Abstract
Background
National coverage of neonatal screening for hyperphenylalaninaemia (HPA) in China is still low and tests to differentiate causes of HPA are not performed in many centres. This study aimed to describe the demographics, geographic distribution, diagnosis, treatment and clinical outcomes of treatment, including intellectual development, in patients with tetrahydrobiopterin (BH4) deficiency in mainland China.
Methods
This was a retrospective, multicentre, chart review in patients with BH4 deficiency across mainland China born 1985–2010.
Results
Two hundred fifty six patients were included; 59.9 % (267/446) of parents were from eastern China. Median (interquartile range) age at diagnosis decreased from 12.0 (5.5, 102.0) months to 2.0 (1.0, 3.5) months in patients born 1985–1999 (n = 28) and 2005–2010 (n = 152), respectively. 6-Pyruvoyl-tetrahydropterin synthase (PTPS) deficiency was the primary cause of BH4 deficiency (96.0 %); four hotspot mutations accounted for 76.6 % of PTS gene mutations; two novel variants in the QDPR gene were identified. Most patients (83.6 %) received treatment with BH4, l-dopa, 5-hydroxytryptophan and/or diet therapy. Target blood Phe concentration was confirmed at 88.9 % of visits; median (Q1, Q3) blood Phe concentration was 106.8 (73.0, 120.0) μmol/L during therapy and 117.0 (67.1, 120.0) μmol/L at last visit. Median (Q1, Q3) WISC IQ score was 80.0 (69.0, 90.0) in 33 patients. DQ scores were within normal range (≥85) for 37/59 (62.7 %) patients. Physical development indicators were within normal ranges. Treatment-related adverse events, reported in 20/256 (7.8 %) patients, were mild-to-moderate in severity.
Conclusion
This study provides valuable information on the current and historical situation of BH4 deficiency in mainland China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A rare form of hyperphenylalaninaemia (HPA) can arise through the deficiency of an essential cofactor, tetrahydrobiopterin (BH4), which leads to decreased metabolism of phenylalanine (Phe) into tyrosine. BH4 deficiency is caused by defects in one of four enzymes responsible for its synthesis and recycling: BH4 is synthesized from guanosine triphosphate (GTP) by GTP cyclohydrolase I (GTPCH), 6-pyruvoyl-tetrahydropterin synthase (PTPS) and sepiapterin reductase, and recycled from pterin-4α-carbinolamine by pterin-4α-carbinolamine dehydratase (PCD) and dihydropteridine reductase (DHPR) (Blau et al 2005). In addition to being a cofactor for phenylalanine hydroxylase, BH4 is also an essential cofactor for tyrosine hydroxylase and tryptophan hydroxylase, enzymes involved in the synthesis of monoamine neurotransmitters (Blau 2010). Thus, BH4 deficiency results in neurotransmitter deficits and patients with BH4 deficiency show signs of progressive neurological impairment when treated with a Phe-restricted diet alone (Wang et al 2006). Differential diagnosis of BH4 deficiency from phenylketonuria (PKU) is essential, and treatment with BH4 and neurotransmitter precursors (5-hydroxytryptophan [5-HTP] and l-dopa) should be initiated as soon as possible to reduce neurological deterioration. Patients with DHPR deficiency also require folinic acid supplements (Shintaku 2002).
Neonatal screening for the detection of HPA has been available since the 1960s (Seymour et al 1997) and was introduced in China in 1981 (Gu et al 2008; Zheng et al 2010). National coverage of neonatal screening in China is gradually expanding and increased from 31 % in 2006 to 40 % in 2007 (Cao et al 2009). According to data obtained during the screening of 18 million newborns from 2000 to 2009, the overall incidence of HPA in China is 1:11,763, with BH4 deficiency accounting for 12.9 % of patients with HPA in Shanghai (Gu et al 2008).
There is geographic diversity in the prevalence of neonatal screening, with screening coverage in 2007 varying from approximately 20 % in western China to more than 80 % in eastern China, and up to 95 % in large cities (Cao et al 2009; Gu and Chen 1999). Increasing screening coverage is therefore a priority. Following identification of HPA at neonatal screening, BH4 deficiency may be differentiated from PKU using BH4 oral loading tests, urine pterin analysis and DHPR activity (Blau et al 1996; Zurfluh et al 2005). Characteristic urinary patterns of pterins and erythrocyte DHPR activity in dried blood spots allow the differentiation of BH4 deficiency subgroups (Shintaku 2002; Zurfluh et al 2005). Urinary pterin analysis, which was introduced in China in 1990 (Ye et al 2002), became routine for patients with HPA in 1999. The BH4 oral loading test and detection of DHPR activity in dried blood spots were introduced in mainland China in 2003, leading to the diagnosis of the first patient with DHPR deficiency in mainland China in 2007 (Ye et al 2008).
