Abstract
Though the control of blood phenylalanine (Phe) levels is essential for minimizing impairment in individuals with phenylketonuria (PKU), the empirical basis for the selection of specific blood Phe levels as targets has not been evaluated. We evaluated the current evidence that particular Phe levels are optimal for minimizing or avoiding cognitive impairment in individuals with PKU. This work uses meta-estimates of blood Phe-IQ correlation to predict the probability of low IQ for a range of Phe levels. We believe this metric is easily interpretable by clinicians, and hence useful in making recommendations for Phe intake. The median baseline association of Phe with IQ was estimated to be negative, both in the context of historical (median = −0.026, 95 % BCI = [−0.040, −0.013]) and concurrent (−0.007, [−0.014, 0.000]) measurement of Phe relative to IQ. The estimated additive fixed effect of critical period Phe measurement was also nominally negative for historical measurement (−0.010, [−0.022, 0.003]) and positive for concurrent measurement (0.007, [−0.018, 0.035]). Probabilities corresponding to historical measures of blood Phe demonstrated an increasing chance of low IQ with increasing Phe, with a stronger association seen between blood Phe measured during the critical period than later. In contrast, concurrently-measured Phe was more weakly correlated with the probability of low IQ, though the correlation is still positive, irrespective of whether Phe was measured during the critical or non-critical period. This meta-analysis illustrates the utility of a Bayesian hierarchical approach for not only combining information from a set of candidate studies, but also for combining different types of data to estimate parameters of interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenylketonuria (PKU) is a metabolic disorder in which a buildup of phenylalanine (Phe) in the blood results from an inability to properly metabolize that amino acid. This buildup, in turn, becomes neurotoxic and can lead to intellectual disability, delayed speech, seizures and behavioral abnormalities. PKU is typically diagnosed at birth based on newborn screening results. Approximately 1 in 13,500 to 19,000 infants in the United States is born with PKU (Hegge et al 2009; National Institutes of Health Consensus Development Panel 2001). The most severe form of PKU (classic PKU) is typically characterized by blood Phe levels exceeding 1200 μmolL-1 while on a normal diet. Individuals are diagnosed with hyperphenylalaninemia if their Phe levelis above normal (120 μmol L-1) but less than about 1000 μmol L-1 while on a normal diet (exact cutoffs vary in the literature and in practice). The mainstay for treatment of PKU is a special diet that restricts the intake of Phe in order to maintain a low Phe concentration in the blood. With adherence to a Phe-restricted diet, adverse cognitive outcomes can be mitigated. However, management of PKU can be burdensome for the patient and their family, so there is interest in identifying alternative ways of managing this lifelong condition effectively. Further, questions remain as to the empirical basis for the selection of specific blood Phe levels as targets to reflect good dietary control.
Individuals with PKU can tolerate varying quantities of Phe intake. Their Phe levels are monitored frequently, allowing physicians to recommend appropriate modifications to Phe intake in order to determine their ideal Phe tolerance. Historically, Phe levels were only monitored closely during the first 6 years of life (the “critical period”) because elevated Phe was not believed to be detrimental in older individuals. However, based on accumulated evidence over the last few decades, it is now standard of care to recommend strict adherence to a Phe-restricted diet and routine monitoring of Phe levels throughout life (National Institutes of Health Consensus Development Panel 2001; Koch et al 2002).
In general, the treatment goal is a Phe level below 360 μmol L-1 for children, though the goal treatment range for adolescents and adults is controversial and varies between centers and among countries (National Institutes of Health Consensus Development Panel 2001; Giovannini et al 2007). However, there is little empirical basis for selecting a particular Phe level as a target, and specifically, on an optimal range for minimizing the clinical and cognitive effects of hyperphenylalaninemia among individuals of varying age.
A meta-analysis by (Waisbren et al 2007) employed random effects models (DerSimonian and Laird 1986) to relate IQ measurement to Phe levels, based on within-study correlations. Neuropsychological or other (non-IQ) cognitive outcomes were deemed by the authors to be too disparate to combine. Of the studies included in the analysis, only 40 presented within-study correlations and could be used in the quantitative meta-analysis. A weakness of this review is that there was no accounting for the variance of the within-study correlations for each study. This ignores a potentially important source of variation, and likely results in overly-precise meta-estimates.
In a separate, more recent meta-analysis, (Albrecht et al 2009) examined the evidence for neuropsychological effects varying with Phe concentration. They restricted their meta-analysis to studies where response data were collected with computer-based measurement devices (942 individuals in total), and where PKU subjects were compared to healthy controls. The reaction time for any of a suite of neuropsychological tests was the chosen response variable. Seven different classes of test were used across studies, and combined in the meta-analysis using a standardized effect size measure (Rosenthal 1994). Results suggested that age was an important covariate, along with Phe and interactions of Phe with age and Phe with test type. Though no estimate of maximum Phe intake could be obtained for adults, the authors estimated an upper threshold of 320 μmol L-1 for children (7–13 years) and 570 μmol L-1 for adolescents (14–18 years).
