Abstract
Combined D,L-2-hydroxyglutaric aciduria (DL-2HGA; OMIM #615182) is a rare neurometabolic disorder clinically characterized by muscular hypotonia, severe neurodevelopmental dysfunction, and intractable seizures associated with respiratory distress. Biochemically, DL-2HGA patients excrete increased amounts of D- and L-2-hydroxyglutarate (D2HG and L2HG, respectively), with predominance of D2HG, and α-ketoglutarate, and show a decrease in urinary citrate. Impaired function of the mitochondrial citrate carrier (CIC) due to pathogenic mutations within the SLC25A1 gene has been identified as the underlying molecular cause of the disease. CIC mediates efflux of the mitochondrial tricarboxylic acid (TCA) cycle intermediates citrate and isocitrate in exchange for cytosolic malate. Thus, depletion of cytosolic citrate as well as accumulation of citrate inside mitochondria have been considered to play a role in the pathophysiology of DL-2HGA. Here, we report for the first time on a patient with a genetically confirmed diagnosis of DL-2HGA and treatment with either malate or citrate. During malate treatment, urinary malate concentration increased, but beyond that, neither biochemical nor clinical alterations were observed. In contrast, treatment with citrate led to an increased urinary excretion of TCA cycle intermediates malate and succinate, and by trend to an increased concentration of urinary citrate. Furthermore, excretion of D2HG and L2HG was reduced during citrate treatment. Clinically, the patient showed stabilization with regard to frequency and severity of seizures. Treating DL-2HGA with citrate should be considered in other DL-2HGA patients, and its effects should be studied systematically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic analyses in infants and children with neurological impairment have detected patients with 2-hydroxyglutaric aciduria (2HGA). Differentiation of the two enantiomers, D-2- and L-2-hydroxyglutaric acid (D2HG and L2HG, respectively) resulted in the classification of affected patients into two subgroups, with accumulation of either D2HG [D-2-hydroxyglutaric aciduria (D2HGA)] or L2HG [L-2-hydroxyglutaric aciduria (L2HGA)]. Three different genetic variants have been identified (Kranendijk et al. 2012): D2HGA has been attributed to either a deficiency of D2HG dehydrogenase (D2HGA type I, OMIM #600721) or to different gain-of-function mutations in a single codon of the isocitrate dehydrogenase-2 gene (IDH2; D2HGA type II, OMIM #613657), whereas patients with L2HGA display a deficiency of L2HG dehydrogenase (OMIM #236792).
In 2000, three patients with severe neonatal-onset encephalopathy, muscular hypotonia, intractable seizures, recurrent respiratory distress, lack of developmental progress, and early death were described (Muntau et al. 2000). In contrast to the known disorders of D2HGA and L2HGA, these patients showed an accumulation of both enantiomers of 2-hydroxyglutaric acid (2HG), with a predominance of D2HG, but did not display mutations in any of the genes mentioned above. This biochemical condition has been designated “combined D,L-2-hydroxyglutaric aciduria” (DL-2HGA; OMIM #615182). Since then, nine additional patients have been described, and mutations in the SLC25A1 gene encoding the mitochondrial citrate carrier (CIC) were identified as the molecular cause of DL-2HGA in all 12 patients (Nota et al. 2013). Independently, another group identified a deficiency of SLC25A1 as the underlying genetic defect in a single patient with a similar neurologic presentation and high excretion of 2HG (Edvardson et al. 2013).
No treatment for DL-2HGA has been established so far, and eight of the 13 patients reported died at a median age of 7.5 months. Due to theoretical considerations discussed previously (Nota et al. 2013) and elaborated in the “Discussion” section and depicted in Fig. 1 of this paper, malate and citrate supplementation were considered a potentially helpful treatment approach. Here, we report for the first time on the clinical course of a patient with genetically proven DL-2HGA. In particular, we document the clinical and biochemical response to successive treatment with malate and citrate in one of only five DL-2HGA patients known to be alive at the time of this report.
