supplied by Brain Voyager. Using a predictor time course according to the linear model of the relation between neural activity and hemodynamic response was generally accepted although the relationship between neural activity and hemodynamic response could vary in different functional systems (Hesselmann et al. 2004). Significance was reported at a corrected P < 0.001 level by the multiple comparison method (Bonferroni’s correction). A region of interest (ROI) was determined by selecting a “significant” voxel cluster in the active region. ROIs were placed on the activated and isolated sensorimotor cortex corresponding to the mouth area, which involved the Tarailach coordinate sets (−41, −18, 41) or (52, −18, 46) evaluated by the cluster centers of spatial gravity related to facial stimulation as Hesselmann et al. (2004) reported. The statistical max-value points and their 3D coordinates were obtained for both the left and right sensorimotor cortex cluster. To compare the mean number of activated voxels on the left sensorimotor cortex with that on the right sensorimotor cortex, a non-parametric test, the Wilcoxon signed rank test, was used from statistical software (StatView 5.0, Windows edition). The level of statistical significance was set at P < 0.05. Moreover, two-factor factorial ANOVA including the hemisphere (left and right) and sex (men and women) was used for testing statistical interaction to the number of activated voxels and Fisher’s PLSD (protected least significance difference) method was carried out for a multiple comparison test among all the combinations of the two factors. The level of statistical significance was set at P < 0.05 (StatView 5.0, Windows edition). For group analysis, anatomical specification (VMRs) and time courses of the clusters (VTCs) at individual subject level were extracted and added, and then averaged by using the Average 3D data sets in the Talairach tab of the 3D Volume Tools dialog. One VMR voxel had the resolution of 1 mm3 and one VTC voxel encompassed 3 × 3 × 3 mm3. After using a GLM single study in which the predictor was used after the box-car function of the stimulation was convolved with the canonical hemodynamic response function, activated areas were superimposed on the averaged anatomical brain images in the Talairach space. Significance was set at an uncorrected P < 3.1294 e−12 level by the multiple comparison method (Bonferroni’s correction) in order to see the activated area isolated in the primary sensorimotor cortex corresponding to the mouth area.
ROIs were selected on the activated and isolated sensorimotor cortex as well as in the individual data analysis, and their activation time-course during lip protrusion was obtained by event-related averaging. The statistical max-value points, statistical max-value and average of statistical values were obtained for both the left and right sensorimotor cortex cluster in the two groups, respectively. In addition, analysis of BOLD signals between the groups by using a GLM (multi-study, multi-subject) was carried out in Brain Voyager in order to compare activations across tasks. Briefly, all the pairs of single-study data and design matrix files were listed sequentially in the multi study list box and z-normalization of the time course of each study prior fitting the GLM model was performed since the variance of voxel time courses might vary strongly between studies. After deriving statistical maps, a contrast asking for those brain regions where male was more active than female was obtained by using the “Overlay GLM” dialog. Significance was set at an uncorrected P < 3.9429 e−14 level by the multiple comparison method (Bonferroni’s correction). To compare the distribution of age and laterality quotient, a non-parametric test, the Mann-Whitney U-test, was used from statistical software (StatView 5.0, Windows edition). The level of statistical significance was set at P < 0.05.
