Abstract
Background
Late-onset glycogenosis type II (GSD II) is a rare, multisystem disorder mainly affecting limb and respiratory muscles due to acid alpha glucosidase deficiency. Despite evidence at autopsy of glycogen accumulation in the brain, no study exploring brain functions is yet available.
Objective
Our objective in this study was to assess brain changes in late-onset GSD II.
Methods
Each patient underwent a standardized neuropsychological assessment, regional grey-matter (GM) atrophy, and resting-state functional magnetic resonance imaging (RS-fMRI). Functional connectivity maps of the salience (SN) and default-mode (DMN) networks were considered. A group of age- and gender-matched healthy controls was enrolled for MRI comparisons. P values family-wise error (FWE) cluster level corrected inferior to 0.05 were considered.
Results
Nine GSD II patients (age 46.6 ± 8.0; 55 % male) were recruited. No significant GM atrophy was found in patients compared with controls (n = 18; age 48.0 ± 9.8,;40 % male). Functional connectivity within the SN was selectively reduced in patients, and cingulate gyrus and medial frontal cortex were mainly involved. Accordingly, patients had significant impairment of executive functions (as measured by Wisconsin Card Sorting test), whereas other cognitive domains were within mean normal ranges.
Conclusions
Our findings extend the clinical spectrum of GSD II by indicating that brain changes occur in this muscular disorder. Above all, these results should lead to better examinations of therapeutic approaches and perspectives for the affected patients. Further studies evaluating in depth these issues are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Late-onset glycogenosis type II (GSD II), also named late-onset Pompe disease or glycogen storage disease type II (GSD II; OMIM #232300), is a rare, autosomal-recessive metabolic disorder caused by an accumulation of glycogen in the lysosome due to deficiency of the l-acid alpha-glucosidase enzyme (van der Ploeg et al. 2010). A number of mutations in both copies of the acid alpha-glucosidase (GAA) gene have been reported, with varying degrees of GAA deficiency. In late-onset GSD II, some residual enzymatic activity (from 1 % to 40 %) is usually present compared with infantile onset in which it is totally absent (van der Ploeg et al. 2010). Compared with the infantile form, late-onset GSD II is more slowly progressive and is clinically characterized by muscle weakness, predominantly in proximal lower limbs, variable respiratory involvement, and generally with spared heart function (American Association of Neuromuscular and Electrodiagnostic Medicine 2009; Kishnani et al. 2006; Wokke et al. 2008). Even though skeletal and smooth muscles are mainly involved, other tissues may be affected by the disease process (Braunsdorf 1987; Laforet et al. 2008). Glycogen storage is also autoptically present in the brain, brainstem, and anterior horns (Di Rocco et al. 2007; Raben et al. 2002). Accordingly, a few reports in cases of infantile-onset disease have highlighted the presence of delayed cerebral myelinization (Chien et al. 2006; Ebbink et al. 2012) and a variable degree of cognitive development ranging from normal to mild retardation (Ebbink et al. 2012; Spiridigliozzi et al. 2012). However, no systematic research on this issue has been carried out, and no data in late-onset GSD II are yet available. Therefore, it remains unknown whether—and if so, which—cognitive and brain changes may occur in these patients.
Imaging genetics is a rapidly emerging field that is opening up a new landscape of discovery in neuroscience (Thompson et al. 2010). In this context, magnetic resonance imaging (MRI) has become an increasingly popular tool for human brain investigation in vivo. MRI has the unique ability to provide quantitative information on both brain-tissue structure and functioning. Voxel-based morphometry (VBM) is currently regarded as a robust MR technique suitable for assessing structural gray matter (GM) modifications in an unbiased fashion (Borroni et al. 2008). On the other hand, resting-state functional MRI (RS-fMRI) has shown the ability to provide measures of functional brain connectivity (De Luca et al. 2006; Fox and Raichle 2007). Functional connectivity is a concept based on evidence that different brain regions present with synchronous patterns of activity at rest. Those regions are likely to be part of common networks subserving complex brain functions. From RS-fMRI data (i.e., fMRI time series collected while patients lie vigilant but at rest in the scanner), several networks can be extracted at the same time in a data-driven fashion using independent component analysis (Greicius et al. 2003). The default-mode network (DMN) is by far the most extensively studied network. This is believed to be relevant for specific higher-level functions, such as the working memory, mind wandering, and goal-directed behaviors (Fox and Raichle 2007). The so-called salience network (SN) is another extensively studied RS-fMRI component, which is believed to be particularly informative when investigating executive functions (Zhou et al. 2010).
