Abstract
Bio-actuated micro-pumps do not need any external power source and pose no risk of electrical or heat shock for the biological materials in lab-on-chip systems. Several different designs of bio-actuated micro-pumps based on the use of the contractile force of cultured cardiomyocites have been proposed earlier. Here we present a novel type of a bio-actuated micro-pump representing a microfluidic channel with a contractile wall. The flow inside the channel is generated by the peristaltic movement of its wall caused by the propagation of an excitation-contraction wave along the channels surface. The directional flow generated by the pump was demonstrated by tracking of polystyrene microspheres, moving in the direction of the propagation of the excitation-contraction wave with an average velocity of 6–8 μm/min. The suggested design of a micro-pump allows the control of pumping direction, which might be useful for targeted delivery of fluids and substances in lab-on-chip systems. Prospects of future development and implementation of this kind of bio-actuated peristaltic pumps are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Creation of bio-actuated microdevices is a relatively new and rapidly developing branch of microfabrication (Ricotti and Menciassi 2012; Kamm and Bashir 2013; Duffy and Feinberg 2014). This type of devices not only serves as an inspiring example of the possibility of application of the top-down approach for biological tissues engineering (Nawroth et al. 2012; Park et al. 2016) but also have a variety of potential applications, ranging from the research of fundamental mechanisms of biological motility (Williams et al. 2014; Park et al. 2016) to targeted drug delivery (Carlsen and Sitti 2014; Felfoul et al. 2016) and drug testing in organ-on-a-chip models (Grosberg et al. 2012; Agarwal et al. 2013; Gossmann et al. 2016).
Another emerging area of research in this field is the creation of bio-actuated micro-pumps (Pilarek et al. 2011). Recently several designs of such pumping devices have been suggested (Tanaka et al. 2006; Park et al. 2007; Tanaka et al. 2007, 2011). All of them have a common feature: the flow of the liquid in them is created by a contractile force of cardiomyocytes deflecting thin PDMS membranes. Although the pumping capacity of this sort of micro-pumps is much less then the capacity of their electromechanical counterparts (Laser and Santiago 2004), nevertheless their further development seems to be quite promising. Taking into account their high biocompatibility and absence of the need for any external power source (except from the glucose dissolved in the culture medium), it seems that this kind of cell-based pumps might find their future application in the creation of complex bio-hybrid structures and organ-on-a-chip systems (Uzel et al. 2014; Zhang et al. 2015).
In the present work, we have developed and tested a new type of bio-actuated micro-pump consisting of a microfluidic channel with a thin flexible PDMS wall seeded with cardiomyocytes. We demonstrated that peristaltic motion of the channels wall creates a flow of the liquid inside the channel. A distinctive feature of such a bio-actuated pump is the absence of large-scale contractile parts present in all its previously designed analogues. This makes it much more compact and creates unique possibilities for its incorporation inside artificial tissue-engineered structures.
2 Materials and methods
2.1 Materials and reagents
Collagenase type II, penicillin/streptomycin, L-glutamine, Hank’s Balanced Salt Solution (HBSS) media, Dulbecco’s Modified Eagle’s Medium (DMEM), Leibovitz’s L15 medium, Phosphate Buffered Saline (PBS), Fetal Bovine Serum (FBS) and Trypan Blue Solution were purchased from Gibco, Thermo Fisher Scientific Inc. (USA). Human Plasma Fibronectin was purchased from IMTEK, Russian Cardiology Research and Production Complex (Russia). PDMS Sylgard 184 and Sylgard 527 kits were purchased from Dow Corning (USA). Lidocaine, norepinephrine and Tyrode’s salt were purchased from Sigma-Aldrich (USA). Cover glass slips (22 × 22 mm) and culture flasks were purchased from Corning (USA). Petri dishes were purchased from Greiner (Austria).
2.2 Fabrication of PDMS scaffolds
A blend of two types of PDMS, Sylgard 527 and Sylgard 184, was used to create thin polymer films with increased flexibility (Palchesko et al. 2012). Sylgard 184 PDMS was prepared according to manufacturer’s instructions by mixing10 parts base to 1 part of curing agent for 5 min followed by defoaming for 15 min at mild vacuum. Sylgard 527 was prepared by mixing equal weights of part A and part B using the same mixing and defoaming cycle. Finally, a PDMS mixture with 1:5 mass ratio of Sylgard 184 and Sylgard 527 was prepared, and then underwent an additional mixing and defoaming cycle.