Results
It could be ascertained by camera monitoring that all the subjects performed the lip protrusion task perfectly and symmetrically. The BOLD signals data analysis with Brain Voyager showed that the sensorimotor cortex was activated strongly and widely in all the subjects. No significant activation in Broca’s or Wernicke’s areas was observed. Table 1 shows the summary of the number of activated voxels in the sensorimotor cortex at a corrected P < 0.001 by the multiple comparison method, and the Talairach coordinates at the most active regions. In men, the results of individual data analysis revealed a larger number of activated voxels in the left sensorimotor cortex than that in the right, and there was a statistically significant difference in the number of activated voxels between in the left and the right side (Wilcoxon signed rank test, P = 0.0051) (Fig. 1). On the other hand, no significant difference was found between the left and right side in women (Fig. 2). There was a statistically significant difference in interaction between the hemisphere and sex (Two-factor factorial ANOVA, P = 0.041) and multiple comparison test showed statistically significant differences between “men and right” and “men and left”, and between “men and left” and “women and left” (Fisher’s PLSD method, P = 0.018, 0.026, respectively). The results of group data analyses indicated bilateral strong activation of the sensorimotor cortex during lip protrusion at an uncorrected P < 3.1294 e−12 level (corrected P-values could not be seen in the statistical maps; i.e, P < 0.000) by the multiple comparison method (Bonferroni’s correction) (Figs. 3 and 4). In general, the cluster size of the activated area in the group data analysis was larger than that in the individual data analysis because of the averaging of each individual data set. The areas of activation on the left side were larger than those on the right side in men, though no significant laterality was observed in women. Activation in the bilateral supplementary motor cortex and cerebellum was also observed, but no laterality was found (Wilcoxon signed rank test, P > 0.05, respectively). Detailed analysis of the averaged time-course of changes in the BOLD responses for the ROIs in the sensorimotor cortex revealed that the peak %BOLD signal in the left sensorimotor cortex was approximately twice as large as that in the right in the male group (Fig. 3), although no laterality was found in the female group (Fig. 4). In detail, the peak value of the percent changes in BOLD signals for the right sensorimotor cortex was approximately 0.5, whereas it was 1.2 for the left in men. In a GLM (multi-study, multi-subject) analysis, the left primary sensory area was significantly activated (yellow), though the right one was not activated (right, Fig. 5). A cluster center of spatial gravity was evaluated at the Talairach coordinate sets (−51, −22, 36) corresponding to the primary sensory area related to the face area. On the other hand, green regions were also activated as brain regions where female activation was greater than male. A cluster center of the left hemisphere green region near the yellow one was evaluated at the Talairach coordinate sets (−40, −21, 42) and that of the right one was (47, −19, 44). Statistical analysis of data in the group study and the male and female characteristics (mean age, laterality quotient, past history of facial nerve diseases or cerebrovascular diseases) are shown in Table 2. There was no statistically significant difference in the R/L amplitude ratio of the facial muscle action potential between men and women as shown in Table 2.
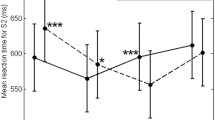
Comparison of the mean number of activated voxels in the left and right sensorimotor cortices in men. There is a statistically significant difference in the mean number of activated voxels between the left and the right sides (Wilcoxon signed rank test, * P = 0.0051). (Error bars indicate one standard error)
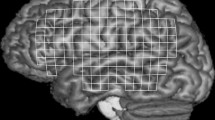
Activation maps and time courses of activation in men during lip protrusion. Above. Activation maps were overlaid on coronal anatomical images at the setting of the statistical max value point. Below. Averaged time-course of changes in the BOLD responses for the regions of interest in the right and the left sensorimotor cortex. The primary sensorimotor cortex is significantly activated bilaterally and the area of activation on the left side is larger than that on the right side. The peak value of the percent changes in BOLD signals for the right sensorimotor cortex is approximately 0.5, although that for the left is 1.2. ROIs have been placed on the right (upper left) and the left (upper right) primary sensorimotor cortex as shown by the red cross. (Error bars indicate standard error)
