Abstract
Ectomycorrhizal fungi hold a potential role in bioremediation of heavy metal polluted areas because of its metal accumulation and detoxification property. We investigated the cadmium (Cd) induced bioaccumulation of glutathione (GSH) mediated by γ-glutamylcysteine synthetase (γ-GCS) in the ectomycorrhizal fungus Hebeloma cylindrosporum. In H. cylindrosporum, a demand driven synthesis of GSH has been observed in response to Cd. The expression and enzyme activity of H. cylindrosporum γ-GCS (Hcγ-GCS) increased as a function of external Cd stress resulting in increased GSH production. The function of Hcγ-GCS in providing heavy metal tolerance to H. cylindrosporum was justified by complementing the gene in gsh1Δ mutant of Saccharomyces cerevisiae. The metal sensitive mutant gsh1Δ successfully restored its metal tolerance ability when transformed with Hcγ-GCS gene. Sequence analysis of Hcγ-GCS showed homology with most of the reported γ-GCS proteins from basidiomycetes family. The active site of the Hcγ-GCS protein is composed of amino acids that were found to be conserved not only in fungi, but also in plants and mammals. From these results, it was concluded that Hcγ-GCS plays an important role in bioaccumulation of GSH, which is a core component in the mycorrhizal defense system under Cd stress for Cd homeostasis and detoxification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is considered as the global environmental threats due to its high bioaccumulation and biomagnifications into the diverse ecosystems (Dietz et al. 2000). It is toxic to living systems even at low concentrations and interferes with many metabolic pathways resulting in cellular damage because of its strong affinity for the sulphydryl residues (Gallego et al. 2012). Cd is the potent inducers of oxidative stress inside the cells and these oxidative damages in cell are usually caused by the imbalance between accumulation of reactive oxygen species (ROS) and the protective cellular antioxidant system (Foyer and Noctor 2011). Therefore it is crucial to maintain adequate level of ROS in the cell. This is attained by the complex antioxidant homeostatic system composed of metabolites like glutathione (GSH) and ascorbate (ASC) (Foyer and Noctor 2011).
Glutathione is a ubiquitous intracellular peptide with multifarious functions like detoxification of various heavy metals and xenobiotics, antioxidant defense, sulfur assimilation; cell signaling and regulate cell growth and death (Lu 2009; Khullar and Reddy 2016). GSH is a key component in metal scavenging, due to the high affinity of metals for its thiol (–SH) group and also as a precursor of phytochelatins (PCs). GSH is reported as the most abundant thiol present in the living system, with the average concentration in millimoles (Pócsi et al. 2004). It is a tripeptide (l-γ-glutamyl-l-cysteinyl-glycine) (~ 307 Da) composed of three amino acids, glutamate, cysteine and glycine. GSH is synthesized in two sequential ATP dependant reactions mediated by two enzymes, γ-glutamylcysteine synthetase (γGCS; E.C.6.3.2.2) and glutathione synthetase (GS; E.C.6.3.2.3). In the first reaction, γGCS catalyzes the formation of γ-glutamylcysteine by combining l-glutamate and l-cycteine, followed by the condensation of glycine to the C-terminal of γ-glutamylcysteine by GS, resulting in the formation of glutathione. Both reactions are ATP dependant. The two major determinants of GSH biosynthesis in the cell are: availability of cysteine (the sulfur amino acid precursor), and the activity of γ-glutamylcysteine synthetase (the rate limiting enzyme) (Lu 2009). During the metal stress, both factors increase in order to cope with the desired GSH demand for the detoxification and survival (Jozefczak et al. 2012). γGCS is a rate limiting enzyme in GSH synthesis and its activity is enhanced by Cd, As, Hg, Cr ions (Vido et al. 2001; Thorsen et al. 2007; Galant et al. 2011; Sobrino-Plata et al. 2014). It has also been reported that the mutation in the γGCS gene resulted in increasing metal sensitivity in Arabidopsis thaliana and Candida glabrata (Sobrino-Plata et al. 2014; Gutiérrez-Escobedo et al. 2013). Many studies have reported the GSH-mediated induction of the transcription of genes involved in Cd transport and detoxification (Sengupta et al. 2012; He et al. 2015).
