Abstract
Background
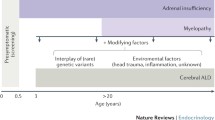
X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal metabolic disorder. Male patients develop adrenocortical insufficiency (80 % before 18 years), a chronic myelopathy (adrenomyeloneuropathy (AMN); all in adulthood), or progressive cerebral demyelination (cerebral ALD; 40 % before 18 years). Cerebral ALD is treated with haematopoetic cell transplantation (HCT). It is unknown if AMN still develops in patients with X-ALD that underwent HCT for cerebral ALD in childhood.
Patients and methods
A retrospective observational study was performed by selecting all adult patients with X-ALD in our cohort that underwent HCT in childhood.
Results
This retrospective study found that three out of five patients in our cohort who underwent HCT in childhood developed signs of myelopathy in adulthood.
Conclusion
These data suggest that HCT for cerebral ALD in childhood does not prevent the onset of AMN in X-ALD in adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
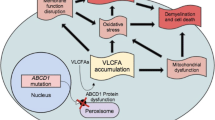
X-linked adrenoleukodystrophy (X-ALD), a progressive neurometabolic disease caused by mutations in ABCD1 gene, is characterized by impaired degradation of very-long-chain fatty acids (VLCFA; >C22) and, (Singh et al 1984) consequently, VLCFA accumulation in plasma and tissues (Moser et al 1981; Engelen et al 2012). Over 80 % of patients develop adrenocortical insufficiency in childhood (Dubey et al 2005). The disease can also manifest as rapidly progressive cerebral demyelination (cerebral-ALD); this is estimated to occur in 30-40 % of boys before the age of 18 years (Moser et al 2001). Mean survival after the appearance of cerebral symptoms is approximately two years if untreated (Engelen et al 2012). Allogeneic hematopoietic cell transplantation (HCT) is the only clinically effective therapeutic intervention in cerebral-ALD. It can arrest progression of cerebral demyelination provided the procedure is performed when boys are neurologically asymptomatic, or exhibit only minor symptoms (Miller et al 2011). Therefore, boys and men with X-ALD are monitored with MRI for the onset of cerebral-ALD (Engelen et al 2012). In adulthood, virtually all men with X-ALD eventually develop slowly progressive myelopathy (adrenomyeloneuropathy; AMN) (Engelen et al 2012). Onset of AMN is typically between the age of 20 to 30 years. Early signs are urge incontinence and a subtle gait disorder, with signs of pyramidal tract and dorsal column dysfunction on examination. Pathologically, this chronic axonopathy found in AMN is quite distinct from the inflammatory demyelination of cerebral-ALD (Powers et al 2000). Recently, we showed that women with X-ALD are not merely carriers, but that >80 % also develop AMN (Engelen et al 2014). Patients often ask if HCT performed in childhood prevents AMN in adulthood. This is still an unresolved question. Therefore, we retrospectively studied patients with X-ALD from our cohort who were transplanted in childhood for the presence of signs of myelopathy.
Patients and methods
Virtually all patients with X-ALD in the Netherlands visit the outpatient clinic of the department of neurology of the Academic Medical Center in Amsterdam. All adult male patients in this cohort that received HCT for cerebral ALD in childhood were identified and included in this study. All patients were examined by one of the authors (BMG or ME). The medical records of all these patients were reviewed. The presence of myelopathy was defined as complaints of urge incontinence and/or a gait disorder with pathological reflexes or dorsal column dysfunction on neurological examination. For this project the IRB issued a waiver since the study is retrospective and only anonymized data was used.