Here, we seek to evaluate the current evidence that any specific Phe levels are optimal for minimizing or avoiding cognitive impairment in individuals with PKU. The (Waisbren et al 2007) review was a step in this direction, as it sought to estimatethe correlation between blood Phe and IQ, but only indirectly addresses our question. Our work uses meta-estimates of blood Phe-IQ correlation to predict the probability of low IQ for a range of Phe levels. We believe this metric is more easily interpretable by clinicians, and hence is potentially more useful in making recommendations for Phe intake. We agree with (Waisbren et al 2007) that other measures of cognitive outcomes (though perhaps objectively better than IQ) are either used non-uniformly across studies or are too difficult to combine.
Literature search methods
We employed a systematic search to retrieve research on the treatment of PKU, as part of a larger systematic review of the disease. Our primary literature search employed five databases: MEDLINE®via the PubMed interface, PsycINFO (CSA Illuminainterface; psychology and psychiatry literature), EMBASE, the Cumulative Index of Nursing and Allied Health Literature (CINAHL) database, and the National Agricultural Library (AGRICOLA) database. Our search strategies used a combination of subjectheading terms appropriate for each database and keywords relevant to PKU (e.g., phenylketonuria, pharmaceutical preparations, phenylalanine). We limited searches to the English language but did not set a date limit.
Studies needed to provide adequate information to ensure that participants were in the target study population. We only included studies with human participants who had any form of PKU or hyperphenylalaninemia. We did not include studies with participants who had primary tetrahydrobiopterin deficiency. As we recognize that classification of the severity of PKU varies across countries and clinics (National Institutes of Health Consensus Development Panel 2001), we did not impose a specific classification of PKU types (e.g., classic versus moderate or mild), but instead allowed the definitions of PKU as they were operationalized by study authors.
We included only English language studies that had a minimum sample size of ten subjects. We included randomized controlled trials (RCTs) and uncontrolled open label trials, prospective and retrospective cohort studies, as well as cross-sectional studies. Because we sought to identify specific Phe levels at which low-IQ outcomes were observed, we required papers to include either individual-level data on both Phe and IQ measurement or a group mean/median and some measure of variance (usually standard deviation) for both. Papers where IQ measurements preceded Phe measurement, or where the timing was unclear, were excluded.
Meta-analytic methods
The association of blood phenylalanine levels with IQ was meta-analyzed using a hierarchical mixed-effects model, estimated using Markov chain Monte Carlo methods (Gelman et al 2003). The advantages of using a Bayesian approach to meta-analysis were recognized over a decade ago (Smith and Spiegelhalter 1995), and they have been applied extensively ever since (Tweedie et al 1996; Sutton and Abrams 2001; Brophy and Joseph 2001; Brophy and Bélisle 2003; Babapulle et al 2004; Kaizar et al 2006; Afilalo et al 2008). It allows for straightforward, probabilistic inference across studies, and readily combines both fixed and random effects. In contrast to the more indirect measures of inference afforded by classical methods, all inference from Bayesian models is in the form of probability statements (p) that describe the uncertainty in the unknown quantities of interest (θ), given the information at hand (y):
This is Bayes’ formula. The left-hand side is the posterior distribution of all unknown parameters in the model, which is proportional to (∝) the product of a data likelihood (that is, the probability of the data conditional (|) on the values of the parameters) and the prior distribution (i.e., before data are observed) of the model on the right-hand side. While the use of priors allows for the incorporation of extant information into the analysis, we used non-informative priors on all parameters (i.e., distributions that are relatively flat, which suggests little or no prior preference for any particular values), allowing the results from the included studies to provide all the evidence.
Here, we define a fixed effect as a single (usually unknown) parameter value that is fixed throughout the population. In contrast, a random effect is drawn from a random distribution (here a normal distribution) for each individual in the population. Random effects therefore allow for some variation among individuals or groups of individuals in a population, due to factors that are not or cannot be measured. Hierarchical models usually imply the use one or more random effects. A hierarchical model is one in which model components are nested according to particular units of organization. For example, individual attributes can be modeled at one level of the hierarchy, while other attributes may be associated with particular groups of individuals, and still others with entire populations. For example, in this meta-analysis, we model Phe and IQ at the level of individuals or groups, but the expected values of these observations are hypothesized to vary by study, according to any number of unmeasured factors. Hence, we use a random effect to describe this study-level variation.
