Abstract
This paper presents a contactless detection method for detecting prostate specific antigen with a giant magnetoresistance sensor. In contactless detection case, the prostate specific antigen sample preparation was separated from the sensor that prevented the sensor from being immersed in chemical solvents, and made the sensor implementing in immediately reuse without wash. Experimental results showed that applied an external magnetic field in a range of 50 Oe to 90 Oe, Dynabeads with a concentration as low as 0.1 μg/mL can be detected by this system and could give an approximate quantitation to the logarithmic of Dynabeads concentration. Sandwich immunoassay was employed for preparing PSA samples. The PSA capture was implemented on a gold film modified with a self-assembled monolayer and using biotinylated secondary antibody against PSA and streptavidinylated Dynabeads. With DC magnetic field in the range of 50 to 90 Oe, PSA can be detected with a detection limit as low as 0.1 ng/mL. Samples spiked with different concentrations of PSA can be distinguished clearly. Due to the contactless detection method, the detection system exhibited advantages such as convenient manipulation, reusable, inexpensive, small weight. So, this detection method was a promising candidate in biomarker detection, especially in point of care detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the first type of magnetic biosensor was developed by Kriz (Kriz et al. 1996), Magnetic biosensor have gain more increasing interests from scientists and researchers due to their possess a number of advantages such as low molecules detection limits, flexibility and the direct availability of an electronic signal suitable for further automated analysis. This makes magnetic biosensors a promising choice for the detection units of future widespread and easy to use lab-on-a-chip systems or biochips (Issadore et al. 2014). Since the idea of using a giant magnetoresistance (GMR) in combination with magnetic beads working as magnetic labels for detecting molecular recognition events (antigen—antibody interaction, ligand—receptor binding) has have been successfully applied to the detection of biological events (Baselt et al. 1998; Edelstein et al. 2000; Rife et al. 2003) in the form of protein (Graham et al. 2003; Ferreira et al. 2003; Mujika et al. 2009), pathogenic bacteria (Arana et al. 2009) and DNA assays (Miller et al. 2001; Schotter et al. 2004) with great speed, sensitivity, selectivity and economy. The resistance of a GMR sensor changes with the traditional magnetic field applied to the sensor, so a magnetically labeled biomarker can induce a signal. Compared with the traditional optical detection now widely used in biomedicine, GMR sensors are more sensitive, portable and give a fully electronic readout (Zhi et al. 2014). Compared to the superconducting quantum interference device (SQUID)-based ultrasensitive magnetic bio-detection (Chemla et al. 2000; Lee et al. 2002), the GMR technology has advantages of room-temperature operation, less complex instruments, and hence more portable and flexible implementation.
However, in the present biomarker detection methods based on GMR sensor, the sensors surface almost require modified by chemical and bio solutions for biomarker capture (Graham et al. 2003; Ferreira et al. 2003; Mujika et al. 2009; Arana et al. 2009; Miller et al. 2001; Schotter et al. 2004; Zhi et al. 2014), that led to the sensor can did not avoid being contaminated and damaged by chemical reactive layer during the biomarker preparing, although there was some protective layer such as SiO2, Si3N4 (Ferreira et al. 2003; Mujika et al. 2009; Arana et al. 2009; Zhi et al. 2014). Secondly, the sensor needs to wash for reuse (Schotter et al. 2004; Zhi et al. 2014); that is not convenient for successively detecting in practical application. In addition, biochips based GMR need to be integrated into a μTAS structure, including microfluidic pumps for low flow velocities; this increases production costs for the chips, often preventing a successful market introduction.
As a mature magnetic sensor, GMR has found an increasingly wide utilization in industry fields such as position, distance, speed, angle, sense of rotation detection, almost those detections were contactless (Rieger et al. 2001). In contactless detection case, the GMR sensor can avoid being immersed in many chemical solutions, keep more stability and reusability; make the detection conveniently and easily, the detection systems can be enhanced more Industrialization and automation.