SN is built around paralimbic structures, most prominently, the dorsal anterior cingulate and anterior insula, which underlie interoceptive–autonomic processing to guide behavior (Mesulam 1998; Seeley et al. 2007). These regions coactivate in response to emotion (Craig 2009, 2002), pain (Peyron et al. 2000) and are active in cognitive and homeostatic control (Menon and Uddin 2010). So far, SN is considered to be related to socially and emotionally relevant information processing (Seeley et al. 2007).
In this work, we aimed at evaluating whether—and if so, which—brain changes occur in patients with late-onset GSD II using VBM and RS-fMRI. We hypothesized that central nervous system (CNS) involvement is a common feature of the disease. We further corroborated neuroimaging data with a careful assessment of neuropsychological performances.
Methods
Study participants
Participants entering this study were recruited at the Neurology Unit, University of Brescia, Italy, from September 2008 to September 2012. Patients were selected by three inclusion criteria: (a) confirmed diagnosis of GSD II, either by reduced GAA activity <35 % of controls in skeletal muscle, leukocytes, or fibroblasts; or by confirmed pathogenetic mutations in the GAA gene; (b) adult age at disease onset; (c) presence of signs or symptoms of the disease (e.g., at least grade 1 on the Walton scale) (American Association of Neuromuscular and Electrodiagnostic Medicine 2009; Bembi et al. 2008). All patients underwent a standardized neuropsychological evaluation and neurological assessment, genetic analysis, and alpha 1,4 glucosidase activity measurement. To characterize pathogenetic mutations within the GAA gene, DNA was isolated from whole blood samples and direct sequencing of the entire coding regions (20 exons) as well as intronic flaking sequences performed with standard procedures. Nucleotides are numbered according to the GeneBank Accession N° NM_000152. GAA dosage in lymphocytes was performed as previously reported (Jack et al. 2006). A group of 18 age- and gender-matched healthy controls (patients:controls 1:2) were included for MRI analyses (mean age 48.0 ± 9.8; 40 % male). Each control was screened for global cognitive functions, which were within normal range; MRI scan was unremarkable. Written informed consent was obtained for each procedure before study initiation, including MRI scanning. The work conformed to the Declaration of Helsinki.
Neuropsychological assessment
A neuropsychological battery of tests was administered to each patient by trained neuropsychologists 48 h before acquisition of the MRI. It included a general cognitive evaluation using the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) and tests specific for each cognitive domain: Wisconsin Card Sorting Test (WCST) (Berg 1948) as a measure of reasoning ability and shifting cognitive strategies; Stroop Color-Word Test (Stroop 1935) as a general measure of cognitive flexibility and control; Controlled Oral Word Association Test and Category Fluency Test (Isaacs and Kennie 1973) as a measures of verbal fluency; Trail Making Test Parts A and B (Reitan 1955) as measures of executive functions; Clock Drawing Test as a measure of several skills, including visuospatial construction ability and executive functioning (Sunderland et al. 1989). For each administered test, appropriate adjustments for age and education were applied according to Italian normative data. Further, scores were expressed as equivalent scores (ES) (Capitani and Laiacona 1997); ES is a 5-point scale (from 0–4, worst to best). We considered ES ≤1 as poor performances and ES ≥2 as good performances.