A powerful aspect of using random effects for meta-analyses is the notion of partial pooling (Gelman et al 2003). This permits us to abandon the tenuous assumption that the effects across studies are independent and identically distributed. Rather, we view them as exchangeable samples from a population of possible PKU studies. Using a random effect to partially pool across studies expresses our desire neither to combine studies in a single estimate (which assumes they are identical) nor to keep them entirely separate (which assumes they are completely different), but rather, some intermediate of the two extremes. In contrast, fixed effects models imply one of these two unlikely extremes, with either a pooled effect size, or individual, study-specific estimates. Moreover, the degree to which studies share information via the population random effect is dictated by the heterogeneity across studies as represented in the data, rather than via arbitrary weighting factors.
In an effort to partially pool the information from the set of studies obtained in the literature search, we specified random effects for the intercept and slope parameters of a linear relationship between blood Phe level and IQ. Importantly, this allowed each study to have its own parameters, each sampled from a conceptual population of parameters. Those with smaller sample sizes were automatically shrunk toward the population means for each parameter, with larger studies influencing the estimate of the population mean more than being influenced by it. In turn, the magnitude of the effect (i.e., slope) was specified partly as a function of a fixed effect that accounted for whether measurements of Phe were carried out during the critical period. Hence, the overall model was a hierarchical mixed effects model. Bayesian hierarchical models are easily estimated using Markov chain Monte Carlo (MCMC) methods (Brooks et al 2010).
We developed two meta-analytic models. The first represents the relationship of blood Phe and IQ when Phe was measured historically with respect to IQ measurement (more than 12 months before IQ measurement), while in the second model, Phe and IQ were measured concurrently (Phe measured within 6 weeks prior to IQ measurement). Note that there were no studies in which Phe measurements were taken 6 weeks to 12 months prior to IQ measurements. We believed it to be unreasonable to combine historical and concurrent measurements in the same model, given the potential for a drastically different relationship between the two variables depending on how far apart they were taken. Studies with both historical and concurrent measurement data were included in both models.
The core of each model is a linear relationship between the expected IQ (μ) and Phe (x):
The subscript j[i] denotes parameters for study j corresponding to observation i. Hence, both the intercept β 0 and slope β 1 are allowed to vary by study. Note that by “observation” we refer here not to individuals, but to groups of individuals within a study that share a covariate. For example, within the same study, one group of individuals might have been measured for Phe in the critical period, and others not; these groups were considered separate observations in this analysis. One study (Seashore et al 1985) reported a range of Phe measurements, rather than a single value, so we imputed values by randomly sampling at every iteration from a uniform distribution across the reported range.
Though age was included as an additional predictor in early versions of the model, it did not appear to be a suitable covariate, and models in which it was included did not exhibit good convergence. Hence, age was omitted from the final model. We suspect that the important aspects of age are adequately characterized by the four combinations of historical or concurrent Phe measurement and measurement in or outside the critical period. For example, concurrent measurements during the critical period implies that the subject was young, while historical measurements during the non-critical period typically applies to older subjects.
The intercept was modeled as a random effect, where each study is assumed to be an exchangeable (i.e., conditionally independent) sample from a population of PKU studies:
Thus, μ β is the expected (population) mean and τ β the associated variance, describing the expected differences within the population. This random effect was included to account for a variety of potential sources of variation in IQ, such as the IQ measurement scale in each study or geographic location.
The slope of the relationship included a study-level random effect and a fixed effect corresponding to whether the Phe measurement was taken during the critical period (via indicator function I):
Finally, the expected value of IQ was used to model the distribution of observed IQ values IQ i , with error described by the variance τ
Twelve studies provided only summarized data, with no individual measurements of Phe or IQ. For studies that provided only data summaries, we were unable to directly estimate the quantities as specified above. Instead, we employed reported correlation coefficients to obtain inference regarding the relationship of these variables. Inference regarding the linear relationship (slope) between Phe and IQ can be obtained from the correlation coefficient (ρ), using the Fisher transformation. Here, the hyperbolic function can be used to transform the correlation to a normally-distributed random variable:
where r j is the reported Pearson correlation from study j, with a standard error that is solely a function of the corresponding sample size n (for a Spearman correlation, the standard error is the inverse square root of n − 2). This provides a measure of precision for the reported correlations, which in turn becomes a measure of precision for the slope of the relationship between Phe and IQ. The expected value of the slope was obtained in the model by converting ρ using the fundamental relationship:
where S xj and S yj are the reported standard deviations of the Phe levels and IQs, respectively, for study j, the availability of which was an inclusion criteria for the selected studies.
The full model structure is illustrated in Fig. 1. Note the distinction between the influence of studies with group-summarized data and that of studies with individual-level data on the estimate of the relationship between Phe and IQ. Both types of data influence the estimate of the hyperparameters of α 0.