The response of ectomycorrhizal (ECM) fungi to toxic metals is important, since these organisms are present at contaminated sites, participate in crucial symbiotic relationships with plants that grow at these sites, and alleviate metal toxicity for their host plants (Jentschke and Goldbold 2000). The mechanisms that are involved in metal homeostasis and detoxification of heavy metals in ECM fungi includes extracellular mechanisms such as precipitation, chelation and cell-wall binding and intracellular mechanisms such as binding to organic acids, sulfur compounds, polyphosphates, peptides and transport into intracellular compartments (Bellion et al. 2006). The main molecules responsible for the sequestration of metals include glutathione, phytochelatins and metallothioneins (Cobbett and Goldsbrough 2002). It has been reported that in ECM fungi, metallothioneins have been induced in response to copper stress than Cd (Reddy et al. 2014, 2016; Khullar and Reddy 2016). However, accumulation of GSH in ECM fungi was reported when exposed to Cd suggesting the role of GSH in Cd detoxification (Courbot et al. 2004).
The aim of the present study was therefore to gain insight into the molecular basis of Cd detoxification in the ectomycorrhizal fungus Hebeloma cylindrosporum by investigating enzyme Hcγ-GCS of GSH metabolism. The relative expression of Hcγ-GCS gene was studied by qPCR under different Cd stress conditions. Further, the functional characterization of Hcγ-GCS gene in response to Cd stress was carried out using yeast mutant gsh1Δ.
Materials and methods
Biological material, stress treatment and metal accumulation
The ectomycorrhizal fungus Hebeloma cylindrosporum was maintained on modified Melin-Norkrans medium (MMN) (Melin 1953) supplemented with Heller’s micronutrients (Gay 1990) at 25 °C. The tolerance of H. cylindrosporum to cadmium was monitored by growing the fungus in MMN broth supplemented with different concentrations of cadmium (0, 3, 6, 9, 12 and 15 µM) for 21 days at 25 °C. After 21 days, the mycelium was harvested and washed with saline water (0.9% NaCl) and 0.1 M EDTA water followed by three washings with distilled water and dried at 60 °C for 24 h. The dry weight of each sample was recorded and the dried mycelium was further digested with nitric acid and perchloric acid (HNO3/HCLO4) in the ratio 3:1 (Kalsotra et al. 2018) and the total metal uptake by mycelium at different Cd concentrations was determined using atomic absorption spectroscopy.
For the functional complementation, S. cerevisiae mutant gsh1Δ-Y07097 (BY4741; MATa; ura3Δ0; leu2Δ0; his3Δ1; met15Δ0; YJL101c::kanMX4) γ-GCS was procured from Indian Institute of Science Education and Research (IISER, Mohali, Punjab, India).
Total glutathione production and Hcγ-GCS activity
Total glutathione produced by H. cylindrosporum in response to the elevated levels of Cd was determined by the method described in Rahman et al. (2006). The fungus was grown on MMN agar plates overlaid with cellophane sheets for 14 days at 25 °C. The cellophane sheets were then transferred on MMN medium supplemented with increasing concentrations of cadmium (CdSO4: 0, 10, 20, 30, 40 µM) for 48 h. After 48 h, the mycelium was scrapped from the cellophane sheets and crushed with liquid nitrogen. Total glutathione was estimated from these crushed samples by enzymatic recycling. Firstly, the cell extract was prepared by homogenizing the mycelium in sulfosalicylic acid-TritonX solution (0.6% sulfosalicylic acid and 0.1% Triton-X solution in 0.1 M Potassium phosphate buffer) with 5 mM EDTA disodium salt at pH 7.5 (KPE) followed by the centrifugation of homogenized sample at 8000×g for 10 min at 4 °C. Clear supernatant obtained was used for GSH estimation. The amount of GSH produced by the mycelium under various stress conditions was quantified spectrophotometrically by enzymatic recycling method, using the sulfydryl reagent 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) to form the yellow colored derivative 5′-thio-2-nitrobenzoic acid (TNB) measurable at 412 nm (Rahman et al. 2006). Cell extract (100 µl) was mixed with 700 µl of KPE buffer followed by addition of 120 µl of freshly prepared DTNB and glutathione reductase (GR) mix. The mixture was kept at room temperature for 30 s. 60 µl of β-NADPH (2 mg β-NADPH in 3 ml KPE buffer) was added to the above mix and absorbance was measured at 412 nm. The amount of GSH produced was measured using the GSH (Sigma-Aldrich; St. Louis, USA) standard curve prepared by the same procedure.