Prostate cancer is the second most common cause of cancer and the sixth leading cause of death by cancer among the male population worldwide (Grönberg 2003). Currently, prostate specific antigen (PSA) is the most reliable tumor biomarker for prostate cancer diagnosis and for monitoring disease recurrence (Özen and Sözen 2006). A total PSA of 4 ng/mL (more recently 2.5 ng/mL) is a highly probable indication for prostate cancer (Linton et al. 2003). Until now, several methods including enzyme-linked immunosorbent-assay (ELISA) (Matsumoto et al. 1999), chemiluminescent immuno-assay (Zhao et al. 2009), bioluminescent immunoassay (Ito et al. 2007) and electrochemical immunoassay (Chuah et al. 2012) have been conducted on clinical serum sample measurements. Although those methods are reliable and precise, it was time consuming, requires complex equipment and trained personal, not convenient for large-scale use in developing country (Devkota et al. 2015). So, it is still a critical demand on simple, low cost, portable, convenient manipulating detection technology for the earlier and sensitive profiling of PSA.
In this paper, we developed a contactless detection method using a GMR-based biosensor system. GMR sensor was fabricated by microfabrication. The contactless detection method was investigated. Dynabeads samples with different concentrations (0.1 μg/mL, 1 μg/mL, 5 μg/mL and 10 μg/mL) were prepared to characterize the performance of the detection system. Combined with double-antibody sandwich assay, the detection and quantification of PSA antigens with different concentrations were performed by the biosensor.
2 Experimental details
2.1 Fabrication of GMR sensor
The photograph of the fabricated GMR sensor is shown in Fig. 1. The GMR sensor used in our experiment has a top spin valve structure: Si/Ta(5)/seedlayer/IrMn(8)/ CoFe(2)/Ru(0.8/CoFe(2)/Cu(2.3)/CoFe(1.5)/Ta(3), all numbers in parenthesis are in nanometers. Each chip sensor consists of 200 strips in serial connection. Each strip has an electrical active area of 300 μm × 3 μm.
The manufacturing process consisted of the following steps: The sensor fabrication started with the spin valve thin film deposition on a 3-in. silicon wafer with the pinned magnetization (Mp) set in a selected direction. This selected direction will make Mp aligned in the width or transverse (y) direction of the patterned spin valve sensors, as illustrated in Fig. 1. E-Beam lithography (Hitachi HL-700F) was used to pattern sensor stripes on a submicron scale. After stripping off the e-beam resist, optical lithography was used to overlay leads pattern on the spin valve sensor stripes. Thick (150 nm) Cu layer was then deposited on the wafer by ion beam deposition. Electrical leads and pads to the spin valve sensors were formed after lift-off. To protect the sensors and leads from corrosion, one passivion layer of SiO2 80 nm) was deposited on top of the sensors and leads, exposing pad area. Figure 1 shows the optical micrograph of a fabricated spin valve sensor. Figure 2 shows the Fabrication steps of the GMR sensor.
2.2 Contactless detection system based on GMR
Here, “contactless” means the biological sample such as CEA, AFP was modified on the surface of a small glass instead of the surface of GMR sensor as shown in Fig. 2b. Figure 2a shows the biomarker labeled by Dynabeads was captured on the sensor surface.
The contactless detection system includes three parts, detection circuit, GMR sensor system, external magnetic field system. Keithley 4250 Source-Measure was used for powering and detecting the signal of the GMR sensor, GMR sensor system including GMR sensor and biological sample, external magnetic field was produced by Helmholtz coil, and the intensity of magnetic field was controlled via a current that generated form DC power supply.