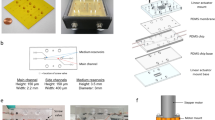
Sugar threads were manually pulled out from melted sugar (Solo Ds, Russia). With the aid of optical microscope, threads with the diameter from 150 to 300 μm were selected and attached to preheated glass cover slips of 22 × 22 mm (Fig. 1a). After cooling at a room temperature, a sugar thread became tightly glued to the surface of the cover slip forming a spacer for future micro-channel fabrication.
Step-by-step illustration of the pump-on-chip fabrication process: (a) - a sugar thread was thermally bonded to the surface of a cover glass; (b) - a thin layer of a PDMS mixture was spin-coated onto the template; (c) - PDMS was baked at 70 °C for 1 h; (d) - the inlet and outlet PDMS blocks were bonded to the micro-construct; (e) – the sterilized micro-construct was incubated with a solution of fibronectin overnight; (f) - cells were seeded on the dried fibronectin at the concentration of 1 million cells per ml; (g) - cells were incubated at 37 °C under humidified CO2 conditions
Cover slips with pre-attached sugar threads were covered with PDMS mixture (Fig. 1b), spin-coated at 5000 rpm for 60 s to produce a thin PDMS film and baked for 1 h at 70 °C (Fig. 1c). Inlet and outlet PDMS blocks with pre-cut channel ports were bound to the micro-channel with a minute amount of PDMS and baked for an extra 1 h at 70 °C (Fig. 1d). PDMS scaffolds were UV-sterilized for 30 min and then incubated with 10 μg/ml Human plasma fibronectin in PBS overnight before cell seeding (Fig. 1e). Inlets and outlets edges were cut to let the sugar spacer dissolve.
2.3 Cell isolation, seeding and cultivation
All procedures were performed according to the institutional requirements for the care and use of laboratory animals. All studies conformed to the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (Publication No. 85–23, revised 1996) and approved by Moscow Institute of Physics and Technology Life Science Center Provisional Animal Care and Research Procedures Committee, Protocol #A2–2012–09-02. Cardiac cell isolation, seeding and cultivation was performed according to Worthington protocol (http://www.worthington-biochem.com/NCIS/default.html). Cardiac cells were isolated from the ventricles of 1–3 day old neonatal Wistar rats. Obtained cell suspension was pre-plated for 1 h to reduce non-myocyte cell concentration and seeded on the fibronectin pre-coated micro-channel at a concentration of 1 million cells per ml of culture medium DMEM supplemented with 10% FBS, 2 mM of L-glutamine, and 100 U/ml of penicillin/streptomycin (Fig. 1f), and incubated at 37 °C under humidified 5% CO2 (Fig. 1g). After 1 day, samples were washed twice with PBS and reincubated in culture medium with 5% FBS.
2.4 Experiments and analysis
During experiments, micro-constructs were kept in a Petri dish with Tyrode’s solution supplied with 100 μM norepinephrine to increase cardiomyocytes contractile force. Temperature was maintained at 37 °C using a Thermo-plate Tokai Hit TP-S (Japan). Cathodal point stimuli (4 V, 20 ms duration) were applied at a rate of 1–2 Hz using the tip of a 500 μm platinum wire placed about 1 mm above the surface of the construct near one of the micro-channel’s ends with a looped platinum wire return electrode placed along the dish wall. Microfluidic channels wall motion was observed with Olympus Microscope IX 71 equipped with a DP72 camera. Videos were recorded with a resolution of 1360 × 1024 at a frame rate of 12.8 fps. Special code in Mathematica 11 (Wolfram Research, USA) was developed to analyze walls displacement along the channel (see Supplementary materials 3).
The flow inside micro-channels was assessed by the movement of 6 μm polystyrene beads (Bangs Laboratories, Inc., USA) injected into the channel through the inlet with a syringe. Video recording of beads movement was started 10 min after their injection to avoid possible effects of inertial movement of the liquid inside the channel. During the tracking of beads, the microscope was focused on the center of the micro-channel. The absence of a passive flow inside the channel was demonstrated by suppressing cardiomyocyte contractions with 3 mM lidocaine. Video records of beads movement inside the channel were analyzed with Mathematica 11 (Wolfram Research, USA). Displacement plots for either individual beads or their ensembles were built. Averaged plots for particle ensembles were smoothed by moving average with a window of 5 points length (390 ms).