Suggested and observed effects of mitochondrial citrate carrier (CIC) deficiency and supplementation of malate and citrate. Under noncatabolic conditions, CIC exchanges cytosolic malate for mitochondrial citrate, which is impaired upon CIC deficiency. Suggested effects of CIC deficiency are illustrated in red. Intramitochondrial accumulation of citrate leads to an increase of tricarboxylic (TCA)-cycle intermediates downstream of citrate, i.e., α-ketoglutarate (KG), which is further converted into L-2-hydroxyglutarate (L2HG) and D-2-hydroxyglutarate (D2HG). Additionally, high intramitochondrial amounts of citrate inhibit citrate synthase. In the cytosol, citrate depletion leads to decreased oxaloacetate concentration, followed by a drop in reduced nicotinamide adenine dinucleotide phosphate (NADPH) + hydronium (H+) and depletion of acetyl-coenzyme A (CoA), with subsequent reduction of fatty-acid and sterol synthesis. Suggested effects of malate supplementation (green). Increased cytosolic malate concentrations may (1) promote residual CIC transport activity and/or (2) increase the amount of functional CIC protein due to stabilization of mutant protein, both leading to attenuation of mitochondrial citrate accumulation. (3) Administration of citrate (yellow) may ameliorate cytosolic depletion of citrate and its downstream consequences. CS citrate synthase, HOT hydroxyacid-oxoacid transhydrogenase, IDH isocitrate dehydrogenase, IMM inner mitochondrial membrane, LDH lactate dehydrogenase, MDH malate dehydrogenase, PC pyruvate carboxylase
Results
Case presentation and initial investigations
We report on a girl (subject no. 12 in Nota et al. 2013), the third child of nonconsanguineous, healthy parents of Italian and Polish descent. Labor was induced at 38 weeks of gestation due to prenatal growth retardation. Birth weight was 2,385 g (300 g below third percentile), height was 44 cm (1 cm below third percentile), and head circumference 32 cm (tenth percentile). No dysmorphism was detected. APGAR scores were 9 (1′)–6 (5′)–10 (10′). Postnatally, the girl developed asymptomatic hypoglycemia (min 1 mmol/l), but blood glucose concentrations stabilized on regular feedings. At 10 days of age, she presented with an attack of tachycardia followed by apnoa with deep cyanosis, respiratory insufficiency, and subsequent bradycardia; this was classified as an “acute, life-threatening event.” These attacks recurred two to three times per week. At the same time, muscular hypotonia and poor feeding were noted. A small atrial septal defect and a grade I intracerebral hemorrhage were detected. An initial metabolic workup (analyses of amino acids and acylcarnitines in blood) in a secondary-care pediatric hospital reported normal results, and genetic testing for Prader–Willi syndrome revealed normal findings.
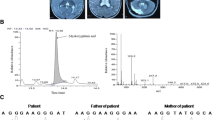
The girl was referred to our hospital at the age of 5 months due to recurrence of the aforementioned attacks–now, however–, with increasing frequency (average two to three times per day up to ten times daily) and intensity, eventually necessitating resuscitation and repeated admissions to the ICU. On examination, the girl had secondary microcephaly, severe muscular truncal hypotonia, primary psychomotor delay, horizontal nystagmus, and absence of visual contact. Her developmental age was estimated to be 1.5 months. An abnormal distribution of fat tissue, with increased amounts at the arms and the back of hands and feet, together with inverted nipples were found. Ophthalmologic examinations, including funduscopy and visual evoked potentials, were normal, as was audiometry. Repeated analyses of amino acids in blood and cerebrospinal fluid (CSF), acylcarnitines in blood, as well as transferrin isoelectric focussing, a screening test for congenital disorders of N-glycosylation, all revealed normal findings. Of note, elevation of lactate in blood (max 5 mM) and CSF (max 5 mM) was detected repeatedly, suggestive of a mitochondrial disorder. Respiratory chain enzymes measured in muscle tissue revealed normal activities of complexes I–IV in relation to the mitochondrial reference enzyme citrate synthase; however, citrate synthase activity was reduced, as were complex I and IV activities when related to noncollagen protein (NCP) (Table 1). Tests for mitochondrial DNA (mtDNA) depletion, deletions, and insertions were normal in this tissue. Light-microscopic examination of muscle tissue was unremarkable, but electron micrographs indicated degenerated mitochondria and fat droplets. Eventually, analysis of organic acids in urine repeatedly showed an increased excretion of 2HG with a concentration of up to 440 mmol/mol creatinine (normal <25); the differentiation of stereoisomers of 2HG revealed the biochemical diagnosis of DL-2HGA. Subsequently, our patient was included into the study of Nota et al. (2013), confirming the disorder genetically by detecting compound heterozygosity for two frameshift mutations within the SLC25A1 gene (c.517_526del [p.Arg173GlyfsX2] and c.821C > T [r.820_821delGC; p.Ala274IlefsX24]), both predicting truncated dysfunctional citrate carriers, if inserted into the mitochondrial membrane at all.