The GMR detection was performed in the in-plane DC mode with the external magnetic field H applied in the transverse direction as shown in Fig. 3. The Dynabeads detection was performed in the two modes. The first mode was with the magnetic excitation field Hext1 applied in the transverse direction as shown in Fig. 3a; the second mode was with the magnetic excitation Hext2 was perpendicular to the sensor surface as shown in Fig. 3b, the GMR sensor was placed in the center of the Helmholtz coil, and the sample was placed on the surface of sensor, the gold film of the glass which captured the Dynabeads was covered on the surface of sensor as shows in Fig. 3b. A Helmholtz coil was used to provide a DC magnetic field ranging up to 90 Oe and perpendicular to the sensing strips of GMR sensor. In addition, in Fig. 3a, Hext1 was the H. We have measured the performance of GMR sensor under the two modes; we found that there was a clear output signal in Fig. 3a mode. However, there was very small output signal form the Fig. 3b mode. This may due to the weak magnetic field induced from Dynabeads was low, and the Hext2 increasing the magnetic anisotropy of free layer in the longitudinal direction (Wang and Li 2008). So, Fig. 3a mode was employed for Dynabeads detection.
3 Results and discussion
3.1 Detection of dynabead by GMR sensor
A 1 μA DC signal was generated by the Keithley 4250 Source-Measure and fed into the GMR sensor, and an R-H loop of the sensor obtained at room temperature. The basic resistant was 29.73kΩ. The maximum MR ratio was defined as (Rmax –R0)/R0, was equal to 10.1 %.
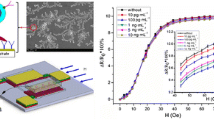
Dynabeads in this work was purchased from Invitrogen with a diameter of 1 μm. The process of Dynabead capture was achieved on a small glass which deposited an Au film on the surface. Dynabeads solutions with concentrations of 0.1 μg/mL, 1 μg/mL, 5 μg/mL and 10 μg/mL were respectively dropped on 4 Au films, and the quantity of each solution was 10 μL. Figure 4 shows the Scanning Electron Microscopy (SEM) photographs of the 10 μg/mL.
Dynabeads (1 μm) were detected by this GMR bio-sensing system with the external magnetic field was up to 90 Oe, and concentrations of Dynabeads within the range of 0.1 μg/mL, 1 μg/mL, 5 μg/mL and 10 μg/mL)). Label(x) was external magnetic field (Oe); Label (Y) was ratio of resistance change about GMR sensor (%)
The basic principle on which magnetic bead detection based was that when Dynabeads (on the surface of gold film) was exposed to the dc magnetic field, a magnetic dipole was induced in the magnetic beads aligned to the external DC field. The field in the in-plane case was mostly unidirectional and opposite to the external DC field. So, in this case, the presence of magnetic beads can decrease the effective DC magnetic field experienced by the GMR sensor, and consequently alters the measured results of the sensor. Figure 3 shows the curves representing the relationship between output signals and external magnetic field under the influences of Dynabeads with different concentrations.
As evident from Fig. 4, there is no significant change in the GMR ration under weak magnetic fields less than 30 Oersted (Oe). This might be due to the fact that the external magnetic field was very weak, resulting in very low-level magnetization of Dynabeads; hence, the induced magnetic field was too weak to affect the GMR sensing elements. For the field ranging from 30 Oe to 40 Oe, these GMR ratios were almost equal to each other for the sample concentrations of 0.1–5 μg/mL; but the difference between 5 μg/mL and 10 μg/mL was not enough pronounced for biomarker detection. For the field ranging from 50 Oe to 90 Oe, we observed a clear difference between the curves of different Dynabeads concentrations. It indicates that Dynabeads with different concentrations can be distinguished from others by this biosensor system based GMR.
Compared with detection method that the biomarker was captured on the sensor surface, this detection method was convenient and easy to manipulate, low power and costs, immediately reuse without clean. However, this method has relatively low sensitivity. In our opinion, there was a small gap between the sensor and the sample results from the contact was rigidity; secondly, the influence of the weak magnetic fields induced by Dynabead to GMR sensor also plays a significant role in detection performance, the influence is up to the distance between the Dynabead and the sensitive element (GMR strips) (Li and Wang 2004; Wang et al. 2005). So, if the sensor’s protect layer (such as SiO2) was significantly reduce from the utilization of MEMS technologies, that can be further enhanced to further improve the detection capability of this detection method.