3 Results and discussion
3.1 Formation and evaluation of micro-construct
The photo of the developed pump on a chip is presented in Fig. 2a. The micro-pump represents a 10 mm long channel with a diameter of 150–300 μm and a wall thickness of about 10 μm equipped with an inlet and outlet for liquid perfusion. The impermeability of the walls of the channel and absence of leakages in the formed constructs were tested by injecting a dyed liquid (Trypan blue solution) inside the micro-channel (see Fig. 2a).
The phase contrast image showing cardiac cells morphology on the surface of a micro-channel on the fourth day of cells incubation is presented in Fig. 2b. The cell monolayer exhibits full confluency throughout the entire length of the micro-channel with a predominant orientation of cells along the channels axis. The next day after cells seeding, the cardiac cells started to deflect the polymer membrane, reaching the maximum contractile force on the fourth day of incubation.
3.2 Assessment of contractile motion of constructs wall
A record of contraction wave propagation along the micro-channel under electrical pacing is presented in the Supplementary video 1. It could be seen that the wave propagates from left to right in this video. Although the membrane contractions amplitude was not measured directly in our experimental setup, we estimated the contractions of the micro-channels wall basing on the video record filmed from the top of the construct applying a specially developed Wolfram Mathematica script for image clusters tracking (Supplementary material 3). Obtained plots of the time course of the displacements for three different regions of micro-channels wall are presented in Fig. 3. The phase delay between these displacement plots indicates the contraction wave propagation along the micro-channel. This fact is essential for peristaltic mass transfer and it should be noted that the contraction wave propagation along the micro-channel was never observed in our experiments without electrical pacing.
Propagation of a contraction wave along the surface of the micro-channels wall. A phase-contrast image of the micro-pump taken at 4× magnification (top). Diagrams of the channels wall displacement for three selected regions (bottom). Colors of the plots correspond to the markers on the top picture. Scale bar = 200 μm
3.3 Evaluation of the flow generated by micro-pump
An example of real-time recording of polystyrene beads movement inside the micro-channel is presented in Supplementary Video 2. First part of the video represents the pulsatile movement of the particles under the electrical pacing of the construct with a frequency of 2 Hz. It could be seen that the particles gradually moves from left to right. This direction accords with the direction of the contraction wave propagation in this experiment. The displacement time-course for a single particle selected near the center of the channel is plotted in Fig. 4a in red.
Time course of micro-particles displacement inside the micro-pump: red – in case of peristaltic contraction of the channels wall (2 Hz stimulation); blue – in case of spontaneous asynchronous contractions (without external stimulation); green – in the absence of contractions (under 3 mM lidocaine); (a) – for a single particle near the central axis of the micro-channel; (b) - averaged for an ensemble of micro-particles
Movement of micro-particles inside the channel without electrical stimulation is demonstrated in the part II of Supplementary Video 2. In this case contraction of the micro-constructs walls is not synchronized and the particles oscillate around their initial positions without any pronounced directional movement. A displacement time-course for the case of spontaneous contraction of the wall of the construct is plotted in blue in Fig. 4a.
Finally, to demonstrate that the movement of the particles is governed by the contraction of micro-channels wall, a complete inhibition of contractile activity was induced by addition of 3 mM lidocaine. Absence of micro-particle movement under lidocaine treatment could be seen in part III of Supplementary video 2. The corresponding displacement plots are presented in Fig. 4a in green.
The displacement plots averaged for a particles ensemble from Supplementary video 2 are shown in the Fig. 4b. An average flow velocity of particles ensembles observed in our experiments was within the range of 6–8 μm/min, while the maximal velocity of directional movement of a single polystyrene bead reaches the values of 12–30 μm/min in different experiments.