MRI acquisition and analysis
All imaging was obtained using a 1.5-T MR scanner (Siemens Symphony, Erlangen, Germany) equipped with a circularly polarized transmit–receive coil. In a single session, the following scans were collected from each studied individual: (1) dual-echo turbo spin-echo (TSE) [repetition time (TR) = 2,500 ms, echo time (TE) = 50 ms] to exclude the presence of macroscopic brain abnormalities, according to exclusion criteria; (2) 3D magnetization-prepared rapid-gradient-echo (MP-RAGE) T1-weighted scan [TR = 2,010 ms, TE = 3.93 ms, matrix = 1 × 1 × 1, in-plane field of view (FOV) = 250 × 250 mm2, slice thickness = 1 mm, flip angle = 15°]; and (3) T2*-weighted echo planar imaging (EPI) sensitized to blood-oxygen-level-dependent (BOLD) contrast [TR = 2,500 ms, TE = 50 ms, 29 axial slices parallel to anterior commissure–posterior commissure line (AC-PC) line, matrix = 64 × 64, field of view = 224 mm, slice thickness = 3.5 mm] for RS-fMRI. BOLD EPI images were collected during rest for an 8-min period, resulting in a total of 195 volumes. During this acquisition, participants were instructed to keep their eyes closed, not to think of anything in particular, and not to fall asleep. Dual-echo TSE images were carefully reviewed to exclude the presence of signs suggestive of cerebrovascular disease, and T1-weighted images from all participants were visually inspected for qualitative assessment to check data quality before carrying out a quantitative volumetric analysis. Intracranial mass was considered an exclusion criterion for further analysis of MRI scans.
Voxel-based morphometry
MP-RAGE data were processed using the VBM protocol in Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Imaging Neuroscience; www.fil.ion.ucl.ac.uk/spm/). For each participant, an iterative combination of segmentations and normalizations (implemented within the Segment SPM8 module) produced a GM probability map (Ashburner and Friston 2005) in Montreal Neurological Institute (MNI) coordinates. To compensate for compression or expansion during warping of images to match the template, GM maps were modulated by multiplying the intensity of each voxel by the local value derived from the deformation field (Jacobian determinants) (Ashburner and Friston 2001). All data were then smoothed using a 12-mm full-width half-maximum (FWHM) Gaussian kernel. Modulated and smoothed GM were analyzed in SPM8 using a full factorial design. Age and gender were added as covariates of no interest. GM map analysis was also adjusted for total intracranial volume (ICV = GM volume + white-matter volume + cerebrospinal fluid volume). Contrasts were designed to assess the effect of glycogenosis type II in regional GM volumes by comparing patients with controls. P values were accepted as statistically significant for p values family-wise error (FWE) cluster level corrected inferior to 0.05.
fMRI data analysis
RS-fMRI data were analyzed using SPM8 for image preprocessing and statistical comparison, and the Group Independent Component Analysis (ICA) for fMRI Toolbox (GIFT, icatb.sourceforge.net/) for network identification. For each participant, the first four volumes of the fMRI series were discarded to allow for T1 equilibration effects. The preprocessing steps included correction for head motion, compensation for slice-dependent time shifts, normalization to the EPI template in Montreal Neurological Institute coordinates provided with SPM8, and smoothing with a 3D Gaussian kernel with 8-mm3 FWHM. Then, all images were filtered by a phase-insensitive bandpass filter (pass band 0.01–0.08 Hz) to reduce the effect of low-frequency drift and high-frequency physiological noise. ICA analysis was employed to identify 20 independent components. Briefly, group ICA for fMRI Toolbox first concatenates the individual data across time and then produces a computation of participant-specific components and time courses. For all participants grouped together, Toolbox performed the analysis in three steps: (1) data reduction, (2) application of the FastICA algorithm, and (3) back-reconstruction for each individual (De Luca et al. 2006). Results were converted to Z scores. The 20 components were reviewed and compared by computing the spatial correlation coefficient to customized templates of the SN and DMN. These templates were obtained from an independent sample of 28 healthy controls [13 women, mean age 38.6 years; standard deviation (SD) 7.5 years]. This procedure was performed using the tool for spatial sorting of the components available with GIFT. Every individual’s Z-score maps corresponding to these two resting-state networks were used for cross-subject analyses. Differences between patients and controls were considered in each network. Age and gender were entered as covariates of no interest. P values were accepted as statistically significant for p values FWE cluster level corrected inferior to 0.05.