Hcγ-GCS activity was determined according to the method described in Ruiz et al. (2003) and Sengupta et al. (2012). The liquid nitrogen crushed samples of H. cylindrosporum stressed with different concentrations of Cd (0, 10, 20, 30, 40 µM) were homogenized with lysis buffer (100 mM Tris–HCl (pH 8.0), 1 mM DTT and 10 mM MgCl2). Hcγ-GCS activity was assayed by mixing 100 µl of supernatant with 500 µl of assay mix consisting of 100 mM Hepes (pH 8.0), 50 mM MgCl2, 5 mM ATP, 5 mM phosphoenolpyruvate, 5 mM DTT, 20 mM glutamate, 1 mM cysteine, and 10 U ml−1 pyruvate kinase. The mixture was incubated at 37 °C for 60 min followed by addition of 100 µl of 50% TCA to stop the reaction. The mixture was centrifuged and the supernatant was used for phosphate estimation by phosphomolybdate method. The amount of phosphate released is directly proportional to the enzyme activity.
RNA isolation, cDNA synthesis and gene amplification
Total RNA was isolated from the liquid nitrogen crushed samples of H. cylindrosporum using the QiAzol lysis reagent (Qiagen; Valencia, CA), followed by the RNase free DNase I treatment. cDNA was synthesized from approximately 5 µg of total RNA using “The RevertAID™ First Strand cDNA synthesis Kit, (Thermo Fisher Scientific, Waltham, USA)” as per the manufacturer’s instructions. The cDNA so obtained was used to amplify the Hcγ-GCS gene using the gene specific primers. The gene specific primers were designed using the sequence retrieved from JGI portal http://genome.jgi.doe.gov/ (Supplementary Table 1). Restriction sites NdeI (CATATG) and NotI (GCGGCCGC) were added to the 5′ end of the forward and reverse primer respectively. PCR amplification of Hcγ-GCS gene was carried out in a 25 µl reaction consisting of 1 X reaction buffer, 2 µl (dNTPs, 2 mM), 1 µl (forward primer, 10 µM), 1 µl (reverse primer, 10 µM), template DNA (100 ng), 1.5 U Taq polymerase and nuclease free water to make 25 µl. The PCR program was set as: initial denaturation at 95 °C for 3 min followed by 30 cycles of 1 min at 94 °C, 1 min at 62 °C, 1 min at 72 °C and final extension at 72 °C for 8 min. The amplified product so obtained was purified and sequenced. The sequence was submitted to GenBank of NCBI under the Accession Number MH892339.
Expression of γ-GCS by real time PCR (RT-PCR) analysis
Total RNA was isolated from the liquid nitrogen crushed samples treated with Cd using the QiAzol lysis reagent (Qiagen; Valencia, CA) and cDNAs were synthesized using “The RevertAID™ First Strand cDNA synthesis Kit, (Thermo Fisher Scientific, Waltham, USA)” as per the manufacturer’s instructions. The relative expression of Hcγ-GCS was determined by Real time PCR analysis using SYBR® Green JumpStart™TaqReadyMix™ (Sigma-Aldrich; St. Louis, USA) in Mastercycler®eprealplex system (Eppendorf; Hamburg, Germany) using the following program: initial denaturation at 95 °C for 20 s followed by 40 cycles of 95 °C for 15 s, 55 °C for 15 s and 72 °C for 20 s. The E value was obtained in the range of 0.97 to 1.08 using the equation E [10(−1/slope)] − 1. The E value was used to calculate Ct1 value using the equation Ct1 = Cte × [log (1 + E)/log2]. Among the three reference genes α-actin, β-tubulin and adenosine kinase, α-actin showed minimum stability value with NormFinder algorithm (Andersen et al. 2004). Hence α-actin was used as a reference gene for studying the relative gene expression of Hcγ-GCS using the comparative CT Method (ΔΔCT Method) − 2−ΔΔCt1” (Livak and Schmittgen 2001).