Activation maps and time courses of activation in women during lip protrusion. Above. Activation maps were overlaid on coronal anatomical images at the setting of the statistical max value point. Below. Averaged time-course of changes in the BOLD responses for the regions of interest in the right and the left sensorimotor cortex. The primary sensorimotor cortex is activated bilaterally and almost equally. The peak value of the percent changes in BOLD signals for the right and left ensorimotor cortex is approximately 0.9, respectively. ROIs have been placed on the right (upper left) and the left (upper right) primary sensorimotor cortex as shown by the red cross. (Error bars indicate standard error)
Contrast activation maps overlaid on coronal images in a GLM (multi-study, multi-subject) analysis. A “yellow” region shows a contrast asking for those brain regions where male is more active than female. The left primary sensory area is significantly activated (right, yellow). The “green” regions indicate brain areas where female activation is greater than male. The primary sensorimotor areas are almost symmetrically activated
Discussion
Lip protrusion requires bilateral coordinated movements of the facial muscles, which are controlled by the cerebral sensorimotor cortex via the corticobulbar tract. Although a digital video study showed mild asymmetry of lip movement in voluntary puckering (Schmidt et al. 2005), it has been reported that the lip protrusion task elicited high correlation values among all electrode pairs of the perioral quadrants in electromyographic recordings, suggesting coordinated equivalent activation of all the quadrants (Wohlert and Goffman 1994). Lip protrusion is a midline movement, and therefore probably involves an equivalent contribution from the cerebral sensorimotor cortex of both sides. An electroencephalographic (EEG) study reported that non-speech movement of a midline structure was under bilateral cortical control, and that control of lip movement was apparently not necessarily a dominant hemisphere function (Wohlert and Larson 1991). Salmelin et al. also reported in an MEG study that no interhemispheric correlations were found for the non-verbal mouth movements (Salmelin and Sams 2002). As regarding fMRI studies, some authors showed no lateralization to the left hemisphere during articulation or swallowing with lip movements in healthy subjects (Kern et al. 2001; Lotze et al. 2000; Riecker et al. 2000; Wohlert 1993). These reports suggest that oral movements in the midline involve coordinated equivalent responses of the facial muscles under the control of the primary sensorimotor cortex of both sides. On the other hand, Kimura and Watson (1989) hypothesized that systems of neural control for all movements of the hand and mouth developed under a pattern of hemispheric dominance because both these parts of the body require coordination of many muscles for fine voluntary actions. Higashino et al. reported from an MEG study that voluntary lip movements without speech were controlled mainly by the left motor cortex (Higashino et al. 2002). Hesselmann et al. showed in an fMRI study that the left-sided lateralization for lip motor activity was established in the context of the group analysis, although it could not be proven within the scope of the single subject analysis (Hesselmann et al. 2004). Thus, the symmetry of the sensorimotor cortex activation during lip movements has been controversial. In the present study, the mean number of activated voxels and the magnitude of the BOLD signal responses in the left sensorimotor cortex were higher than those in the right in men, but this was not the case in women. In addition, a multiple comparison test revealed a statistically significant difference associated with hemisphere and sex. The left primary sensory area related to the face area was significantly activated as a region where male was more active than female in a GLM (multi-study, multi-subject) analysis. On the other hand, the anatomical localizations of the “green” clusters at the sensorimotor areas, as brain regions where female was more active than male, were almost symmetric in Fig. 5. These localizations were similar to those of the Hesselmann study as stated in the Sect. “Material and Methods”. This activation can account for primary sensorimotor activation within Brodmanns areas 4 (M1) and 1, 2, and 3 (S1) (Hesselmann et al. 2004). Although it is not clear why the localization of the activated voxels differed between men and women, it is interesting that the activation of the left sensory area was observed alone as a region where male was more active than female. These findings suggest that the laterality to the left of the function of the sensory cortex during lip protrusion may be associated with sex-related differences. To the best of our knowledge, ours is the first study to reveal the dominance of the left sensory cortex during non-verbal lip protrusion in healthy right-handed men with 3-tesla fMRI study. At the same time, for group data analysis, a random effects analysis should be performed in order to generalize the obtained fMRI results to the population level. However, 50 or more subjects per experimental group have to be included for a random effects analysis and so that analysis is not thought to be very informative for our study.