Repeated electroencephalogram (EEG) examinations revealed persistent slowing but no epileptic discharges. The clinical picture of the girl’s attacks, however, impressed as convulsive seizures and thus considered epileptic in the sense of brainstem fits, without detectability in the superficial EEG conduction. Hence, treatment with levetiracetam was initiated. Consecutively, the patient could be weaned from assisted ventilation but still showed on average two severe attacks per day. Add-on therapy with topiramate did not alter her condition. After notification of the diagnosis and pathophysiological considerations, a therapeutic trial with malate and citrate was initiated.
Clinical and biochemical course before and during treatment
The parents gave informed consent to off-label treatment with malate and citrate, which are approved medications in some other medical conditions. Blood gas analyses and organic acid determinations in urine were regularly performed before and during the trial. We found persistent compensated metabolic acidosis [pH 7.36 ± 0.1; base excess −7.1 ± 3.5 mmol/l; bicarbonate (HCO3) 16.3 ± 1.6 mmol/l], with no differences before and during treatment with either malate or citrate. Organic acid analyses showed that urinary concentrations of malate, succinate, and lactate were in the normal range before treatment (Figs. 2 and 3a, b, e). Concentrations of 2HG and α-ketoglutarate (αKG) in urine, although with fluctuations (Fig. 2), were markedly increased in comparison with the normal range (Fig. 3d, f); however, citrate concentrations were significantly reduced (Fig. 3c). Malate (Kalium-L-malat 17.21 %, B. Braun Melsungen AG, Germany) was administered orally at a maximum dose of 400 mg (3 mmol) per kg body weight per day according to the dosage scheme given in Fig. 2. During this period, no alteration in the patient’s clinical course was noted, and the frequency of seizures remained unchanged. Biochemically, a significant increase in urinary excretion of malate was notable upon malate administration (Figs. 2 and 3a), whereas concentrations of other organic acids were constant compared with the untreated state.
Metabolite profile before and during treatment with malate and citrate. Organic acids in urine were measured repeatedly before and during treatment with either malate or citrate. Urinary concentrations of malate (green squares), citrate (black rhombs), and total 2-hydroxyglutarate (2HG; red triangles) refer to the left y-axis, concentrations of α-ketoglutarate (KG; blue circles) are shown on the right y-axis. Treatment periods are marked by shaded background (green, malate treatment; yellow, citrate treatment), with dosages of the respective substances given below. Concentrations of 2HG stereoisomers D-2-hydroxyglutarate (D2HG) and L-2-hydroxyglutarate (L2HG) were determined as indicated (#, D2HG; *, L2HG) before (day −22) and during (day 64) citrate treatment
Excretion of organic acids before and during treatment with malate or citrate. Median urinary excretion of different organic acids [a, malate; b, succinate; c, citrate; d, total 2-hydroxyglutarate (2HG); e, lactate; f, α-ketoglutarate]. Medians were calculated from all measurements performed before (no treatment) or during treatment with either malate or citrate. Box plots represent median (horizontal black line), interquartile range (boxes), and range (vertical bars) of 7, 10, and 12 measurements, respectively. Circles mark outliers. Shaded areas indicate normal ranges. Levels of significance were tested using Mann–Whitney U tests; *p < 0.05
Three days after cessation of malate treatment, oral administration of citrate (Blemaren®, Aristo Pharma, Berlin, Germany) was started, as illustrated in Fig. 2. Subsequently, the dose was increased up to a maximum of 1,500 mg (7.8 mmol) per kg body weight per day. Higher citrate doses were not tolerated due to gastrointestinal side effects. No change of urinary lactate or αKG levels was observed (Fig. 3e, f). In comparison with the untreated state, significantly increased urinary concentrations of malate and succinate were detected on citrate administration (Fig. 2 and 3a, b). Regarding the diminished excretion of citrate observed before and during malate administration, a trend toward higher concentrations was visible during citrate treatment (Figs. 2 and 3c), although not statistically significant. Of note, the excretion of 2HG was reduced upon citrate treatment compared with malate treatment (Figs. 2 and 3d). Determination of the urinary concentrations of D2HG and L2HG revealed that their concentrations were reduced upon citrate administration compared with the untreated state (D2HG: 97 vs. 427 mmol/mol creatinine; L2HG: 60 vs. 122 mmol/mol creatinine; Fig. 2). Clinically, we observed a stabilization of the girl’s condition during citrate treatment. The number of the aforementioned attacks decreased–they now occurred only every third day–and they were markedly less intense, so that intensive care procedures were no longer necessary. Additionally, the exacerbation of attacks could now be prevented by rectal administration of diazepam when the girl showed tachycardia known to precede the attacks. The improvement of her clinical condition permitted discharge to home care at 12 months. Two weeks later, the anticonvulsive treatment with levetiracetam and topiramate was extended with phenobarbitone by the family’s pediatrician, although no deterioration had occurred.