3.2 Detection of PSA by GMR sensor
Double-antibody sandwich assay was employed for PSA sample preparation that process was achieved on a small glass with the area was equal to the detection area of the giant magnetoresistance sensor. The experiment details were elaborated in supplement material. Six test samples with different PSA concentrations, 0 ng/mL(BSA), 0.1 ng/mL, 0.5 ng/mL, 1 ng/mL, 5 ng/mL and 10 ng/mL, were prepared and labeled as sample 1, 2, 3, 4, 5 and 6 respectively. Additionally, we prepared five copy samples for each concentration, and each sample was tested for three times. Figure 5 shows the change in the MR in presence of the external magnetic field ranging from 40 Oe to 90 Oe; a clear difference is evident in the MR in presence of PSA labeled by Dynabead. Furthermore, each sample can clearly be distinguished, thus verifying the feasibility of the contactless detection method based GMR sensor in detecting the pathogen. However, as evident from Fig. 5, the MR signals of sample (1) and sample (2) are almost indistinguishable at the external magnetic field less than 40 Oe. Therefore, employing our newly developed GMR-based immunosensor, one can detect and quantify PSA from any sample containing >0.1 ng/mL PSA labeled with Dynabeads in presence of an applied DC magnetic field ranging from 40 Oe to 90 Oe.
The MR of the GMR bio-sensing system in absence of PSA sample. Double antibody sandwich immunoassay and streptavidin–biotin binding assay were employed for immobilizing and labeling of PSA with the concentrations of 0.1 ng/mL, 0.5 ng/mL, 1 ng/mL, 5 ng/mL and 10 ng/mL. From the inset figure, each sample can clearly be distinguished in presence of the external magnetic field ranging from 40 Oe to 90 Oe; Label(x) was external magnetic field (Oe); Label (Y) was the ratio of resistance change about GMR sensor (%)
In other words, the minimum detectable limit of this GMR based sensor for PSA is 0.1 ng/mL, and the samples containing different PSA concentrations can clearly be distinguished from others. This newly developed GMR based contactless detection technique exhibits lower minimum detection limit compared with the reported minimum detectable PSA concentration of 1 ng/mL achieved by the electrochemical impedance spectroscopy (Jolly et al. 2015) and 4.5 ng/mL achieved by dark field microscopy.(Poon et al. 2014) Although the minimum detectable concentration of 0.24 ng/mLhas been achieved by biosensors based on electrochemical sensor (Kokkinos et al. 2013), our GMR sensor is convenient for manipulation and reusable without cleaning. In the experiment, due to the PSA capture was achieved on the small glass surface; in comparison to the other biosensors based on GMR that the sensor ‘s surface need functionalization (Ferreira et al. 2003; Mujika et al. 2009; Zhi et al. 2014; Tondra et al. 2002) the surface of our sensor does not need chemical modification for capturing target biomarker, thus preventing contamination and damage by chemical reactive layer of samples. Thus, this new GMR based immunosensor not only possesses good stability, it also offers rapid, convenient sample change. In addition, the small glasses can be arrayed for automation detection with the GMR as the detector. Considering this special detection performance, this biosensor system can be a promising candidate for clinical PSA or other biomarkers detection, especially in the point of care application.
A relative standard deviation (RSD) of 0.95 % for 8 successive measurements (1 ng/mL) was observed, which indicates good reliability and stability of measurement results. Specificity tests were carried using carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP) instead of PSA antigens. As shown in Fig. 6. This confirmed that PSA antigen selectively bonds to PSA antibody. Besides, the respond times of this biosensor system for detecting the sample was less than 12 s.
Figure 7 presents the MR ratio of the bio-sensing system corresponding to the concentrations of PSA antigen in presence of the external DC magnetic field of 66 Oe. MRno sample refers to the MR of the bio-sensing system in absence of any sample, while MRsample refers to the MR of the bio-sensing system in presence of the target sample; ΔMR is the difference between them.