Functional complementation of Hcγ-GCS in S. cerevisiae
The Hcγ-GCS gene was amplified from cDNA using primers Hcbam1F and Hceco1R. BamH1 and EcoR1 restriction sites were added to the 5′ end of both forward and reverse primer respectively (Supplementary Table 1). The amplified PCR product was ligated into the yeast expression vector pFL61 under the control of the PGK promoter from the yeast phosphoglycerokinase encoding gene (Minet et al. 1992). For the functional complementation, S. cerevisiae mutant gsh1Δ (γ-GCS mutant) was transformed with empty vector pFL61 and pFL61-Hcγ-GCS by lithium acetate method (Stearns et al. 1990). The transformants along with wild type BY4741 were then pre-grown on SD-ura medium for 24 h at 30 °C with constant shaking at 220 rpm. The OD600 of each culture was adjusted to 1.0. For each culture 4 serial dilutions were made 100, 10−1, 10−2 and 10−3. 5 μl serial dilutions of each culture was spotted on SD-Ura plates with and without Cd (CdSO4: 40 μM). The plates were incubated at 30 °C for 3 days and photographed. In a consecutive experiment, all the three cultures, gsh1Δ (pFL61 + Hcγ-GCS), gsh1Δ (pFL61) and wild type BY4741 were inoculated in 50 ml SD-ura broth and allowed to grow at 30 °C till the OD600nm reached 0.05. The cultures were then subjected to different concentrations of Cd and allowed to grow for next 24 h and the OD at 600 nm was recorded.
Sequence analysis and structure prediction of Hcγ-GCS
The ORF of the sequence was identified using ORF finder and subjected to BLAST (http://www.ncbi.nlm.nih.gov/BLAST) for homology search. The homologous sequence so obtained were aligned by ClustalW. Phylogenetic tree was reconstructed by using MEGA software. Further, Expasy tool was used to calculate the molecular weight and pI of Hcγ-GCS protein. The protein domains were identified using Interpro (http://www.ebi.ac.uk/interpro/sequence-search). Various conserved domains like catalytic domain, ATP binding domains, glycosylation sites, phosphorylation sites were identified by Motif-Scan and ATPint (crdd.osdd.net/raghava/atpint/index.html). The secondary structure of Hcγ-GCS protein was predicted using PSSpred (http://zhanglab.ccmb.med.umich.edu/PSSpred), where all the possible α-helix, β-sheets and coils were identified. The tertiary structure of Hcγ-GCS protein was constructed by I-TASSER. The structure template was identified using threading program LOMETS from PDB library with Normalized Z-score greater than 1. I-TASSER generated 5 predicted models and the model with highest confidence value (C value) was selected. The structure so obtained was verified by aligning it with all structures in PDB library using TM-align. Various ligand binding sites in the predicted structure were identified using COFACTOR and COACH programs.
The data were analyzed by One way analysis of variance the significant differences among the means were compared with Tukey’s test at P < 0.05. All the analysis was performed by using Graph Pad Prism 5.1 software.
Results
Metal tolerance and accumulation
The growth of H. cylindrosporum decreased significantly with increasing concentrations of Cd. The half minimum inhibitory (IC50) of Cd was recorded at 8 µM (Fig. 1a). However, the Cd accumulated inside the fungus was found to increase with increase in external metal concentrations. Maximum Cd accumulation of 0.9 µg mg−1 dry mycelium was recorded at 9 µM and decreased hereafter (Fig. 1b).
Glutathione production and Hcγ-GCS activity
The total GSH production increased as a function of external Cd stress. Total GSH production increased significantly from 0.118 to 0.85 nmol mg−1 mycelium when the stress was increased from 0 to 40 µM (Fig. 2a). Approx. 8.4 folds increase in glutathione concentration was observed, when the Cd stress was increased from 0 to 40 µM. Since Hcγ-GCS is an ATP dependant enzyme, it liberated one molecule of inorganic phosphorus every time it catalyze the synthesis of γ-glutamylcysteine, converting ATP to ADP. Therefore the amount of inorganic phosphorus liberated per minute per mg of protein gives direct evidence of the enzyme activity of Hcγ-GCS. The 10 µM Cd stressed mycelium liberated 0.75 µmol mg−1 min−1 of inorganic phosphorus, which is three times the phosphorus liberated by control mycelium (0.25 µmol mg−1 min−1). At 40 µM Cd stress, the concentration of inorganic phosphorous increased to 1.06 µmol mg−1 min−1, which is 5 times that of control mycelium (Fig. 2b).