Results
Patients
Demographic characteristics of GSD II patients are reported in Table 1. Patients had a mean age of 46.6 (SD = 8), 55.5 % were men, and mean age at disease onset was 31.6 (SD = 12.6). The most common mutation, c-32 13 T/G (IVS1), was found in all patients In regard to the other allele, four patients were 2237G/A mutation carriers, three had 525delT genetic variation, two were delexon18 carriers, and one had 1927G/A mutation. Endogenous GAA activity levels from lymphocytes ranged from 2 % to 25 %. All patients except one were in treatment with enzymatic replacement therapy (therapy initiation range 1–5 years).
Neuropsychological assessment
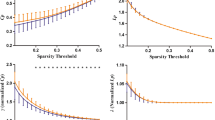
A number of patients with late-onset GSD II reported pathological performances in the WCST, a test assessing strategic planning, organized searching, utilizing environmental feedback to shift cognitive sets, and modulating impulsive responding (see Table 2). As shown in Fig. 1, when ES were considered, 66.7 % of patients showed poor performances (≤1) at WCST global score and at nonperseverative errors of WCST, whereas 55.5 % of patients showed impaired perseverative errors at WCST. No significant deficits (>50 % of patients) in the other tests were reported.
Percentage of patients with poor cognitive performances: equivalent scores (ES) ≤1, indicative of poor performances (see text for details). MMSE Mini-Mental State Examination, Phon. phonemic, Sem semantic, TM trail making, IT interference time, IE interference errors, WCST Wisconsin Card Sorting Test, tot global score, per perseverative errors, nper nonperseverative errors
Visual inspection of T1-weighted and T2-FLAIR images
T1-weighted images did not reveal any macroscopic abnormality and according to experienced radiologist were indistinguishable from those of healthy controls of comparable age. In this series of patients, ectasia of the basilar artery in two cases was reported, as already documented in the literature (Laforet et al. 2008). In eight of nine patients with GSD II, no alterations were found on fluid attenuated inversion recovery (FLAIR) images were found; in one patient, a chronic ischemia event with no clinical relevance was detected.
VBM
There was no significant difference in regional GM volumes between patients and age-matched controls at the pre-established threshold.
RS-fMRI
Among the 20 components modelled in the ICA analysis, several well-known resting-state networks were identified, including the SN and the DMN.
Analysis of the SN revealed a significantly reduced functional connectivity in patients compared with controls. As shown in Fig. 2 and Table 3, these clusters of reduced functional connectivity were localized in the middle frontal lobe, bilaterally, and in the cingulate gyrus.
Reduced salience network (SN) connectivity in late-onset glycogenosis type II patients compared with healthy controls. Results are superimposed on a 3D brain template. L left. P < 0.001 uncorrected, only cluster survived at family-wise error (FWE) < 0.05 cluster level were reported. Threshold = 100 voxels
The inverse correlation (i.e. controls < patients) showed no cluster above the pre-established threshold in the SN. Analysis of the DMN showed a reduced connectivity in patients with GSD II compared with controls in the left caudate. Again, the inverse correlation (i.e., controls < patients) showed no cluster above the pre-established threshold in the DMN. No significant correlation was found between functional connectivity within the SN and WCST, residual enzymatic activity, and disease duration.
Discussion
In the last 30 years, a growing number of patients with late-onset GSD II has been described (Winkel et al. 2005) and the clinical picture carefully detailed. Late-onset GSD II is a multisystem disorder primarily characterized by muscle weakness but with other emerging signs and symptoms that need further attention to counteract disease progression properly. In this study, we aimed at evaluating whether GSD II may also be considered a brain disease, as there is evidence at autopsy of glycogen accumulation in the brain (Di Rocco et al. 2007; Raben et al. 2002). We therefore performed a comprehensive study of brain structure and function using VBM and RS-fMRI. Our neuroimaging findings along with neuropsychological assessment confirmed the main hypotheses of our work.