Lip protrusion task was proved to be approximately symmetrical by electromyogram in this study, although slight asymmetry of lip movement in voluntary puckering was observed in a previous report (Schmidt et al. 2005). The difference may be due to the difference of measurement method between electromyogram and digital video. Why was the left hemisphere activated dominantly regardless of symmetrical muscle movements? A GLM (multi-study, multi-subject) analysis showed the left dominant activation in the primary sensory area in the men group. The finding suggests that bilateral primary motor cortices might be symmetrically activated in men as well as women. On the other hand, Foki et al. reported that a strong left-hemispheric dominance for symmetrical chin movements was shown in the group of right-handed healthy subjects at an fMRI study and a possible explanation of the lateralization results could be the existence of a general left-hemispheric motor dominance in right-handed persons (Foki et al. 2007). According to this opinion, it could be also considered that the symmetrical oral movements themselves may be associated with the left dominant activation of the sensorimotor cortex. However, it was reported that no laterality of sensorimotor cortical activation was observed during non-verbal lip protrusion as if preparing to kiss someone (‘imagine a nice person in front of you whom you kiss on the cheek’) and while articulating silently the Finnish vowel ‘o’ with lip protrusion (Salmelin and Sams 2002). This finding suggests that the symmetrical oral movements might not produce the left dominance of sensorimotor cortical responses. At the same time, only seven subjects were enrolled in that study. Therefore, it has to be considered that any laterality of sensorimotor cortical function remains under discussion. In order to resolve this question, a larger study would be required.
Why is the left sensorimotor cortex activated more strongly and widely in men, but not in women? Higashino et al. suggested in an MEG study in men that the left-sided laterality might be related to the fact that speech is controlled by the motor speech center of Broca’s area in the left hemisphere (Higashino et al. 2002). Speech production requires bilateral and symmetrical oral muscle movements as well as lip protrusion. The difference between lip protrusion and speech production is that lip protrusion is a simple movement without sound, words, images or complex tongue movements. Speech production needs syllable strings in addition to lip and tongue movement. Interestingly, an fMRI study found that significant brain activation was restricted to the sensorimotor cortex during production of syllable strings and as compared to the monosyllables “ta” and “stra”, the lexical item “Tagebau” yielded a considerably more pronounced lateralization effect toward the dominant hemisphere in healthy German natives (five males and five females) (Riecker et al. 2000). These findings suggest that the displayed activation patterns reflect speech motor processes. On the other hand, in our study, a non-verbal task was performed to investigate the sensorimotor cortex function during lip protrusion and no significant activation in Broca’s or Wernicke’s area was observed, suggesting that a direct relationship between non-verbal lip movement and the speech control center remains unclear. This finding of laterality to the left hemisphere might not be fully explained only by the opinion that the speech center is located mainly at the left hemisphere, because simple soundless lip protrusion differs from the complex oral muscle movements associated with speech and location of the speech center really remains controversial.
We speculate that the sex-related difference may be associated with stronger activation on the left sensorimotor cortex during lip protrusion because distribution of the dominant hemisphere with regard to the speech center is known to differ frequently between men and women. In women, interhemispheric transmission times of verbal information were similar for the left-to-right and right-to-left directions, whereas in men, those were directionally asymmetrical and were considerably longer in the left-to-right direction (Nowicka and Fersten 2001). The finding corresponds to bilateral activation of the female brain and a predominantly left hemisphere activation of the male brain. Ikezawa et al. recently reported that with phonetic auditory mismatch negatives using dichotic listening tasks, EEG findings revealed significantly larger amplitude in females than males, especially in the right hemisphere, while scalp current density findings revealed left hemisphere dominance and contralateral dominance in males alone. In conclusion, while males exhibited left-lateralized activation, females exhibited more bilateral activity (Ikezawa et al. 2008). These findings suggest that functional lateralization subserving verbal information process and preattentive detection of phonetic change differs between the genders. Thus, our finding of left lateralization (especially to the primary sensory area) in men during lip protrusion may be also associated with the gender difference in the basic neural circuit of speech because silent lip protrusion is thought to be one of preliminary steps toward verbal and phonetic action in human communication. The left primary sensory cortex might play a special role in the preliminary step of a speech act in men.