The primary objectives of this retrospective study were to describe the demographics, geographic distribution, diagnosis, treatment and clinical outcomes of patients with BH4 deficiency in mainland China.
Methods
Study design
This was a retrospective multicentre chart review of patients with BH4 deficiency born between 1985 and 2010 who had visited nine treatment centres across mainland China. This study, performed from October 2010 to February 2011, was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Participants
Patients at participating treatment centres were eligible if they had been diagnosed with BH4 deficiency through blood Phe concentration, urine pterin analysis and detection of DHPR activity. In some cases, data on DHPR activity, the BH4 loading test and gene mutation analysis were also available. Patients with PTPS deficiency, whose urinary biopterin to pterin ratio (B%) was ≥10 %, required confirmation of BH4 deficiency using gene mutation analysis. Patients were excluded if they or their parents did not give permission to use their data.
Demographics
Demographic data and details of parental geographical heritage were obtained from medical records where available for all patients.
Diagnosis
When available, the following laboratory data were obtained: neonatal screening status, urinary pterin profile, DHPR activity in dried blood spots, blood Phe concentration following a BH4 loading test (20 mg/kg; performed in patients with blood Phe concentration >600 μmol/L at baseline), and blood Phe concentration following a combined Phe and BH4 loading test (Phe 100 mg/kg before BH4 loading test in patients with blood Phe concentration <600 μmol/L at baseline). Baseline was defined as the maximum blood Phe concentration before initiation of treatment. Where possible, results of gene mutation analyses, which were used to confirm BH4 deficiency in patients with an abnormal urinary pterin profile or abnormal DHPR activity, were obtained. Information related to the signs and symptoms of BH4 deficiency was collected, and magnetic resonance imaging (MRI) was performed in some patients.
Treatment and outcomes
Data on BH4 deficiency treatment, including information about diet therapy, BH4 (Schircks Laboratories, Switzerland), l-dopa and 5-HTP supplementation, were obtained. Changes in blood Phe concentration from baseline and percentage rate of Phe control within target concentration during BH4 therapy were assessed.
Intelligence and physical outcomes
Where available, the following information was sought: intelligence quotient (IQ) assessed by Wechsler Intelligence Scale for Children (WISC); physical development status assessed using the infant Gesell developmental quotient (DQ) for children aged under 3 years, chosen because it is widely used for assessing early child development in China (Cui et al 2001; Perera et al 2008; Zhu et al 2005); indicators of physical development at last visit, including height, weight and head circumference; and education and school grades following treatment for BH4 deficiency. Data were analysed by dividing patients into two subgroups: those with DQ scores <85 and those with scores ≥85, the latter of which were considered within the normal range.
Statistical analyses
As this was a retrospective study, there was no predetermined sample size. Data were pooled across centres and summarized using descriptive statistics. No adjustments for multiplicity were performed. Duplicate patient records were identified using name, sex and birth date. Missing data were handled as follows: if age at diagnosis was missing, age at first l-dopa administration was used; if baseline blood Phe concentration was missing, Phe concentration at confirmatory testing was used. For change in Phe concentration during the BH4 loading test, data were pooled for patients receiving combined BH4 and Phe loading tests and those receiving BH4-only loading tests; for patients receiving the combined test, baseline Phe concentration was defined as the concentration at the start of the BH4 loading test (3 h after Phe loading). Paired t-tests and Wilcoxon signed-rank tests were performed to compare baseline and endpoint data; comparisons between groups were performed using a two-sample t-test or Wilcoxon rank-sum test. Qualitative data were analysed using the chi-squared test. All analyses were performed using SAS Software version 9.1 or later (SAS Institute Inc, NC, USA) and significance was set at 5 %. All values are given as median (interquartile range [Q1, Q3]) unless stated otherwise.
Results
Study population
Records from a total of 294 patients were screened and 256 patients were included in the study; 38 patients were excluded: 37 owing to duplicate records and one owing to incomplete diagnostic information.
Demographics
The median (Q1, Q3) patient age was 39.0 (16.0, 76.0) months (range 2–241 months); 59.8 % (153/256) were male and the mean (SD) birth weight was 3.1 (0.5) kg (Table 1). Based on geography relative to the Yangtze River, 47.8 % (213/446) of parents were from northern China and 52.0 % (232/446) were from southern China. According to a national geographic division, 59.9 % (267/446) of parents were from eastern China, 34.1 % (152/446) from central China and 5.8 % (26/446) from western China. Among 114 patients who provided information about family history, 28 (24.6 %) had a familial history of BH4 deficiency (Table 1).