When looking at differences in regional brain atrophy, VBM analysis revealed no significant cluster above the pre-established threshold, in line with autopsy studies, which reported no evidence of cortical atrophy (van der Walt et al. 1987). Conversely, significant changes in connectivity of the explored functional brain networks were reported. Patients with GSD II showed SN disruption. This network is implicated in executive functioning, planning, and abstract reasoning, and is consistent with findings of the neuropsychological profile. Indeed, disconnection of cortical regions is a potential mechanism for cognitive dysfunction in various neurological disorders (Dineen et al. 2009) and may also be responsible for the reported impairment at WCST, as this test maps frontal-lobe functions. Despite the absence of direct associations between cognitive data and changes in functional connectivity within the SN, which is likely due to the relative low number of patients, we can speculate that there is a direct correlation between cognitive impairment and fMRI findings. Indeed, we applied two tests that are able to detect dysfunctions of the frontal lobe—namely, the WCST and Stroop tests—and found impaired performances in the former. Indeed, this mismatch may be explained by differences in the explored functions. The WCST is more complex and evaluates set shifting abilities, cognitive flexibility, and problem solving, whereas the second measures selective attention and the ability to inhibit automatic responses when necessary.
The relationship between our current neuroimaging and cognitive findings and the molecular mechanisms underlying neuronal firing patterns are complex and still not elucidated. Few studies on glycogen accumulation in the human brain are available as yet (Di Rocco et al. 2007; Taksir et al. 2007), and results of our study suggest that future work is warranted to increase our understanding of this issue. For the first time, our work reported here extends the clinical spectrum of this multisystem disorder and might provide a model by which to characterize in vivo the brain changes occurring in patients with GSD II. Indeed, recent studies suggest that nonspecific demyelinating white-matter changes in infantile-onset disease were observed (Chien et al. 2006). Accordingly, experimental mouse models of Pompe disease have widely confirmed the presence of glycogen accumulation in the CNS (Taksir et al. 2007).
The association between glycogen accumulation and the selective disruption of the SN found in our study needs to be further investigated. Furthermore, even without a clear stringent correlation with clinical symptomatology, DMN alterations also should be considered in future studies. Indeed, we found RS-fMRI brain abnormalities without grey-matter atrophy, suggesting functional disruption of neuronal networks without structural changes. These findings might have potential consequences on the current treatment approach (Angelini et al. 2012). Enzyme replacement therapy has a beneficial impact upon muscle manifestations of late-onset disease, but the enzyme does not cross the blood–brain barrier and cannot overcome CNS changes (Ebbink et al. 2012; Begley et al. 2008). Nevertheless, a previous study indicated that long-term enzyme replacement therapy ameliorated white-matter changes in infantile-onset disease (Chien et al. 2006). Therefore, the effect of current pharmacological treatment in covering CNS manifestations needs to be further investigated.
This study has some limitations: First, as this is a rare disorder, the sample size is relatively small. We overcame this limitation by applying stringent statistical analysis corrected for multiple comparisons. In the same way, we were unable to detect associations between measures of global cognition and the strength of connectivity in the evaluated functional networks. Second, a more detailed cognitive assessment might be of help to evaluate in depth the subtle cognitive deficits and to further explore executive functions. Third, a comprehensive, in-depth work evaluating white-matter abnormalities is desirable. Future studies on larger samples evaluating the effect of different mutations and different rates of GAA deficiency are needed to clarify whether and how the human brain may cope with glycogen accumulation.