Terumitsu et al. has shown that for simple speech utterances the primary motor cortex exhibited specialization strongly suggesting a differential role of the right and left primary motor cortices in speech production, apparently independent of gender (Terumitsu et al. 2006). Our study which suggests a different hemispheric lateralization for lip protrusion based on gender might seem at odds with the Terumitsu finding. Terumitsu et al., to be sure, clearly showed that, within the dominant hemisphere, spatially and functionally independent components corresponding to phonation and verbalization formed distinct clusters, and this result means that greater functional specialization of the primary motor cortex in the dominant hemisphere indicates its predominant role over that of the non-dominant hemisphere for speech production.
However, there is an important difference between the Terumitsu study and our study. In the Terumitsu study, participants performed the three types of motor task: [T] articulatory tongue movements without phonation (producing tongue movements for the syllables/lalelilulelolalo/silently without sound), [P] phonation without articulatory tongue movements (voicing the Japanese vowel/e/), and [V] simultaneous phonation and articulatory tongue movements (voicing the syllables/lalelilulelolalo/). These tasks consisted of tongue movements and/or phonation. On the other hand, the present task, lip protrusion, does not involve tongue movements or phonation, because lip protrusion consists of only facial muscle symmetrical movements, which is the most important difference in the motor task between the Terumitsu study and the present study. Therefore, we consider that the Terumitsu findings and our results are not inconsistent with each other. The most interesting point in our study is that the left sensorimotor cortex (especially, sensory area) predominantly functions during lip protrusion without phonation and tongue movements.
Wohlert reported that differences between the waveforms recorded at the right- and left-hemisphere sites were not significant by recording EEG potentials from scalp electrodes in seven right-handed female subjects before a non-speech task (lip pursing), a speech-like task (lip rounding), and a speech task (single word production) (Wohlert 1993). It can be understood that the sex-related difference may affect the results because the study involved only female subjects. There is also some evidence that women have less EEG-detected lateralization in a speech task (Rippon 1990). Lip pursing movement showed no significant lateralization to the dominant hemisphere, but the number of activated voxels (350 voxels) in the left sensorimotor cortex was a little higher than that (284 voxels) in the right sensorimotor cortex in seven healthy subjects (four males and three females) (Lotze et al. 2000). It can be surmised that one of the reasons why no significant lateralization was found in the study may be because the study included females. An MEG study which revealed the dominant activation of the left motor cortex involved only males (Higashino et al. 2002). Concerning motor function, Hesselmann et al. argued that gender differences were not thought to be important and could consequently be neglected with reference to the Corfield study (Hesselmann et al. 2004), however only eight subjects (four men and four women) were enrolled in the Corfield study (Confield et al. 1999) and so gender differences should not be neglected in a larger study.
Therefore, it may be fully explainable by contemplating our results and other studies that laterality to the left sensorimotor cortex (especially, sensory area) during lip protrusion may be associated with sex-related differences. For all that, our group data analysis might not be very useful for generalization to the whole population level because a random effects analysis could not be accepted.
Conclusion
The present 3- Tesla MRI study clearly demonstrated that the left primary sensorimotor cortex was activated more widely and strongly than the right during the lip protrusion task in healthy right-handed men, but this finding was not observed in women. Above all, the left primary sensory area was significantly activated as a region where male was more active than female. These findings may be associated with gender differences. It suggests that the left primary sensory cortex might play a special role in the preliminary step of a speech act in men.