Sensitivity and linear range analysis with the external magnetic field was 66 Oe. In the concentration range of 0.1–10 ng/mL, there was a good linear relationship between the logarithm of PSA antigen concentration and MR of the bio-sensing system. The detection limit was 0.1 ng/mL. Label(x) was the concentration of PSA; Label (Y) was the difference between the MR of the bio-sensing system in absence of any sample and the target sample
In Fig. 7, the blue line indicates the relation of the measured outputs to the PSA antigen concentrations; we found a good linear relationship between the logarithm of PSA antigen concentration and MR of the bio-sensing system in the concentration range of 0.1–10 ng/mL, which can be used for further quantitative analysis.
During the experiment, our GMR based sensor allows convenient changing of the PSA samples. In comparison to the present detection method based GMR that the sensor surface need modified by chemical solutions for biomarker capture, contamination and damage of this newly developed sensor by chemical reactive layer of samples can be avoided, thus allowing stable performance. This sensor can immediately be reused without washing as the design allows contactless detection. So, the performance of sensor can keep good stability and reusable. In addition, the bio-samples can be conveniently and easily changed in detection without need training person. If the small glasses that used for preparing bio-sample were arrayed, the detection system can be enhanced more automation. Therefore, our sensor has the potential to point to point detection of various kinds of biomarkers at different concentrations. Additionally, to capture biomarkers, our GMR based biosensor does not need bio-modification.
4 Conclusion
In this work, we develop a contactless biomarker detection system based on GMR sensor, the system was characterized by Dynabeads, and successfully applied in PSA detection. In contactless detection case, this new detection method possesses several advantages such as convenient manipulation; avoid being damaged by chemical solution and implementing in immediately reuse without wash. So, it is of considerable interest towards the biomedical application based on known specific binding of target and labels. In future studies, we will focus on enhancing the detection capability of this detection method via reducing sensor’s protect layer (such as SiO2) from the utilization of MEMS technologies, and expanding the bio-sensing system to multi-target biomolecules.
References
M. S. Arana, E. Casta˜noa, M. Tijero, R. Vilares, J. M. Ruano-López, A. Cruz, L. Sainz, J. Berganza, Biosens Bioelectron 24, 1253–1258 (2009)
D. R. Baselt, G. U. Lee, M. Natesan, S. W. Metzger, P. E. Sheehan, R. J. Colton, Biosens. Bioelectron 13, 731–739 (1998)
Y.R. Chemla, H.L. Crossman, Y. Poon, R. McDermott, R. Stevens, M.D Alper, J. Clarke. Proc. Natl. Acad. Sci. U.S.A., 97, 14268–14272. (2000)
K. Chuah, L. M. H. Lai, I. Y. Goon, S. G. Parker, R. Amal, J. J. Gooding, Chem. Commun. 48, 3503–3505 (2012)
J. Devkota, G. Kokkinis, M. Jamalieh, M. H. Pan, H. Srikanth, S. Cardoso, F.A. Cardoso, I. Gioiroudi. Proc. SPIE 9518, Bio-MEMS and Medical Microdevices II, 95180 W (2015).