References
American Association of Neuromuscular & Electrodiagnostic Medicine (2009) Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve 40:149–160
Angelini C, Semplicini C, Ravaglia S et al (2012) Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 259:952–958
Ashburner J, Friston KJ (2001) Why voxel-based morphometry should be used. NeuroImage 14:1238–1243
Ashburner J, Friston KJ (2005) Unified segmentation. NeuroImage 26:839–851
Begley DJ, Pontikis CC, Scarpa M (2008) Lysosomal storage diseases and the blood–brain barrier. Curr Pharm Des 14:1566–1580
Bembi B, Cerini E, Danesino C et al (2008) Diagnosis of glycogenosis type II. Neurology 71:S4–S11
Berg EA (1948) A simple objective technique for measuring flexibility in thinking. J Gen Psychol 39:15–22
Borroni B, Alberici A, Premi E et al (2008) Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res 11:585–595
Braunsdorf WE (1987) Fusiform aneurysm of basilar artery and ectatic internal carotid arteries associated with glycogenosis type 2 (Pompe’s disease). Neurosurgery 21:748–749
Capitani E, Laiacona M (1997) Composite neuropsychological batteries and demographic correction: standardization based on equivalent scores, with a review of published data. The Italian Group for the Neuropsychological Study of Ageing. J Clin Exp Neuropsychol 19:795–809
Chien YH, Lee NC, Peng SF, Hwu WL (2006) Brain development in infantile-onset Pompe disease treated by enzyme replacement therapy. Pediatr Res 60:349–352
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3(8):655–666
Craig AD (2009) Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci 364(1525):1933–1942
De Luca M, Beckmann CF, De SN, Matthews PM, Smith SM (2006) fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage 29:1359–1367
Di Rocco M, Buzzi D, Taro M (2007) Glycogen storage disease type II: clinical overview. Acta Myol 26:42–44
Dineen RA, Vilisaar J, Hlinka J et al (2009) Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132:239–249
Ebbink BJ, Aarsen FK, van Gelder CM et al (2012) Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology 78:1512–1518
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258
Isaacs B, Kennie AT (1973) The set test as an aid to the detection of dementia in old people. Br J Psychiatry 123:467–470
Jack RM, Gordon C, Scott CR, Kishnani PS, Bali D (2006) The use of acarbose inhibition in the measurement of acid alpha-glucosidase activity in blood lymphocytes for the diagnosis of Pompe disease. Genet Med 8:307–312
Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D (2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148:671–676
Laforet P, Petiot P, Nicolino M et al (2008) Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology 70:2063–2066
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667
Mesulam MM (1998) From sensation to cognition. Brain 121(Pt 6):1013–1052
Peyron R, Laurent B, García-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30(5):263–288
Raben N, Plotz P, Byrne BJ (2002) Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease). Curr Mol Med 2:145–166
Reitan RM (1955) The relation of the trail making test to organic brain damage. J Consult Psychol 19:393–394
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356
Spiridigliozzi GA, Heller JH, Kishnani PS (2012) Cognitive and adaptive functioning of children with infantile Pompe disease treated with enzyme replacement therapy: long-term follow-up. Am J Med Genet C Semin Med Genet 160:22–29
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Sunderland T, Hill JL, Mellow AM et al (1989) Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc 37:725–729
Taksir TV, Griffiths D, Johnson J, Ryan S, Shihabuddin LS, Thurberg BL (2007) Optimized preservation of CNS morphology for the identification of glycogen in the Pompe mouse model. J Histochem Cytochem 55:991–998
Thompson PM, Martin NG, Wright MJ (2010) Imaging genomics. Curr Opin Neurol 23:368–373
van der Ploeg AT, Clemens PR, Corzo D et al (2010) A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med 362:1396–1406
van der Walt JD, Swash M, Leake J, Cox EL (1987) The pattern of involvement of adult-onset acid maltase deficiency at autopsy. Muscle Nerve 10:272–281
Winkel LP, Hagemans ML, van Doorn PA et al (2005) The natural course of non-classic Pompe’s disease; a review of 225 published cases. J Neurol 252:875–884
Wokke JH, Escolar DM, Pestronk A et al (2008) Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve 38:1236–1245
Zhou J, Greicius MD, Gennatas ED et al (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133:1352–1367
Acknowledgements
The authors kindly thank Prof. C. Danesino from the Unit of Medical Genetics of University of Pavia, Italy, for conducting the genetic studies, and Dr. P. Tonin from the Department of Neurological, Neuropsychological, Morphological and Movement Sciences of University of Verona, Italy, for biochemical assay. We also thank the Italian Group for Glycogenosis II of the Italian Association of Myology.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Gerard T. Berry
Rights and permissions
About this article
Cite this article
Borroni, B., Cotelli, M.S., Premi, E. et al. The brain in late-onset glycogenosis II: a structural and functional MRI study. J Inherit Metab Dis 36, 989–995 (2013). https://doi.org/10.1007/s10545-013-9601-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-013-9601-7