Disease characteristics and diagnosis
Neonatal screening for HPA was performed in 81.8 % (207/253) of patients (Table 1). In total, clinical symptoms of BH4 deficiency prior to diagnosis were reported for 50.4 % (129/256) of patients, with first presentation of symptoms at a median (Q1, Q3) age of 4.0 (3.0, 7.0) months (n = 110); 73.6 % (92/125) of patients for whom detailed information regarding symptoms were available presented with typical symptoms of BH4 deficiency (including hypotonia, apathy, drowsiness, low response, drooling and retardation of motor development), 70.4 % (88/125) with systemic symptoms of HPA (including yellow hair, white skin, characteristic body odour, convulsions, mental retardation, occasional tremors and microcephaly), and 4.0 % (5/125) with other symptoms (including eczema and vomiting). Information about symptoms was missing for four patients and not all patients presented with the same symptoms. Neonatal screening had not been performed in 34.9 % (45/129) of patients who displayed symptoms of BH4 deficiency prior to diagnosis; among patients with symptoms who underwent neonatal screening, 70.2 % (59/84) did not receive prompt diagnosis (before age 3 months) of BH4 deficiency. Patients who underwent neonatal screening were diagnosed at an earlier age (median [Q1, Q3] age 2.0 [1.0, 5.0] months, n = 200) than those who did not undergo neonatal screening (16.0 [7.0, 49.0] months, n = 45). Patients with BH4 deficiency had a median (Q1, Q3) blood Phe concentration of 495 (283.5, 897.0) μmol/L at neonatal screening and 950.1 (480.0, 1200.0) μmol/L at confirmatory testing.
Overall, the median (Q1, Q3) age at diagnosis was 3.0 (1.5, 9.0) months (n = 248) and decreased from 12.0 (5.5, 102.0) months in patients born between 1985 and 1999 (n = 28) to 5.0 (2.0, 17.0) months in patients born between 2000 and 2004 (n = 66), and to 2.0 (1.0, 3.5) months in those born between 2005 and 2010 (n = 152).
Urinary pterin analysis and DHPR activity
For 250 patients diagnosed with PTPS, DHPR and GTPCH deficiency, urinary pterin analysis and DHPR activity data were available for 242 and 114 patients, respectively; eight patients were diagnosed by gene mutation analysis. In 96.0 % (240/250) of patients, BH4 deficiency was due to PTPS defects, while 2.4 % (6/250) of patients had DHPR deficiency, and 1.6 % (4/250) had autosomal dominant GTPCH deficiency (dopa-responsive dystonia; DRD) (Table 2). Mean biopterin and biopterin to pterin ratio (B%) were markedly out of the normal range in patients with PTPS deficiency, compared with patients with DHPR and GTPCH deficiency, respectively (Table 2). Among 224 patients with PTPS deficiency with documented B% and age at diagnosis, B% was <3 % in 159 (71 %), <5 % in 191 (85.3 %), 5–10 % in 31 (13.8 %), and >10 % in 2 (0.9 %) patients. Patients with B% >10 % underwent genotyping for diagnosis. Mean DHPR activity was close to normal in patients with PTPS deficiency and GTPCH deficiency, and decreased to about 6–10 % of normal levels in patients with DHPR deficiency (Table 2).
BH4 loading test
BH4 loading tests were performed in 181 patients with PTPS deficiency (including 96 patients receiving a combined Phe and BH4 loading test) and in five patients with DHPR deficiency (including two patients receiving a combined Phe and BH4 loading test). Mean blood Phe concentration decreased significantly following BH4 loading in patients with both PTPS deficiency and DHPR deficiency (Fig. 1), particularly in patients with PTPS deficiency. Target blood Phe concentration (≤120 μmol/L) was reached by 97.4 % (149/153) of patients with PTPS deficiency within 6 h and by 98.8 % (162/164) within 24 h. In contrast, target blood Phe concentration was achieved in 20.0 % (1/5) of patients with DHPR deficiency within 6 h of BH4 loading and 80.0 % (4/5) of patients failed to achieve the target within 24 h.
Change in blood Phe concentration during 24-hour BH4 loading test. Data were pooled for patients receiving combined BH4 and Phe loading tests and those receiving BH4-only loading tests; baseline Phe concentration (0 h) includes data for 3 h after Phe loading for 98 patients receiving the combined loading test. PTPS deficiency: an=169, bn=180, cn=153, dn=175, en=164. Compared with baseline (0 hours): *p<0.05; **p<0.01; †p<0.001 BH4, tetrahydrobiopterin; DHPR, dihydropteridine reductase; Phe, phenylalanine; PTPS, 6-pyruvoyl-tetrahydropterin synthase; SD, standard deviation
Time taken to reach target Phe concentration in patients with PTPS deficiency was dependent on blood Phe concentration immediately prior to BH4 loading. Patients with baseline blood Phe concentrations of <360 μmol/L (6/181; 3.3 %) took a mean (SD) of 3.0 (1.1) hours to reach the target concentration, compared with 4.4 (2.4) hours for patients with baseline concentrations of 360–1,200 μmol/L (118/181; 65.2 %) and 4.7 (1.3) hours for patients with baseline concentrations of ≥1,200 μmol/L (57/181; 31.5 %).