Results
In our cohort there were five adult male patients with X-ALD that underwent HCT in childhood. Clinical characteristics of these five patients are summarized in Table 1. The median number of years since transplantation was 16 (range: 12–19). Two of the patients were brothers (C1 and C2). All patients were diagnosed because of a positive family history or because of adrenocortical insufficiency. The diagnosis was confirmed by VLCFA analysis and/or ABCD1 mutation analysis (Table 1). In all patients cerebral ALD was found in a clinically asymptomatic stage on follow-up MRIs. Age at the last clinical assessment is indicated. All patients had full donor chimerism (data for one patient not available) and were doing well without signs of chronic graft versus host disease. Two patients were transplanted with bone marrow from a matched unrelated donor, two had a HLA matched sib (for patient A a healthy brother, for patient D a sister who was a carrier). All patients had adrenocortical dysfunction. Three patients showed signs of myelopathy that were not present before HCT or during earlier follow-up visits. VLCFA levels before and after HCT are reported if available.
Discussion
This small-scale observational study shows that three out of five patients developed myelopathy despite HCT. Age of onset of subtle signs of myelopathy (in early adulthood) is consistent with what we know of the natural history of X-ALD (Engelen et al 2012). These data suggest that HCT does not prevent the onset of AMN. However, slowing of the progression of myelopathy by HCT cannot be excluded. If HCT has any disease-modifying effect on AMN remains to be determined by longer follow-up of a large group of transplanted and untransplanted patients. Such a study has been initiated on behalf of the Center for International Blood and Marrow Transplant Research (CIBMTR). From a pathophysiological point of view, the inflammatory demyelination of cerebral ALD and the chronic axonopathy of AMN are distinct (Schaumburg et al 1975; Powers et al 2000; Engelen et al 2012; Kemp et al 2012). Therefore, it is possible that HCT only halts the inflammatory process of cerebral ALD. The chronic axonopathy is most likely the effect of chronic VLCFA toxicity,(Powers et al 1980) and the biochemical defect of X-ALD is not corrected by HCT (also illustrated by the pre- and post-transplant VLCFA levels in Table 1). Therefore it is perhaps not surprising that the myelopathy of AMN is not prevented. Currently no effective VLCFA lowering therapy is available. Lorenzo’s oil normalizes C26:0 levels in plasma and was proven to be ineffective in stabilizing the myelopathy in patients with X-ALD (van Geel et al 1999). This study reinforces the need for the development of an effective VLCFA-lowering treatment to stabilize, or prevent, AMN.
References
Dubey P, Raymond GV, Moser AB, Kharkar S, Bezman L, Moser HW (2005) Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr 146:528–532
Engelen M, Kemp S, de Visser M et al (2012) X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 7:51
Engelen M, Barbier M, Dijkstra IM et al (2014) X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain 137:693–706
Kemp S, Berger J, Aubourg P (2012) X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta 1822:1465–1474
Miller WP, Rothman SM, Nascene D et al (2011) Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 118:1971–1978
Moser HW, Moser AB, Frayer KK et al (1981) Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology 31:1241–1249
Moser HW, Smith KD, Watkins PA, Powers J, Moser AB (2001) X-linked adrenoleukodystrophy. In The metabolic and molecular bases of inherited disease. McGraw Hill, New York 3257–3301.
Powers JM, Schaumburg HH, Johnson AB, Raine CS (1980) A correlative study of the adrenal cortex in adreno-leukodystrophy–evidence for a fatal intoxication with very long chain saturated fatty acids. Invest Cell Pathol 3:353–376
Powers JM, DeCiero DP, Ito M, Moser AB, Moser HW (2000) Adrenomyeloneuropathy: a neuropathologic review featuring its noninflammatory myelopathy. J Neuropathol Exp Neurol 59:89–102
Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP Jr (1975) Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol 32:577–591
Singh I, Moser AE, Moser HW, Kishimoto Y (1984) Adrenoleukodystrophy: impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr Res 18:286–290
van Geel BM, Assies J, Haverkort EB et al (1999) Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with "Lorenzo's oil". J Neurol Neurosurg Psychiatry 67:290–299
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jutta Gaertner
Rights and permissions
About this article
Cite this article
van Geel, B.M., Poll-The, B., Verrips, A. et al. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J Inherit Metab Dis 38, 359–361 (2015). https://doi.org/10.1007/s10545-014-9797-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9797-1