The girl was regularly seen in our outpatient clinic, and on follow-up 16 months later, it was noted that no further emergency visits to the hospital had been necessary. Attacks with respiratory insufficiency occurred only approximately every 3 months. Poor feeding necessitated placement of a gastrostomy tube. Thriving was age appropriate; however, the patient remained microcephalic (1.5 cm below third percentile). The girl made very slow but distinct developmental progress, and no regression in development has been observed. On evaluation, she showed no visual contact but followed a source of light. She reacted to acoustic stimuli and responded to speech with basic vocal sounds. In comparison with the initial neurologic examinations, truncal hypotonia persisted, but the girl showed slightly increased mobility and strength of extremities. Sometimes, she could lift her head against gravity for few seconds, and occasionally she was able to turn herself from the dorsal position to the side, but not beyond. Her developmental age was estimated to be 2–3 months.
MRI findings before and during treatment
Repeated magnetic resonance imaging (MRI) examinations at ages 1, 6, and 18 months revealed hypoplasia of the corpus callosum, prominent inner and outer spaces due to brain atrophy involving white and gray matter, and absence of signal abnormalities within the basal ganglia and brain stem. Myelination was delayed but had already progressed before and during citrate treatment, without any signs of dysmyelination or demyelination (Fig. 4). Findings were similar to the reported MRI and [1H]-MR spectroscopy findings in DL-2HGA (Edvardson et al. 2013; Read et al. 2005), aside from the absence of subependymal cysts and the presence of a lactate peak in our patient.
Magnetic resonance imaging (MRI) findings in D,L-2-hydroxyglutaric aciduria (DL-2HGA). Axial (a, e), coronal (b, f), and sagittal (c, g) T2-weighted images were obtained at 6 months (before citrate treatment; a-d) and 18 months (6 months after initiation of citrate treatment; e-h). At age 6 months, the lateral ventricles and subarachnoid spaces are highly dilated, and the brain looks atrophic. The ventricles are dysfigured. The cerebral mantle is reduced in width, and both the cortex and the white matter look atrophic (a, b, c). The basal nuclei, thalami, brain stem, and cerebellum have a much better volume compared with an MRI obtained at age 1 month (not shown). Diffusion-weighted images show no areas of restricted diffusion (not shown). Myelination age is approximately 3–4 months, which is behind for the age of the child at the time. [1H]-MR spectroscopy displays a peak representing increased lactate (d, inverted doublet at 1.3 ppm), as well as a slightly decreased N-acetylaspartic acid (NAA):choline ratio (d). At age 18 months (e-h), the sagittal images (g) show that the corpus callosum is extremely thin and that the lateral ventricles are dilated. The axial images (e) confirm the dilatation of lateral ventricles and subarchnoid spaces. The remaining cerebral mantle is thin. Not only the white matter but also the cortex looks thin (e, f, g). What remains of the cerebral white matter shows progress of myelination, but myelination is behind for the age. Compared with the MRI at age 6 months, myelination has progressed, whereas the other findings appear unchanged. [1H]-MR spectroscopy reveals no lactate, and a slight decrease of NAA:creatin ratio (h)
Discussion
We report for the first time on a patient with DL-2HGA in whom CIC deficiency was genetically confirmed and who was successively treated with malate and citrate. All known CIC-deficient patients display a primarily neurological phenotype compatible with impaired mitochondrial function. This is surprising, as it had been reported that CIC messenger RNA (mRNA) and protein expression is high in liver, pancreas, and kidney but low in brain, heart, and skeletal muscle. In our patient, mitochondrial dysfunction was evidenced by lactate elevation in blood, CSF, and brain (detected by [1H]-MR spectroscopy), as well as by reduction of complex I and IV activities and reduced citrate synthase activity in muscle tissue (which could be the result of a negative feedback mechanism). The discrepancy between the neurologic phenotype of CIC deficiency and the primarily hepatic, renal, and pancreatic expression of CIC (Palmieri 2004) remains unclear.