Gene mutation analysis
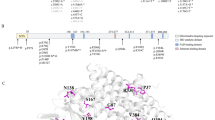
Gene mutation analysis was performed in 143 patients from 141 families: 136 patients with 6-pyruvoyl-tetrahydropterin synthase (PTS) gene mutations (two patients from the same family), four patients with quinoid dihydropteridine reductase (QDPR) gene mutations, and three patients with guanosine triphosphate cyclohydrolase (GCH1) gene mutations (two patients from the same family). In total, 270 gene mutations were detected in 280 alleles (a mutation detection rate of 96.4 %; Table 3). Thirty-nine different mutations were observed: 33 in the PTS gene, five in the QDPR gene, and one in the GCH1 gene. Four hotspot mutations accounted for 76.6 % of PTS gene mutations: P87S (259C>T) (42.9 %), D96N (286G>A) (13.4 %), N52S (155A>G) (11.5 %), and IVS1-291A>G (splicing defect; 8.8 %). All mutations identified in the PTS gene have been reported before. Only two novel variants, 451G>A (G151R) and IVS6-3delC (splicing defect) in the QDPR gene were identified.
Cerebral MRI scans
Fifty-three patients underwent cerebral MRI scanning following diagnosis of BH4 deficiency at a median (Q1, Q3) age of 7.0 (4.0, 18.0) months; white matter signal abnormalities in T2-weighted imaging were detected in 43.4 % (23/53) of patients.
Treatment and adverse events
In total, 214/256 (83.6 %) patients received treatment with BH4, l-dopa, 5-HTP and/or diet therapy; 42/256 (16.4 %) patients (or their parents/caregivers) refused treatment. The median (Q1, Q3) starting age for BH4 therapy decreased from 87.0 (28.0, 156.0) months in children born 1985–1999 (n = 11), to 25.0 (15.0, 37.0) months in those born 2000–2004 (n = 14), and to 2.0 (1.0, 15.0) months in those born 2005–2010 (n = 35). A total of 69/194 (35.6 %) patients received both BH4 and diet therapy, which in some cases was due to an initial misdiagnosis of PKU and in others unaffordability or unavailability of BH4. The median minimal starting dose of BH4 was 1 mg/kg/day for patients with PTPS deficiency, adjusted according to Phe concentrations to achieve the target Phe concentration for the corresponding age. The median (Q1, Q3) starting age for l-dopa and 5-HTP therapy, respectively, decreased from 114.0 (49.0, 156.0; n = 10) and 117 (35.5, 156.0; n = 8) months in children born 1985–1999, to 21.0 (14.5, 34.0; n = 12) and 19.0 (15.0, 32.0; n = 13) months in those born 2000–2004, and to 2.0 (1.0, 15.0; n = 36) and 2.0 (1.0, 14.0; n = 34) months in those born 2005–2010. The median minimal starting doses of l-dopa (25 % carbidopa) or 5-HTP were also 1 mg/kg/day, with increments of 1 mg/kg every 5–7 days as required to achieve target concentrations for the corresponding age and adjusted according to clinical symptoms and blood prolactin concentrations. Patients with DHPR deficiency received a median minimal BH4 starting dose of 10 mg/kg/day, or a low Phe diet, folinic acid supplementation of 15 mg/day, and median maximal doses of 5.0 mg/kg/day of 5-HTP and 7.0 mg/kg/day of l-dopa. Patients with GTPCH-deficient DRD received 1–6 mg/kg/day of l-dopa only. In all patients with BH4 deficiency, mean (SD) minimum and maximum BH4 doses were 1.1 (0.8) and 3.1 (6.4) mg/kg/day, respectively; mean (SD) minimum and maximum l-dopa doses were 2.1 (4.0) and 8.9 (8.2) mg/kg/day, respectively; and mean (SD) minimum and maximum 5-HTP doses were 1.8 (3.2) and 5.6 (4.9) mg/kg/day, respectively.
Twenty treatment-related adverse events (AEs) were reported in 20/256 (7.8 %) patients. The most common AEs were diarrhoea (n = 10), the ‘on-off’ phenomenon (n = 5) and rashes (n = 2). Nausea and vomiting, headache and tics were each reported in one patient. Two AEs were considered probably related to BH4 treatment in the investigator’s judgement: in the first instance, mild diarrhoea from which the patient completely recovered following adequate treatment; and in the second case, mild headache which resolved spontaneously. Other AEs were judged to be related to l-dopa or 5-HTP. All AEs were of mild or moderate intensity and no severe AE was reported.