Flow rate created by our pump could be estimated from the mean velocity of tracked particles (~ 6–8 μm/min) and cross section area of the channel (~ 0.035 mm2) as 0.21–0.28 nl/min which is almost identical to the flow obtained in the work (Park et al. 2007). At the same time, the obtained flow rate is two to three orders of magnitude smaller than the previously reported flow rate for bio-actuated pumps designed in (Tanaka et al. 2006) & (Tanaka et al. 2007). But it should be kept in mind that in those works, flow was generated by contraction of a macroscopic cardiomyocyte monolayer of almost one square centimeter in scale, while in our work, as well as in (Park et al. 2007), the flow was created by deflection of millimeter-scaled membranes.
In the present work it was most essential to demonstrate the principal applicability of the peristaltic mechanism for bio-actuated micro-pumping. Nevertheless, there is no doubt that for the purpose of practical application, an increase of the generated flow rate would be very desirable. Although we already used PDMS mixture with an almost 10-times decreased elastic modulus in comparison to conventional PDMS (Palchesko et al. 2012), and added norepinephrine to increase the contractile force of cardiomyocytes, there are still some extra possibilities for the enhancement of the contractions magnitude. In our future work we hope to manage to increase the flow rate generated by the micro-pump by using micropatterning of the cells (Feinberg et al. 2012) and a less stiff polymer for the scaffold fabrication (Lee and Mooney 2012).
4 Conclusions
In the present work we have demonstrated a novel possibility for performing bio-actuated pumping in artificial microfluidic systems. A novel type of bio-actuated micro-pump was designed, which generates flow by means of peristaltic motion of the wall of a microfluidic channel. The major advantage of the proposed micro-pump design is its compactness. In fact, the micro-channel itself in our model is, in essence, a pump. This creates unique possibilities for potential incorporation of such pumping systems into artificially created tissues.
One of the major challenges of modern tissue engineering is the facilitation of mass-transfer of nutrients and oxygen into the deeper layers of artificially created tissues (Hirt et al. 2014). Novel approaches aimed to solve this problem, including different microvascularization techniques, are constantly developing during the recent years (Zorlutuna et al. 2012; Sekine et al. 2013; Hasan et al. 2014). Methods of creation of micro-vessel-like structures are also getting more and more advanced (Xiao et al. 2014; Hasan et al. 2015) and it seems quite likely that a method for the creation of networks of peristaltic pumping channels might be developed soon. In a way, a development of such an approach to the active transport of nutrients and metabolites through the tissue would be equivalent to the creation of a biomimetic artificial lymphatic drainage system.
Finally, one more key feature of the proposed approach to bio-actuated pumping is worth discussing. As the direction of pumping of the liquid inside the contractile micro-channels is determined by the direction of the propagation of an excitation wave along its wall, the direction of pumping might be easily controlled. Moreover, this can be done not only by applying an external electrical stimulation, but also by means of the recently developed methods of photo-control of excitable tissues (Magome et al. 2011). As a result, micro-vascular networks of peristaltic channels might provide a tool to perform a precisely controlled active mass transfer in an artificial tissue. This might be of a particular interest, for example, for the purpose of controlled address delivery of differentiation factors through the vascular network of artificially engineered tissues.