References
Confield DR, Murphy K, Josephs O, Fink GR, Frackowiak RS, Adams L, Turner R (1999) Cortical and subcortical control of tongue movement in humans. A functional neuroimaging study using fMRI. J Appl Physiol 86:1468–1477
Foki T, Geissler A, Gartus A, Pahs G, Deecke L, Beisteiner R (2007) Cortical lateralization of bilateral symmetrical chin movements and clinical relevance in tumor patients—a high field BOLD-FMRI study. Neuroimage 37:26–39
Goebel R (1996) Brainvoyager: a program for analyzing and visualizing functional and structural magnetic resonance data sets. Neuroimage 3:604
Goebel R, Khorram-Sefat D, Hacker H, Singer W (1996) Going beyond the information given, the neuronal substrate of phi-motion and shape-form-motion as revealed by functional magnetic resonance imaging. NeuroReport 3:174
Hesselmann V, Sorger B, Lasek K, Guntinas-Lichius O, Krug B, Sturm V, Goebel R, Lackner K (2004) Discriminating the cortical representation sites of tongue and lip movement by functional MRI. Brain Topo 16:159–167
Higashino K, Kunihiro T, Goto F, Kanzaki S, Hayashi Y, Honda S, Ogawa I (2002) Magnetoencephalography preceding the task of lip protrusion. Facial N Res. Jpn 22:79–81 (in Japanese)
Ikezawa S, Nakagome K, Mimura M, Shinoda J, Itoh K, Homma I, Kamijima K (2008) Gender differences in lateralization of mismatch negativity in dichotic listening tasks. Int J Psychophysiol 68:41–50
Ishikawa M, Ohira T, Namiki J, Gotoh K, Takase M, Toya S (1996) Electrophysiologic investigation of facial spasm: F-waves of the facial muscles. Acta Neurochir (Wien) 138:24–32
Ishikawa M, Namiki J, Takase M, Kojima A, Kawase T (1999) F-waves of the facial muscles in healthy control subjects and in patients with peripheral facial nerve disturbance. Electromyogr Clin Neurophysiol 39:167–174
Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R (2001) Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280:G531–G538
Kimura D, Watson N (1989) The relation between oral movement control and speech. Brain Lang 37:565–590
Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N (2000) The representation of articulation in the primary sensorimotor cortex. NeuroReport 11:2985–2989
Nowicka A, Fersten E (2001) Sex-related differences in interhemispheric transmission time in the human brain. NeuroReport 12:4171–4175
Ogawa S, Lee TM, Kay AR, Tank DW (1990a) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–9872
Ogawa S, Lee TM, Nayak AS, Glynn P (1990b) Oxygenation sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 14:64–78
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–114
Riecker A, Ackermann H, Wildgruber D, Meyer J, Dogil G, Haider H, Grodd W (2000) Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex. A functional magnetic resonance imaging (fMRI) study. Brain Lang 75:259–276
Rippon G (1990) Individual differences in eletrodermal and electroencephalographic asymmetries. Intern J Psycophysiol 8:309–320
Salmelin R, Sams M (2002) Motor cortex involvement during verbal versus non-verbal lip and tongue movements. Hum Brain Mapping 16:81–91
Schmidt KL, VanSwearingen JM, Levenstein RM (2005) Speed, amplitude, and asymmetry of lip movement in voluntary puckering and blowing expressions: implications for facial assessment. Motor Control 9:270–280
Talairach J, Tournoux P (1988) Co-Planar stereotaxic atlas of the human brain: three-dimensional proportional system. Thieme Medical, New York, NY
Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T (2006) Human primary motor cortex shows hemispheric specialization for speech. NeuroReport 17:1091–1095
Tracy JI, Faro SS, Mohammed FB, Pinus AB, Madi SM, Laskas JW (2001) Cerebellar mediation of the complexity of bimanual compared to unimanual movements. Neurology 57:1862–1869
Wohlert AB (1993) Event-related brain potentials preceding speech and nonspeech oral movements of varying complexity. J Speech Hear Res 36:897–905
Wohlert AB, Goffman L (1994) Human perioral muscle activation patterns. J Speech Hear Res 37:1032–1040
Wohlert AB, Larson CR (1991) Cerebral averaged potentials preceding oral movement. J Speech Hear Res 34:1387–1396
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukunaga, A., Ohira, T., Kamba, M. et al. The Possibility of Left Dominant Activation of the Sensorimotor Cortex During Lip Protrusion in Men. Brain Topogr 22, 109–118 (2009). https://doi.org/10.1007/s10548-009-0101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-009-0101-x