Clinical outcomes
Most patients (194/256; 75.8 %) received BH4 therapy. A significant decrease from baseline in median blood Phe concentration was observed at last visit (p < 0.0001; Table 4). During BH4 treatment, patients had a median (Q1, Q3) blood Phe concentration of 106.8 (73.0, 120.0) μmol/L, and blood Phe concentration was within the target range (<120 μmol/L) at 88.9 % of visits.
Median (Q1, Q3) IQ assessment on the WISC was 80.0 (69.0, 90.0) (range 36.3–112) in 33 patients. The median (Q1, Q3) age at which treatment was started was significantly (p = 0.02) younger in patients with an IQ above 70 (2 [1, 4] months) than in patients with an IQ below 70 (6 [5, 10] months]). DQ was available for 23.0 % (59/256) of patients. The majority of patients (37/59; 62.7 %) had DQ scores within the normal range (≥85) and 74.4 % (29/39) of patients received good or average school grades (Table 5). In multivariate analyses, DQ was significantly negatively correlated with age at treatment initiation (n = 44; p = 0.0009); however, no correlations were found between DQ and mean blood Phe concentration, proportion of visits with Phe concentration within target range for Phe concentration, and average BH4 or l-dopa dosage. The median (Q1, Q3) age at last visit was 42 (16.5, 77.0) months. Indicators of physical development at last visit, including height, weight and head circumference, were within normal ranges for healthy Chinese children of the same age and sex (P3 ∼ P97) (Li et al 2009).
Among the 256 patients included in this study, 17 patients (6.6 %; age range 2 months–5 years) were deceased. The causes of death were worsening disease or complications of BH4 deficiency (n = 10, including eight untreated patients and two who did not receive prompt or adequate treatment), accidental death (n = 2), serious infectious disease (n = 2) and unknown (n = 3). None of the deaths was considered to be related to treatment.
Discussion
The aim of this retrospective chart review was to investigate the demographics, geographic distribution, diagnosis and treatment of BH4 deficiency in China in patients born between 1985 and 2010 following the expansion of national neonatal screening. To our knowledge, this study represents the first attempt to summarize the disease characteristics by collecting data about the disease on a national scale.
Regarding the geographical distribution of BH4 deficiency, the parents of most patients were from eastern China. However, this apparent higher incidence of BH4 deficiency in eastern regions of China is likely to reflect the diverse coverage of neonatal screening among the different regions and limited access outside large cities in China (Cao et al 2009). Neonatal screening and medical technology for the detection of HPA have advanced more rapidly in eastern than western China. However, differential diagnosis from PKU is not a routine requirement in most average-sized or small cities and towns in China.
Most patients (81.8 %) in this study underwent neonatal screening. The median age at diagnosis decreased from 12 months in children born during 1985–1999 to 5 months in those born during 2000–2004 and to 2 months in those born after 2005, suggesting that diagnosis of BH4 deficiency occurred at an earlier age as the national screening rate and awareness of BH4 deficiency increased. However, the median age of diagnosis of BH4 deficiency in patients born after 2005 was higher in China than in many developed countries, where BH4 loading tests are commonly performed at a median age of 3 weeks (Bernegger and Blau 2002). For patients with clinical symptoms at first visit, approximately 35 % of patients had not received neonatal screening. About 70 % did not receive prompt differential diagnosis from PKU after HPA was identified at neonatal screening and displayed progressive neurological dysfunction associated with untreated BH4 deficiency. Neurological symptoms of BH4 deficiency may not become apparent during the neonatal period and are most commonly observed at approximately 4 months of age (Blau et al 1996), consistent with the median age of symptom onset in patients who presented with symptoms before diagnosis in this study. The results emphasize that neonatal screening procedures require standardization in China so that every patient with HPA undergoes further differential diagnosis, including the BH4 loading test, urinary pterin analysis and DHPR activity, to identify BH4 deficiency (Blau et al 2005).