Directed acyclic graph (DAG) showing the meta-analysis model structure, which is used for both the concurrent and historical Phe measurement models. Unfilled circles represent stochastic nodes (RE = random effect), shaded circles represent data, triangles represent deterministic nodes and squares represent factor potentials (arbitrary log-probability terms). The large enclosing square represents the collection of n unique studies in the meta-analysis; the smaller enclosing box represents the distinct groups (i.e. subsets that had distinct covariates) within each study. Different information was contributed depending on whether the study provided group-summarized data (η 1 studies) or individual-level data (η 2 studies), as indicated by the dashed boxes; group-level data provided inference on the slope parameter only, while individual-level data informed both the slope and intercept. The data node I crit is an indicator variable that equals one for studies with Phe measurements in the critical period, and zero otherwise
All stochastic parameters were specified using non-informative prior distributions. For continuous parameters on the real line (e.g., linear model coefficients), a normal distribution with mean zero and precision (inverse-variance) 0.01 was used. For variance parameters, the standard deviation was modeled uniformly on the interval (0, 1000).
In order to evaluate the effect of particular levels of Phe on the likelihood of cognitive impairment, we chose a threshold value of IQ to bound the definition of impairment. We assume that for a standardized measure like IQ, a boundary of one standard deviation below the mean (IQ = 85) was a reasonable choice. This threshold value was used to define indicator variables that were set to one if the value of the predicted IQ was below 85 during the current iteration of the MCMC sampler, and zero otherwise. Hence, for each combination of predictors, the total number of ones divided by the number of MCMC iterations represents a posterior predictive probability of observing IQ <85. This corresponds to the integral of the posterior predictive distribution of IQ up to an 85 score. To illustrate the variation of this probability in response to Phe, this probability was calculated for a range of blood Phe levels from 200 to 3000 μmol L-1 , in increments of 200. This was done for critical period and non-critical period Phe measurement, under both the historical and concurrent measurement models (Fig. 2). To assess the performance of the models for a lower threshold value, we also estimated the probability of IQ lower than 70 (two standard deviations below the population mean), characterizing those with intellectual disabilities.
This model was coded in PyMC version 2.1 (Patil et al 2010), which implements several MCMC algorithms for fitting Bayesian hierarchical models. The model was run for 1 million iterations, with the first 900,000 conservatively discarded as a burn-in interval. The remaining sample was thinned by a factor of 10 to account for autocorrelation, yielding 10,000 samples for inference. Convergence of the chain was checked through visual inspection of the traces of all parameters, and via the (Geweke et al 1992) diagnostic. Posterior predictive checks (Gelman et al 2003) were performed, which compare data simulated from the posterior distribution to the observed data. This exercise showed no substantial lack of fit for any of the studies included in the dataset.
Results
Seventeen unique studies (reported in 21 publications) met our criteria and addressed the relationship between blood Phe levels and IQ (Table 1). Age ranges and IQ levels varied widely across studies. Ten studies were conducted in Europe (Cerone et al 1999; Griffiths et al 2000; Jones et al 1995; Leuzzi et al 1998; Pfaendner et al 2005; Rupp et al 2001; Schmidt et al 1994; Weglage et al 1999, 2000, 2001), six in the United States (Anastasoaie et al 2008; Ris et al 1997; Seashore et al 1985; Viau et al 2011; Wasserstein et al 2006; Welsh et al 1990), and one in Iran (Azadi et al 2009).
The number of participants in individual studies ranged from 10 to 57. The studies included a total of 432 individuals with PKU. Of the studies that reported on disease classification, ten included only participants with classic PKU, and the remainder did not provide the classification or included individuals with less severe PKU. Results are therefore most clearly applicable to individuals with classic PKU.
Participant ages ranged from 2 to 35 years. A majority of studies included primarily participants under age 25 at intake (Anastasoaie et al 2008; Azadi et al 2009; Cerone et al 1999; Griffiths et al 2000; Leuzzi et al 1998; Ris et al 1997; Schmidt et al 1994; Seashore et al 1985; Weglage et al 1999, 2000; Welsh et al 1990; Viau et al 2011), with five studies including only participants under age 15 at intake (Cerone et al 1999; Griffiths et al 2000; Seashore et al 1985; Weglage et al 1999; Welsh et al 1990). Dietary control varied among the studies, with five studies reporting that all participants adhered to a restricted diet (Anastasoaie et al 2008; Azadi et al 2009; Griffiths et al 2000; Wasserstein et al 2006; Welsh et al 1990), seven reporting a mix of dietary control (some participants on and some off a restricted diet) (Jones et al 1995; Leuzzi et al 1998; Pfaendner et al 2005; Ris et al 1997; Rupp et al 2001; Schmidt et al 1994; Viau et al 2011), and three reporting that participants had discontinued a restricted diet (Cerone et al 1999; Seashore et al 1985; Weglage et al 2001). Dietary status was not clearly reported in the remaining studies (Weglage et al 1999, 2000).