R. L. Edelstein, C. R. Tamanaha, P. E. Sheehan, M. M. Miller, D. R. Baselt, L. J. Whitman, R. J. Colton, Biosens. Bioelectron 14, 805–813 (2000)
H. A. Ferreira, D. L. Graham, P. P. Freitas, J. M. S. Cabral, J. Appl. Phys 93, 7281–7286 (2003)
D. L. Graham, H. A. Ferreira, P. P. Freitas, J. M. S. Cabral, Biosens Bioelectron 18, 483–488 (2003)
H. Grönberg, Lancet 361, 859–864 (2003)
D. Issadore, Y. I. Park, H. Shao, C. Min, K. Lee, M. Liong, R. Weissleder, H. Lee, Lab on a Chip 14(14), 2385–2397 (2014)
K. Ito, W. Nishimura, M. Maeda, et al., Analytica chimica acta, 588, 245–251 (2007)
P. Jolly, N. Formisano, J. Tkac, P. Kasak, C. G. Frost, P. Estrela, Sens. Actuators B:Chem. 209, 306–312 (2015)
C. Kokkinos, A. Economou, P. S. Petrou, S. E. Kakabakos, Anal. Chem. 85, 10686–10691 (2013)
C. B. Kriz, K. Radevik, D. Kriz, Anal Chemial 68, 1966–1970 (1996)
S. Lee, W. R. Myers, H. L. Grossman, H. M. Cho, Y. R. Chemla, J. Clarke, Appl. Phys. Lett 81, 3094–3096 (2002)
G. Li, S. X. Wang, IEEE Trans Magn 40, 3000–3002 (2004)
H. J. Linton, L. S. Marks, L. S. Millar, C. L. Knott, H. G. Rittenhouse, D. SMikolajczyk, Clin. Chem 49, 253–259 (2003)
K. Matsumoto, N. Konishi, Y. Hiasa, E. Kimura, Y. Takahashi, K. Shinohara, T. Samori, Clin. Chim. Acta 281, 57–69 (1999)
M. M. Miller, P. E. Sheehan, R. L. Edelstein, C. R. Tamanaha, L. Zhong, S. Bounnak, L. J. Whitman, R. J. Colton, J magnetism Magn mater 225, 138–144 (2001)
M. Mujika, S. Arana, E. Casta˜noa, M. Tijero, R. Vilares, J.M. Ruano-López, A.Cruz, L. Sainz, J. Berganza, Biosens Bioelectron 24, 1253–1258 (2009)
H. Özen, S. Sözen, Eur. Urol. Suppl 5, 495–499 (2006)
C. Y. Poon, H. M. Chan, H. W. Li, Sens. Actuators B:Chem 190, 737–744 (2014)
G. Rieger, K. Ludiwig, J. Hauch, W. Clemens, Sensors Actuators A Phys. 91(1), 7–11 (2001)
J. C. Rife, M. M. Miller, P. E. Sheehan, C. R. Tamanaha, M. Tondra, L. J. Whitman, Sens actuators a 107, 209–218 (2003)
J. Schotter, P. B. Kamp, A. Becker, A. Pühler, G. Reiss, H. Brückl, Biosens. Bioelectron. 19(10), 1149–1156 (2004)
M. Tondra, M. porter, R. J. Lipert, J. Vac. Sci. Technol 18, 1125–1129 (2002)
S. X. Wang, G. X. Li, Advances in magnetics. Vol 44, 1687–1702 (2008)
S. X. Wang, S. Y. Bae, G. Li, S. Sun, R. L. White, J. T. Kemp, C. D. Webb, J Magn Magn Mater 293, 731–736 (2005)
L. Zhao, L. Sun, X. Chu, Trends in. Anal. Chem. 28, 404–415 (2009)
X. Zhi, M. Deng, H. Yang, G. Gao, K. Wang, H. L. Fu, Y. X. Zhang, D. Chen, D. X. Cui, Biosens Bioelectron 54, 372–377 (2014)
Acknowledgments
This work is supported by The National Natural Science Foundation of China (No.61273065), National Science and Technology Support Program (2012BAK08B05), Natural Science Foundation of Shanghai (13ZR1420800), Support fund of Shanghai Jiao Tong University (AgriX2015005), Support fund of Joint research center for advanced aerospace technology of Shanghai Academy of Spaceflight Technology-Shanghai Jiao Tong University (USCAST2015-2), Support fund of aerospace technology (15GFZ-JJ02-05), the Analytical and Testing Center in Shanghai Jiao Tong University, the Center for Advanced Electronic Materials and Devices in Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOCX 478 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Zhi, S., Lei, C. et al. Investigation of contactless detection using a giant magnetoresistance sensor for detecting prostate specific antigen. Biomed Microdevices 18, 60 (2016). https://doi.org/10.1007/s10544-016-0084-9
Published:
DOI: https://doi.org/10.1007/s10544-016-0084-9