Most patients (96.0 %) in this study had PTPS deficiency, while only 2.4 % had DHPR deficiency. Autosomal dominant GTPCH deficiency (DRD) was present in 1.6 % of patients and no patients with autosomal recessive GTPCH deficiency were reported. Internationally, PTPS deficiency is the predominant subgroup of BH4 deficiency (approximately 59 %), followed by DHPR deficiency (approximately 32 %) (Blau and Thony 2012). Thus, the incidence of PTPS deficiency was markedly greater and the incidence of DHPR deficiency was markedly lower in China than internationally. However, because DHPR activity determination was not introduced in China until 2003, it is possible that patients with DHPR deficiency may have been missed before 2003. GTPCH and PCD deficiencies are relatively uncommon, each accounting for approximately 4 % of cases internationally (Blau et al 1996). Urinary pterin profiles were available for most of the patients in this study. Mean biopterin and B% were markedly below the normal range in patients with PTPS deficiency. Approximately 14 % of patients had a borderline B% of 5–10 %, and in such cases further differential diagnosis using the BH4 loading test is required. B% alone, however, is not a reliable indicator of BH4 deficiency and may be affected by the inflammatory response (Murr et al 2002). For patients with DHPR deficiency, urinary pterin profiles are not reliable tests and DHPR activity determination must be performed to confirm diagnosis. Thus, overall, urinary pterin analysis, DHPR activity and the BH4 loading test provided reliable detection of BH4 deficiency. Gene mutation analysis can make further diagnoses at the gene level.
The mutation detection rate in this study was 96.4 %. Gene mutation analysis revealed 39 mutations: 33 in the PTS gene, five in the QDPR gene and one in the GCH1 gene. Four hot spot mutations identified in the PTS gene included P87S, D96N, N52S and IVS1-291A>G. The three missense gene mutations P87S, N52S and D96N are associated with approximately 75–80 % of PTPS mutations in the Chinese population (Liu et al 1998, 2001). P87S, N52S and D96N have also been identified as the most common PTPS mutations in East Asian populations (Chiu et al 2012). The P87S mutation is evenly distributed across northern and southern Chinese populations, while N52S occurs predominantly in the southern Chinese population and D96N occurs predominantly in the northern Chinese population (Liu et al 1998, 2001). While most patients in this study were born from parents from eastern China, the proportions of P87S, N52S and D96N gene mutations identified were similar to those reported in the literature (Liu et al 1998, 2001). IVS1-291A>G may be an additional hotspot mutation in the PTS gene of Chinese patients with PTPS deficiency. Furthermore, all mutations in the PTS gene have been previously reported (Chiu et al 2012) and only two novel variants, G151R and IVS6-3delC, in the QDPR gene were identified.
While most patients (84 %) with BH4 deficiency in this study received combination therapy with BH4, l-dopa and 5-HTP, treatment with BH4 and 5-HTP was most likely delayed following the diagnosis of BH4 deficiency because of unavailability of treatment or high treatment costs in mainland China. Furthermore, the absence of a patient support programme or reimbursement system in China precludes the treatment of BH4 deficiency for many patients. Data collected from 128 patients revealed that during BH4 therapy, blood Phe concentrations were within the target concentration range at 88.9 % of visits, supporting the findings of previous studies on the effectiveness of unregistered BH4 therapy (Kitagawa et al 1990; Schaub et al 1978) and registered BH4 therapy in patients with HPA due to BH4 deficiency (Wasserstein et al 2008). Low daily doses (2–5 mg/kg) were sufficient to maintain blood Phe within normal levels in patients with PTPS deficiency. However, for patients with DHPR deficiency, there were insufficient data to support the efficacy and safety of BH4. Supplementation with BH4 in patients with DHPR deficiency has been associated with accumulation of 7,8-dihydrobiopterin, which may have a negative effect on nitric oxide synthase and aromatic acid hydroxylases (Blau 2010). A low Phe diet is therefore recommended for patients with DHPR deficiency. The overall incidence of AEs in patients receiving treatment for BH4 deficiency was low. All AEs were mild or moderate in severity and most were related to l-dopa or 5-HTP therapy. Thus, treatment with BH4, either alone or in combination, was well tolerated. Data relating to patient deaths reported in this retrospective study serve to demonstrate that BH4 deficiency is a lethal disease if left untreated.
Multivariate analysis revealed that DQ was significantly negatively correlated with age at treatment initiation; however, it must be noted that only 44 patients were included in the multivariate analysis and, hence, these results should be interpreted with caution.
The results of this study should be considered within the context of the study limitations. The accuracy and availability of medical records used for data collection must be taken into account and it is difficult to control for bias and confounders in a retrospective study (Hess 2004). Other limitations of retrospective studies include loss to follow-up, missing data, variations in treatment and in outcomes assessment, and non-standardized methods of assessments by multiple individuals.
Great progress has been made in the diagnosis and treatment of BH4 deficiency with the introduction of neonatal screening in China. However, the screening rate is still low and screening is not universally available (Yang et al 2011; Zhan et al 2009). Furthermore, there is a lack of patient support programmes and a reimbursement system. However, the expansion of neonatal screening in China has allowed early detection and treatment of BH4 deficiency and was associated with a reduction in the incidence of neurodevelopmental impairments.