IQ scores ranged from 44 to 148 across studies. Ten studies reported concurrent measures of Phe levels (blood Phe measurement within 6 weeks of IQ measurement) (Azadi et al 2009; Cerone et al 1999; Jones et al 1995; Ris et al 1997; Rupp et al 2001; Schmidt et al 1994; Viau et al 2011; Wasserstein et al 2006; Weglage et al 2000; Welsh et al 1990) and nine studies reported historical Phe measurements (blood Phe measurements taken more than 12 months before IQ measurement) (Leuzzi et al 1998; Pfaendner et al 2005; Rupp et al 2001; Seashore et al 1985; Wasserstein et al 2006; Viau et al 2011; Weglage et al 1999, 2001; Welsh et al 1990). Phe measurements were also taken in the critical period (blood Phe measurement before age 6) in nine studies (Anastasoaie et al 2008; Griffiths et al 2000; Pfaendner et al 2005; Seashore et al 1985; Viau et al 2011; Wasserstein et al 2006; Weglage et al 2000, 2001; Welsh et al 1990). The one study that included very young children used developmental quotient as the outcome measurement for the young children (Anastasoaie et al 2008). The scale used to measure IQ also varied widely across (and sometimes within) studies (Table 1), though we assume that this is not an important component of variation or bias.
The mean baseline association of Phe with IQ (μ α ) was estimated to be negative, both in the context of historical and concurrent measurement of Phe (Table 2), with the associated 95 % Bayesian credible interval (BCI) also strongly negative. The BCI is a measure of uncertainty in the estimated value, analogous to the classical confidence interval, but more directly interpretable as the probability that the true value lies within the interval (confidence intervals cannot be interpreted this way). The absolute magnitude of this association was stronger for historical measurements than for concurrent. The estimated additive fixed effect of critical period Phe measurement (α 1) was nominally negative for historical measurement and positive for concurrent measurement, though the 95 % BCI included zero for both parameters. So, under the historical model, the overall median Phe effect is the sum of the mean of the random effect (−0.026) and the critical period effect (−0.01), for an estimate of −0.036. In the concurrent model, the positive median critical period effect on average cancels out the negative Phe effect. Estimates of the model intercepts for both models were quantitatively similar to one another, as was the sampling standard deviation (σ).
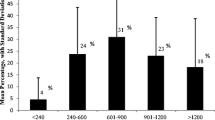
The implications of these estimates on our research question are summarized by the posterior predicted probabilities of low IQ over a range of Phe levels, under each model (Fig. 3). These values are tail probabilities of their respective posterior predictive distributions of IQ, numerically integrated via the MCMC algorithm. Note that because these values are integrals of probability distributions, there is no associated measure of uncertainty, such as a credible interval; we have essentially integrated over the uncertainty. At the lower range of Phe measurement (<400 μmol L-1), all models similarly predicted a low probability of IQ <85, close to the general population value (15 %). The probabilities corresponding to historical measures of blood Phe (two dark red lines in Fig. 3) demonstrate an increasing chance of low IQ with increasing Phe, with a stronger association seen between blood Phe measured during the critical period (solid line) than later (dashed line). In contrast, concurrently-measured Phe is more weakly correlated with the probability of low IQ (two dark blue lines).
Probability of IQ <85 (dark lines) at varying blood Phe levels and Phe measurement times. Solid lines represent critical period Phe measurements and dashed lines represent measurements outside the critical period. In addition, historical measurements are represented by red lines, concurrent measurements by blue. Corresponding lighter lines, using the same line style, represent the probability of IQ <70. The predictive region that extrapolates beyond the range of data provided by the studies in the meta-analysis is shaded gray
Decreasing the threshold value for low IQ to two standard deviations below the population mean similarly showed historical measurements to be more predictive of low IQ than concurrent measurements (lighter lines in Fig. 3). However, conditional on historical measurement, there was a stronger apparent effect of critical period measurement in predicting low IQ. In contrast, concurrent measurements are even more poorly predictive for IQ <70 than for IQ <85, and measurements during the critical period had no effect on the predicted probabilities.
Discussion
Individuals with phenylketonuria, their families and their clinicians update their decisions and treatment regimens based on continual Phe measurements, with little information about the degree to which any course of treatment is providing protection against cognitive impairment. The precise relationship of blood Phe levels to IQ and the timing of the effect have not been fully elucidated, in part because extant studies are small and sample populations in individual studies are sometimes selected to be homogeneous. By combining information from several studies that measured both Phe and IQ in individuals with PKU, we characterized the extant evidence of the relationship between specific blood Phe levels and IQ, the impact of the critical period on cognition and the best timing for Phe and IQ measurement in order to determine these effects. It is well established that high levels of blood Phe are associated with a lower IQ (Waisbren et al 2007) and that dietary control can mitigate the effects of high Phe (Weglage et al 1993; Channon et al 2007; Vilaseca et al 2010). Our meta-analysis provides additional support for continuing dietary control through adolescence and into adulthood, although detailed information about the requisitelevel of control by age group and particularly into older age remains unknown.