References
A. Agarwal, J.A. Goss, A. Cho, M.L. Mc Cain, K.K. Parker, Lab Chip 13, 3599 (2013)
R.W. Carlsen, M. Sitti, Small 10, 3831 (2014)
R.M. Duffy, A.W. Feinberg, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 178 (2014)
A.W. Feinberg, P.W. Alford, H. Jin, C.M. Ripplinger, A.A. Werdich, S.P. Sheehy, A. Grosberg, K.K. Parker, Biomaterials 33, 5732 (2012)
O. Felfoul, M. Mohammadi, S. Taherkhani, D. de Lanauze, Y. Zhong Xu, D. Loghin, S. Essa, S. Jancik, D. Houle, M. Lafleur, L. Gaboury, M. Tabrizian, N. Kaou, M. Atkin, T. Vuong, G. Batist, N. Beauchemin, D. Radzioch, S. Martel, Nat. Nanotechnol. 11, 941 (2016)
M. Gossmann, R. Frotscher, P. Linder, S. Neumann, R. Bayer, M. Epple, M. Staat, A.T. Artmann, G.M. Artmann, Cell. Physiol. Biochem. 38, 1182 (2016)
A. Grosberg, A.P. Nesmith, J.A. Goss, M.D. Brigham, M.L. McCain, K.K. Parker, J. Pharmacol. Toxicol. Methods 65, 126 (2012)
A. Hasan, A. Paul, N.E. Vrana, X. Zhao, A. Memic, Y.S. Hwang, M.R. Dokmeci, A. Khademhosseini, Biomaterials 35, 7308 (2014)
A. Hasan, A. Paul, A. Memic, A. Khademhosseini, Biomed. Microdevices 17, 1 (2015)
M.N. Hirt, A. Hansen, T. Eschenhagen, Circ. Res. 114, 354 (2014)
R.D. Kamm, R. Bashir, Ann. Biomed. Eng. 42, 445 (2013)
D.J. Laser, J.G. Santiago, J. Micromech. Microeng. 14, R35 (2004)
K.Y. Lee, D.J. Mooney, Prog. Polym. Sci. 37, 106 (2012)
N. Magome, G. Kanaporis, N. Moisan, K. Tanaka, K.I. Agladze, Tissue Eng. Part A 17, 2703 (2011)
J.C. Nawroth, H. Lee, A.W. Feinberg, C.M. Ripplinger, M.L. McCain, A. Grosberg, J.O. Dabiri, K.K. Parker, Nat. Biotechnol. 30, 792 (2012)
R.N. Palchesko, L. Zhang, Y. Sun, A.W. Feinberg, PLoS One 7, e51499 (2012). doi:10.1371/journal.pone.0051499
J. Park, I.C. Kim, J. Baek, M. Cha, J. Kim, S. Park, J. Lee, B. Kim, Lab Chip 7, 1367 (2007)
S.-J. Park, M. Gazzola, K.S. Park, S. Park, V. Di Santo, E.L. Blevins, J.U. Lind, P.H. Campbell, S. Dauth, A.K. Capulli, F.S. Pasqualini, S. Ahn, A. Cho, H. Yuan, B.M. Maoz, R. Vijaykumar, J.-W. Choi, K. Deisseroth, G.V. Lauder, L. Mahadevan, K.K. Parker, Science 353, 158 (2016)
M. Pilarek, P. Neubauer, U. Marx, Sensors Actuators B Chem. 156, 517 (2011)
L. Ricotti, A. Menciassi, Biomed. Microdevices 14, 987 (2012)
H. Sekine, T. Shimizu, K. Sakaguchi, I. Dobashi, M. Wada, M. Yamato, E. Kobayashi, M. Umezu, T. Okano, Nat. Commun. 4, 1399 (2013)
Y. Tanaka, K. Morishima, T. Shimizu, A. Kikuchi, M. Yamato, T. Okano, T. Kitamori, Lab Chip 6, 362 (2006)
Y. Tanaka, K. Sato, T. Shimizu, M. Yamato, T. Okano, T. Kitamori, Lab Chip 7, 207 (2007)
Y. Tanaka, Y. Yanagisawa, T. Kitamori, Sensors Actuators B Chem. 156, 494 (2011)
S.G.M. Uzel, A. Pavesi, R.D. Kamm, Prog. Biophys. Mol. Biol. 115, 279 (2014)
B.J. Williams, S.V. Anand, J. Rajagopalan, M.T.A. Saif, Nat. Commun. 5, 1 (2014)
Y. Xiao, B. Zhang, H. Liu, J.W. Miklas, M. Gagliardi, A. Pahnke, N. Thavandiran, Y. Sun, C. Simmons, G. Keller, M. Radisic, Lab Chip 14, 869 (2014)
Y.S. Zhang, J. Aleman, A. Arneri, S. Bersini, F. Piraino, S.R. Shin, M.R. Dokmeci, A. Khademhosseini, Biomed. Mater. 10, 34006 (2015)
P. Zorlutuna, N. Annabi, G. Camci-Unal, M. Nikkhah, J.M. Cha, J.W. Nichol, A. Manbachi, H. Bae, S. Chen, A. Khademhosseini, Adv. Mater. 24, 1782 (2012)
Acknowledgements
This research work was partially supported by the Russian Foundation for Basic Research grant 16-34-60225.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shutko, A.V., Gorbunov, V.S., Guria, K.G. et al. Biocontractile microfluidic channels for peristaltic pumping. Biomed Microdevices 19, 72 (2017). https://doi.org/10.1007/s10544-017-0216-x
Published:
DOI: https://doi.org/10.1007/s10544-017-0216-x