References
Bernegger C, Blau N (2002) High frequency of tetrahydrobiopterin-responsiveness among hyperphenylalaninemias: a study of 1919 patients observed from 1988 to 2002. Mol Genet Metab 77:304–313
Blau N (2010) Phenylketonuria and BH4 deficiencies. In: Blau N, Burton BK, Thöny B, van Spronsen FJ, Waisbren S (eds) UNI-MED Verlag AG, Bremen, Germany
Blau N, Barnes I, Dhondt JL (1996) International database of tetrahydrobiopterin deficiencies. J Inherit Metab Dis 19:8–14
Blau N, Bonafe L, Blaskovics M (2005) Disorders of phenylalanine and tetrahydrobiopterin metabolism. In: Blau N, Duran M, Blaskovics M, Gibson K (eds) Physician’s Guide to the Laboratory Diagnosis of Metabolic Disease. Chapman & Hall, London, pp 89–106
Blau N, Thony B (2012) BIOMDB: Patients with BH4 deficiency. http://www.bh4.org/BH4_databases_biomdb4.asp.
Cao Y, Yuan P, Wang YP, Mao M, Zhu J (2009) The profile of newborn screening coverage in China. J Med Screen 16:163–166
Chiu YH, Chang YC, Chang YH et al (2012) Mutation spectrum of and founder effects affecting the PTS gene in East Asian populations. J Hum Genet 57:145–152
Cui H, Hou J, Ma G (2001) Influences of rearing style on the intellectual development of infants. Wei Sheng Yan Jiu 30:362–364
Gu X, Wang Z, Ye J, Han L, Qiu W (2008) Newborn screening in China: phenylketonuria, congenital hypothyroidism and expanded screening. Ann Acad Med Singap 37:107–114
Gu XF, Chen RG (1999) Current status of neonatal screening in China. J Med Screen 6:186–187
Hess DR (2004) Retrospective studies and chart reviews. Respir Care 49:1171–1174
Kitagawa T, Yamato T, Arashima S et al (1990) Clinical results of using sapropterin hydrochloride (R-tetrahydrobiopterin) for atypical hyperphenylalaninemia. Japan J Pediatr Med 22:1737–1750
Li H, Ji CY, Zong XN, Zhang YQ (2009) Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi 47:487–492
Liu TT, Chiang SH, Wu SJ, Hsiao KJ (2001) Tetrahydrobiopterin-deficient hyperphenylalaninemia in the Chinese. Clin Chim Acta 313:157–169
Liu TT, Hsiao KJ, Lu SF et al (1998) Mutation analysis of the 6-pyruvoyl-tetrahydropterin synthase gene in Chinese hyperphenylalaninemia caused by tetrahydrobiopterin synthesis deficiency. Hum Mutat 11:76–83
Murr C, Widner B, Wirleitner B, Fuchs D (2002) Neopterin as a marker for immune system activation. Curr Drug Metab 3:175–187
Perera F, Li TY, Zhou ZJ et al (2008) Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ Health Perspect 116:1396–1400
Schaub J, Daumling S, Curtius HC et al (1978) Tetrahydrobiopterin therapy of atypical phenylketonuria due to defective dihydrobiopterin biosynthesis. Arch Dis Child 53:674–676
Seymour CA, Thomason MJ, Chalmers RA et al (1997) Newborn screening for inborn errors of metabolism: a systematic review. Health Technol Assess 1:1–95
Shintaku H (2002) Disorders of tetrahydrobiopterin metabolism and their treatment. Curr Drug Metab 3:123–131
Wang L, Yu WM, He C et al (2006) Long-term outcome and neuroradiological findings of 31 patients with 6-pyruvoyltetrahydropterin synthase deficiency. J Inherit Metab Dis 29:127–134
Wasserstein M, Burton B, Cederbaum S et al (2008) Interim results of a Phase II, multicenter, open-label study of sapropterin dihydrochloride in subjects with hyperphenylalaninemia related to primary BH4 deficiency. Abstract presented at the Annual Meeting of the American Society for Human Genetics (ASHG), 2008, Philadelphia, Pennsylvania, USA; abstract 774/T
Yang L, Mao H, Yang R (2011) Delays in referral, and parents refusing treatment for children with PKU. J Med Screen 18:214
Ye J, Liu X, Ma X et al (2002) Screening for tetrahydrobiopterin deficiency among hyperphenylalaninemia patients in Southern China. Chin Med J (Engl) 115:217–221
Ye J, Qiu WJ, Han LS et al (2008) Diagnosis, treatment and gene mutation analysis of the first case with dihydropteridine reductase deficiency in the mainland of China. Zhonghua Er Ke Za Zhi 46:281–285
Zhan JY, Qin YF, Zhao ZY (2009) Neonatal screening for congenital hypothyroidism and phenylketonuria in China. World J Pediatr 5:136–139
Zheng S, Song M, Wu L et al (2010) China: public health genomics. Public Health Genomics 13:269–275
Zhu H, Zhao ZY, Jiang YJ et al (2005) Multifactorial analysis of effects of mothers’ autoimmune thyroid disease on their infants’ intellectual development. Zhonghua Er Ke Za Zhi 43:340–344
Zurfluh MR, Giovannini M, Fiori L et al (2005) Screening for tetrahydrobiopterin deficiencies using dried blood spots on filter paper. Mol Genet Metab 86(Suppl 1):S96–S103