Increasing Phe is negatively associated with IQ, with a probability of IQ less than 85 exceeding the population probability (approximately 15%) at blood Phe over 400 μmol L-1 and leveling off at about 80% at 2000 μmol L-1. This finding supports the typical target goal for blood Phe levels in individuals with PKU (under 360 μmol L-1, (National Institutes of Health Consensus Development Panel 2001)). Notably, the negative association between blood Phe and IQ is strongest when Phe is measured at least 1 year prior to IQ testing. A blood Phe level obtained more than 1 year before IQ testing is likely to be a better indicator of how well Phe has been controlled over the long term, compared to concurrent measurements. This relationship lends support to the principle that cognitive effects accumulate over a long time period, and thus concurrent measurements are relatively poor predictors of a cognitive effect. The strongest associations are seen in the group for which historical measurements were taken during the critical period (<6 years old) and associated with later IQ; historical measurements taken after the critical period are more weakly associated with probability of low IQ. This implies that control of blood Phe levels during the critical period is particularly important, but the need for dietary control continues after the age of six. Current clinical practice is to try to maintain tight Phe control even in adulthood, which is supported by this analysis and is consistent with the NIH recommendations of diet for life (Koch et al 2002).
Further, the lack of strong association in measurements taken concurrently during the critical period suggests that effects are unlikely to be observed during this period, either because the IQ test is not stable for young children (less than 5 years old) or because the reduction in IQ takes time to manifest. From a clinical perspective, this provides a basis for being cautious in interpreting measures of cognitive outcomes during the critical period as they relate to blood Phe, and emphasizes the importance of well-controlled Phe levels during the critical period and over time.
We caution that these estimates may be subject to selection bias because they are based on studies that include non-randomly selected individuals from the PKU population. Insurance coverage and access to care for individuals with PKU, especially adults, is non-uniform across states and insurance companies. This likely results in unequal access to medical care, which is the primary means for being recruited into studies. If the included studies exclude individuals outside their health care systems, our estimates of association may be conservative, since they may be more likely to exclude people who are non-adherent to diet. Thus, we anticipate that clinicians can use these results to encourage parents and patients to maintain dietary control even in the absence of immediate, observable effects. We also caution that, despite an evident relationship of decreasing IQ with increased blood Phe measurement, there is no implication of reversibility for this effect; in other words, there is no mechanism for reversing IQ impairment simply by lowering Phe. Finally, it is important to be aware that IQ is a composite measure of many cognitive abilities, rather than an indicator of ability in specific domains. As such, IQ fails to capture specific difficulties in cognition experienced by individuals with PKU. Though the relationship between Phe levels and IQ can be easily interpreted by clinicians, the use of such an index sacrifices potentially valuable information.
This meta-analysis illustrates the utility of a Bayesian hierarchical approach for not only combining information from a set of candidate studies, but also for combining different types of data to estimate parameters of interest. Several papers reported only summarized data for IQ and Phe measurements from their respective studies, which might have otherwise resulted in their exclusion from the meta-analysis, or at best, forced us to fit separate models and combine them post hoc. Our use of a hierarchical model made it possible for both individual and group-summarized data to inform the linear model parameters for the relationship between Phe and IQ (see arrows from critical period and Phe random effect to data models in Fig. 2). This is particularly helpful for rare diseases like PKU, for which the body of available literature is limited, to be able to include as many studies as possible that are selected in the systematic review.
A second advantage of using Bayesian models for meta-analysis is the utility of the posterior predictive distribution. The posterior predictive distribution makes predictions for observable quantities, conditional on the information available from (in this case) the candidate studies in the meta-analysis. The residual uncertainty in the model parameters is integrated into the predictions, allowing us to fully account for all sources of uncertainty across the model. Hence, our estimated probabilities of low IQ are made in light of the limitations of the body of research used to fit our model. We feel that the ability to readily generate predictions based on meta-analytic models increases the value of systematic reviews to clinicians by providing reliable and interpretable quantitative outputs.
The systematic review that accompanied this meta-analysis revealed several research needs that warrant the attention of the PKU community (Lindegren et al 2012). First is the need for a better understanding of how dietary control requirements change with age. This necessitates complete and accurate measurement of Phe and cognition over long time periods, perhaps through a long-term follow-up study or multi-site collaboration. A second research gap relates to determining which measures of executive function are most appropriate for gaging success with day-to-day activities. It is possible that some measures of executive function are more sensitive to changes in Phe than IQ. Third, the effects of mild hyperphenylalaninemia relative to those of classic, mild, and moderate PKU ought to be clarified. Finally, there is a notable lack of consistent methodologies using standardized tools and measures and consistent data collection across centers. As a result, many candidate studies were excluded from the meta-analysis due to incomplete reporting of data or results. In order to improve inference, it is critical that covariates such as familial IQ, socioeconomic status, maternal education, age at initial treatment, and concurrent medications be fully described so they might be used in a more extensive meta-analysis of Phe-IQ associations.