Our case and all other reported DL-2HGA patients (Nota et al. 2013) showed decreased citrate excretion into urine (Fig. 2 and 3). This is in line with the model that CIC mediates the efflux of the mitochondrial tricarboxylic acid (TCA)-cycle intermediates citrate and isocitrate in exchange for cytosolic malate (Palmieri 2004; Fig. 1). Within the cytosol, citrate is converted into oxaloacetate and acetyl-coenzyme A (CoA). The latter is essential for fatty acid and sterol synthesis and acts as a regulator for glycolysis (Mycielska et al. 2009), whereas oxaloacetate gives rise to malate and pyruvate formation while producing NADPH + H+ (Palmieri 2004).
The pathophysiology of DL-2HGA is unknown. Theoretically, deficiency of CIC function may lead to (I) intramitochondrial accumulation, and/or (II) cytosolic depletion of citrate, and/or (III) direct toxic effects of accumulating D2HG and L2HG (Fig. 1). Any intramitochondrial accumulation of citrate would lead to an accumulation of other TCA-cycle intermediates, such as αKG. It has been discussed that intramitochondrial αKG will be converted to L2HG or D2HG via L-malate dehydrogenase and hydroxyacid-oxoacid transhydrogenase, respectively (Kranendijk et al. 2012). It has been described that L2HG and D2HG share transport characteristics with glucarate (GA) and 3-hydroxyglutarate (3HG) (Hagos et al. 2008; Mühlhausen et al. 2008), which are pathologic metabolites accumulating in glutaric aciduria type 1; and that 3HG impairs the anaplerotic supply of TCA-cycle intermediates from astrocytes to neurons mediated by the sodium-dependent dicarboxylate co-transporters (NaC) 2 and 3 (Lamp et al. 2011). As L2HG and D2HG are also accepted substrates for NaC3 (Hagos et al. 2008), they might contribute to impaired mitochondrial energy production by the same mechanism as 3HG.
At first, the administration of malate was considered a possible therapeutic approach for the treatment of DL-2HGA, as an induced elevation of cytosolic malate should theoretically promote the SLC25A1-mediated citrate–malate antiport mechanism, thus ameliorating an intramitochondrial citrate accumulation as well as a cytosolic depletion of citrate (Fig. 1). The idea behind this was that the splice-site mutation SLC25A1 c.821C > T on one allele of our patient, which had been shown to lead to reduced but not absent levels of the CIC protein (Nota et al. 2013), confers some residual activity as a consequence of the fact that a certain proportion of mutated mRNA is normally spliced (predicting p.Ala274Val), whereas in the other part, the novel splice-site is used (with the consequence of a frameshift p.Ala274IlefsX24). In our patient, malate supplementation led to an increase in urinary malate but neither replenished the reduced urinary citrate levels nor diminished the increased excretion of 2HG and αKG. Also, clinically, no effect of malate supplementation could be observed.
If the cytosolic citrate depletion played the predominant pathophysiologic role, this would lead to a depletion of acetyl-CoA, a lack of NADPH + H+, and a reduced biosynthesis of fatty acids (which are precursors of complex lipids, such as sphingo- or phospholipids or plasmalogens), and to impaired sterol synthesis (Fig. 1). In fact, we found reduced plasma cholesterol levels in our patient (Table 2) but we can only speculate on a risk of cerebral cholesterol and sterol depletion. The brain completely depends on local cholesterol and lipid biosynthesis, as there is no influx from the circulation (Kanungo et al. 2013). Plasma 24-hydroxycholesterol, a specific product of brain cholesterol metabolism (Lund et al. 2003), was not available in our case but might be a valuable marker in future studies.