Acknowledgements
The authors thank Prof. Ming Shen (China-Japan Friendship Hospital) for her assistance with the chart review, data collection and patient follow-up; Dr Qiang Gu, Dr Yupeng Liu and Dr Qiao Wang (Department of Pediatrics, Peking University First Hospital), Dr Xuelian Zhou and Dr Fan Tong (The Children’s Hospital, Zhejiang University School of Medicine), Dr Bei Li, Dr Weifeng Cao, Dr Qianyu Chen, Dr Jia Wu, Dr Xiang Jiang (Women and Children Medical Center of Guangzhou) for their assistance with data collection and patient follow-up; Dr Liqin Zhang (Qingdao Women and Children’s Medical Health Centre, Qingdao, Shandong Province), Dr Zhuoying Li (Maternal and Child Health Hospital, Hei Long Jiang Province), Dr Yijun Sun and Dr Yun Sun (Nanjing Maternal and Child Care Hospital, Nanjing, Jiansu Province) for their assistance with data collection; Prof. Kwang-Jen Hsiao and Dr Tze-Tze Liu (National Yang-Ming University, Taipei, Taiwan) for urinary pterin and gene mutation analyses for some patients enrolled in the study; and Prof. Haruo Shintaku (Osaka City University Graduate School of Medicine, Osaka, Japan) for support with DHPR analyses. The authors also acknowledge Alyson Bexfield, Jane Davies and Katie Lay of Caudex Medical, Oxford, UK, supported by Merck Serono S.A. – Geneva, Switzerland (a branch of Merck Serono S.A., Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany), for assistance in preparing the initial draft of the manuscript, collating the comments of authors and other named contributors, and assembling tables and figures. This study was supported by Merck Serono Co., Ltd, Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. The study was also supported by the National Key Technology R&D Programme (No. 2006BAI05A05 and No. 2006BAI05A07), the National High Technology Research and Development Programme (“863”Program; No. 2007AA02Z447), and Shanghai Jiao Tong University School of Medicine Foundation Technology Programme (No. 06XJ21024).
Conflict of interest
This study was supported by funding from Merck Serono Co., Ltd, Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. Support for manuscript development was provided by Merck Serono S.A. – Geneva, Switzerland (a branch of Merck Serono S.A., Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany). The authors confirm independence from the sponsors; the content of the article has not been influenced by Merck Serono S.A. – Geneva, Switzerland. Jun Ye, Yanling Yang, Weimin Yu, Hui Zou, Jianhui Jiang, Rulai Yang and Xuefan Gu declare no conflicts of interest. Sunny Shang was an employee of Merck Serono China (an affiliate of Merck KGaA, Darmstadt, Germany) at the time the paper was written.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Georg Hoffmann
Details of funding
This study was supported by funding from Merck Serono Co., Ltd, Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. Support for manuscript development was provided by Merck Serono S.A. — Geneva (a branch of Merck Serono S.A., Coinsins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany). The authors confirm independence from the sponsors; the content of the article has not been influenced by Merck Serono S.A. — Geneva.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
Individual patient data for all patients in the study. Typical symptoms of BH4 deficiency were defined as hypotonia, apathy, drowsiness, low response, drooling and retardation of motor development. Systemic symptoms of HPA were defined as yellow hair, white skin, characteristic body odour, convulsions, mental retardation, occasional tremors, hands and feet jitter, microcephaly and microcephaly symptoms. AE, adverse event; B%, biopterin to pterin ratio; BH4, tetrahydrobiopterin; DHPR, dihydropteridine reductase; DHPRD, dihydropteridine reductase deficiency; DQ, developmental quotient; GTPCH, guanosine triphosphate cyclohydrolase I deficiency; HPA, hyperphenylalaninaemia; 5-HTP, 5-hydroxytryptophan; IQ, intelligence quotient; Phe, phenylalanine; PTPSD, 6-pyruvoyl-tetrahydropterin synthase deficiency (XLSX 58 kb)
Rights and permissions
About this article
Cite this article
Ye, J., Yang, Y., Yu, W. et al. Demographics, diagnosis and treatment of 256 patients with tetrahydrobiopterin deficiency in mainland China: results of a retrospective, multicentre study. J Inherit Metab Dis 36, 893–901 (2013). https://doi.org/10.1007/s10545-012-9550-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9550-6