Abbreviations
- BCI:
-
Bayesian credible interval
- E:
-
Expected value
- IQ:
-
Intelligence quotient
- MCMC:
-
Markov chain Monte Carlo
- Phe:
-
Phenylalanine
- PKU:
-
Phenylketonuria
- RE:
-
Random effect
- SD:
-
Standard deviation
References
Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJM, Eisenberg MJ (2008) Statins for secondary prevention in elderly patients. J Am Coll Cardiol 51(1):37–45
Albrecht J, Garbade SF, Burgard P (2009) Neuropsychological speed tests and blood phenylalanine levels in patients with phenylketonuria: a meta-analysis. Neurosci Biobehav Rev 33(3):414–421
Anastasoaie V, Kurzius L, Forbes P, Waisbren S (2008) Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol Genet Metab 95(1–2):17–20
Azadi B, Seddigh A, Tehrani-Doost M, Alaghband-Rad J, Ashrafi MR (2009) Executive dysfunction in treated phenylketonuric patients. Eur Child Adolesc Psychiatry 18(6):360–368
Babapulle M, Joseph L, Bélisle P, Brophy J (2004) A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet
Brooks S, Gelman A, Jones G, Meng XL (2010) Handbook of Markov chain Monte Carlo. Methods and applications. Chapman & Hall/ CRC, Gainesville
Brophy J, Bélisle P (2003) Evidence for use of coronary stents: a hierarchical Bayesian meta-analysis. Ann Intern Med 138:777–786
Brophy J, Joseph L (2001) Î2-blockers in congestive heart failure: a Bayesian meta-analysis. Ann Intern Med 134: 550-560
Cerone R, Schiaffino M, Di Stefano S, Veneselli E (1999) Phenylketonuria: diet for life or not? Acta Paediatr 88:664–6
Channon S, Goodman G, Zlotowitz S, Mockler C, Lee PJ (2007) Effects of dietary management of phenylketonuria on long-term cognitive outcome. Arch Dis Child 92(3):213–218
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Gelman A, Carlin JB, Stern HS, Rubin DB (2003) Bayesian data analysis. In: Statistical Science, 2nd edn. Chapman and Hall/CRC, Gainesville
Geweke J, Berger JO, Dawid AP (1992) Evaluating the accuracy of sampling-based approaches to the calculation of posterior moments. Bayesian Statistics 4
Giovannini M, Verduci E, Salvatici E, Fiori L, Riva E (2007) Phenylketonuria: dietary and therapeutic challenges. J Inherit Metab Dis 30(2):145–152
Griffiths PV, Demellweek C, Fay N, Robinson PH, Davidson DC (2000) Wechsler subscale IQ and subtest profile in early treated phenylketonuria. Arch Dis Child 82(3):209–215
Hegge KA, Horning KK, Peitz GJ, Hegge K (2009) Sapropterin: a new therapeutic agent for phenylketonuria. Ann Pharmacother 43(9):1466–1473
Jones SJ, Turano G, Kriss A, Shawkat F, Kendall B, Thompson AJ (1995) Visual evoked potentials in phenylketonuria: association with brain MRI, dietary state, and IQ. J Neurol Neurosurg Psychiatry 59(3):260–265
Kaizar EE, Greenhouse JB, Seltman H, Kelleher K (2006) Do antidepressants cause suicidality in children? A Bayesian meta-analysis. Clin Trials 3(2):73–90
Koch R, Burton B, Hoganson G, Peterson R, Rhead W, Rouse B, Scott R, Wolff J, Stern AM, Güttler F, Nelson M, de la Cruz F, Coldwell J, Erbe R, Geraghty MT, Shear C, Thomas J, Azen C (2002) Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 25(5):333–346
Leuzzi V, Rinalduzzi S, Chiarotti F, Garzia P, Trasimeni G, Accornero N (1998) Subclinical visual impairment in phenylketonuria. A neurophysiological study (VEP-P) with clinical, biochemical, and neuroradiological (MRI) correlations. J Inherit Metab Dis 21(4):351–364
Lindegren ML, Krishnaswami S, Fonnesbeck C, Reimschisel T, Fisher J, Jackson K, Shields T, Sathe NA, McPheeters ML (2012) Adjuvant treatment for phenylketonuria (PKU): Comparative effectiveness review no. 56. Tech. rep., Agency for Healthcare Research and Quality, Rockville, MD, URL http://www.effectivehealthcare.ahrq.gov/reports/final.cfm
National Institutes of Health Consensus Development Panel (2001) National Institutes of Health Consensus Development conference statement: phenylketonuria: screening and management, 16-18 October 2000. In: Pediatrics, pp 972–982
Patil A, Huard D, Fonnesbeck C (2010) PyMC: Bayesian stochastic modelling in python. J Stat Softw 35(4):1–80