We assumed that citrate supplementation could restore the lowered cytosolic citrate concentrations thus replenishing acetyl-CoA and NADPH + H+ (Fig. 1). Supplementation of citrate in the form of its potassium salt has repeatedly been described, and no adverse effects were reported (McNally et al. 2009). During citrate supplementation, we observed an increased urinary excretion of malate and succinate, TCA-cycle metabolites distal to αKG, suggesting an anaplerotic effect. Taking outliers into consideration, an apparent trend to an increased excretion of urinary citrate (Fig. 3c) and a reduced excretion of 2HG (Fig. 3d), D2HG, and L2HG (Fig. 2) was observed. The reduction of plasma cholesterol concentration was attenuated (Table 2). The high excretion level of urinary αKG, however, remained unchanged. Clinically, the patient’s seizures-like attacks with subsequent respiratory distress had stabilized. We clearly must state, however, that it cannot be ultimately determined whether the beneficial effect was predominantly caused by citrate supplementation or was, rather, due to intensified anticonvulsive treatment, or both. Higher doses of citrate may be necessary to produce stronger biochemical and clinical effects, but they were not tolerated by our patient, as evidenced by gastrointestinal side effects. In addition to citrate, the preparation used in this trial (Blemaren®) also contains a small amount of adipate (35 mg/0.24 mmol per tablet, corresponding to 30 mg or 0.2 mmol/kg body weight per day at the highest citrate dosage). Whether or not the addition of adipate contributed to the effects reported here cannot be concluded from our observations.
Taken together, treatment of DL-2HGA with citrate in our patient led to partial amelioration of biochemical parameters and appeared to contribute to stabilization of the clinical course. Treatment of DL-2HGA with citrate should be studied systematically.
References
Chalmers RA, Lawson AM (1982) Organic acids in man. Chapman and Hall Ltd., London
Edvardson S, Porcelli V, Jalas C et al (2013) Agenesis of corpus callosum and optic nerve hypoplasia due to mutations in SLC25A1 encoding the mitochondrial citrate transporter. J Med Genet 50:240–245
Hagos Y, Krick W, Braulke T, Mühlhausen C, Burckhardt G, Burckhardt BC (2008) Organic anion transporters OAT1 and OAT4 mediate the high affinity transport of glutarate derivatives accumulating in patients with glutaric acidurias. Pflugers Arch 457:223–231
Kanungo S, Soares N, He M, Steiner RD (2013) Sterol metabolism disorders and neurodevelopment - an update. Dev Disabil Res Rev 17:197–210
Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS, Jakobs C (2012) Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 35:571–587
Lamp J, Keyser B, Koeller DM, Ullrich K, Braulke T, Mühlhausen C (2011) Glutaric aciduria type 1 metabolites impair the succinate transport from astrocytic to neuronal cells. J Biol Chem 286:17777–17784
Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russel DW (2003) Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 278:22980–22988
McNally MA, Pyzik PL, Rubenstein JE, Hamdy RF, Kossoff EH (2009) Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics 124:e300–e304
Mühlhausen C, Burckhardt BC, Hagos Y et al (2008) Membrane translocation of glutaric acid and its derivatives. J Inherit Metab Dis 31:188–193
Muntau AC, Röschinger W, Merkenschlager A et al (2000) Combined D-2- and L-2-hydroxyglutaric aciduria with neonatal onset encephalopathy: a third biochemical variant of 2-hydroxyglutaric aciduria? Neuropediatrics 31:137–140
Mycielska ME, Patel A, Rizaner N et al (2009) Citrate transport and metabolism in mammalian cells: prostate epithelial cells and prostate cancer. Bioessays 31:10–20
Nota B, Struys EA, Pop A et al (2013) Deficiency in SLC25A1, encoding the mitochondrial citrate carrier, causes combined D-2- and L-2-hydroxyglutaric aciduria. Am J Hum Genet 92:627–631
Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch 447:689–709
Read M-H, Bonamy C, Laloum D et al (2005) Clinical, biochemical, magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H-MRS) findings in a fourth case of combined D- and L-2 hydroxyglutaric aciduria. J Inherit Metab Dis 28:1149–1150
Struys EA, Jansen EE, Verhoeven NM, Jakobs C (2004) Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin Chem 50:1391–1395
Acknowledgments
We thank Dr. Uwe Ahting, Mitocenter, Institute of Human Genetics, Technische Universität, Munich, Germany, for providing the data of mitochondrial enzyme analyses, and Prof. Dr. Jens Fiehler, Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, for providing MRI images.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Cornelis Jakobs
Rights and permissions
About this article
Cite this article
Mühlhausen, C., Salomons, G.S., Lukacs, Z. et al. Combined D2-/L2-hydroxyglutaric aciduria (SLC25A1 deficiency): clinical course and effects of citrate treatment. J Inherit Metab Dis 37, 775–781 (2014). https://doi.org/10.1007/s10545-014-9702-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9702-y