Pfaendner NH, Reuner G, Pietz J, Jost G, Rating D, Magnotta VA, Mohr A, Kress B, Sartor K, Hähnel S (2005) MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol 26(7):1681–1685
Ris MD, Weber AM, Hunt MM, Berry HK, Williams SE, Leslie N (1997) Adult psychosocial outcome in early-treated phenylketonuria. J Inherit Metab Dis 20(4):499–508
Rosenthal R (1994) Parametric measures of effect size. In: Cooper H, Hedges L (eds) The handbook of research synthesis. The handbook of research synthesis. Sage, New York, pp 231–244
Rupp A, Kreis R, Zschocke J, Slotboom J, Boesch C, Rating D, Pietz J (2001) Variability of blood-brain ratios of phenylalanine in typical patients with phenylketonuria. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 21(3):276–284
Schmidt E, Rupp A, Burgard P, Pietz J, Weglage J, de Sonneville L (1994) Sustained attention in adult phenylketonuria: the influence of the concurrent phenylalanine-blood-level. J Clin Exp Neuropsychol 16(5):681–688
Seashore MR, Friedman E, Novelly RA, Bapat V (1985) Loss of intellectual function in children with phenylketonuria after relaxation of dietary phenylalanine restriction. Pediatrics 75(2):226–232
Smith T, Spiegelhalter D (1995) Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med 14:2685–2699
Sutton A, Abrams K (2001) Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 10(4):277–303
Tweedie R, Scott DJ, Biggerstaff BJ, Mengersen KL (1996) Bayesian meta-analysis, with application to studies of ETS and lung cancer. Lung Cancer (Amsterdam, Netherlands) 14(Suppl 1):S171–194
Viau KS, Wengreen HJ, Ernst SL, Cantor NL, Furtado LV, Longo N (2011) Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J Inherit Metab Dis 34(4):963–971
Vilaseca MA, Lambruschini N, Gómez-López L, Gutiérrez A, Fusté E, Gassió R, Artuch R, Campistol J (2010) Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr Hosp: Organo Oficial Sociedad Española Nutrición Parenteral Enteral 25(1):60–66
Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, Levy H (2007) Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab 92(1–2):63–70
Wasserstein MP, Snyderman SE, Sansaricq C, Buchsbaum MS (2006) Cerebral glucose metabolism in adults with early treated classic phenylketonuria. Mol Genet Metab 87(3):272–277
Weglage J, Fünders B, Wilken B, Schubert D, Ullrich K (1993) School performance and intellectual outcome in adolescents with phenylketonuria. Acta Paediatrica (Oslo, Norway: 1992) 82(6–7):582–586
Weglage J, Pietsch M, Denecke J, Sprinz A, Feldmann R, Grenzebach M, Ullrich K (1999) Regression of neuropsychological deficits in early-treated phenylketonurics during adolescence. J Inherit Metab Dis 22(6):693–705
Weglage J, Grenzebach M, Pietsch M, Feldmann R, Linnenbank R, Denecke J, Koch HG (2000) Behavioural and emotional problems in early-treated adolescents with phenylketonuria in comparison with diabetic patients and healthy controls. J Inherit Metab Dis 23(5):487–496
Weglage J, Wiedermann D, Denecke J, Feldmann R, Koch HG, Ullrich K, Harms E, Möller HE (2001) Individual blood-brain barrier phenylalanine transport determines clinical outcome in phenylketonuria. Ann Neurol 50(4):463–467
Welsh MC, Pennington BF, Ozonoff S, Rouse B, McCabe ER (1990) Neuropsychology of early-treated phenylketonuria: specific executive function deficits. Child Dev 61(6):1697–1713
Acknowledgments
The authors would like to acknowledge the contributions of M. Bauer, J. Fisher, K. Jackson, R. Jerome, S. Jones, K. Lee, N. Sathe, T. Shields and Y. Summers, each of whom contributed importantly to the systematic review.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Nenad Blau
Supplementary materials
Supplementary materials, including model source code and data, are available at http://github.com/fonnesbeck/PKUMetaAnalysis.
Rights and permissions
About this article
Cite this article
Fonnesbeck, C.J., McPheeters, M.L., Krishnaswami, S. et al. Estimating the probability of IQ impairment from blood phenylalanine for phenylketonuria patients: a hierarchical meta-analysis. J Inherit Metab Dis 36, 757–766 (2013). https://doi.org/10.1007/s10545-012-9564-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9564-0