Effect of increasing concentrations of Cd on a total glutathione (GSH) production and b the enzyme activity of Hcγ-GCS. The activity was measured as the amount of inorganic phosphorous released by the enzymes using ATP per mg of isolated protein per minute. Values sharing a common letter among same metals are not significantly different at P < 0.05 (n = 3). Error bars are ± SD
Real time PCR analysis
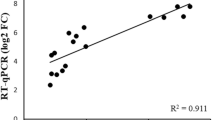
The mRNA accumulation of Hcγ-GCS gene in H. cylindrosporum increased when exposed to increasing concentrations of Cd. The 10 folds increase in mRNA accumulation of Hcγ-GCS gene was observed at 10 µM Cd stress, whereas it increased to 22 folds at 40 µM Cd (Fig. 3). This clearly depicts that Hcγ-GCS gene is highly inducible by Cd stress, resulting in more glutathione biosynthesis.
Fold increase in the relative expression of Hcγ-GCS gene when stressed with increasing concentrations of Cd (CdSO4: 0, 10, 20, 30, 40 µM) for 48 h at 25 °C. Actin was normalized as an internal reference gene. Values sharing a common letter within the gene are not significantly different at P < 0.05 (n = 3). Error bars are ± SD
Functional complementation of Hcγ-GCS gene in yeast mutant
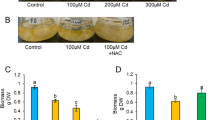
The role of Hcγ-GCS in inducing heavy metal tolerance in ectomycorrhizal fungus H. cylindrosporum was validated by expressing the gene in S. cerevisiae mutant for γGCS (gsh1Δ). The growth of gsh1Δ yeast mutant cells transformed with empty vector pFL61 (EV) and pFL61 + Hcγ-GCS was monitored on SD-Ura medium supplemented with and without Cd. The Drop test analysis of all the three cultures BY4741, gsh1Δ/EV and gsh1Δ/Hcγ-GCS, clearly revealed that the growth of gsh1Δ/EV inhibited at Cd 40 µM, whereas the same mutant when transformed with Hcγ-GCS gene successfully grew under Cd stress like their wild type BY4741 cells (Fig. 4a). Further, the restoration of metal tolerance ability by yeast mutant gsh1Δ was validated by growing all the three cultures BY4741, gsh1Δ/EV and gsh1Δ/Hcγ-GCS in SD-Ura liquid medium supplemented with increasing concentrations of Cd. When subjected under metal stress, gsh1Δ/EV did not show any tolerance to Cd. However gsh1Δ/Hcγ-GCS successfully grew under Cd stress like their parent BY4741 cells (Fig. 4b). This clearly shows that the metal tolerance ability was successfully restored in gsh1Δ, when transformed with Hcγ-GCS gene.
Drop test analysis (a) and liquid broth test (b) of the metal sensitive Saccharomyces cerevisiae mutant gsh1Δ (Accession Number: Y07097), transformed with empty vector pFL61 (EV) and pFL61 + Hcγ-GCS gene on selective media SD-Ura supplemented with and without Cd. BY4741 wild type strain was used as a positive control. For liquid broth test, growth was recorded as optical density at 600 nm after 24 h of incubation at 30 °C and 200 rpm. The values represent an average of three biological replicated with ± SD
Sequence analysis of Hcγ-GCS gene
The full length cDNA sequences of Hcγ-GCS consists of 2040 bp long open reading frame encoding 679 amino acids with predicted molecular weight of 78.09 kDa and pI of 6.6. The protein is a transmembrane protein with three membrane domains: N-terminal from amino acid 1 to 446 forms a non-cytoplasmic domain; amino acids from 447 to 469 are embedded in the membrane forming a transmembrane domain and at the C-terminal from amino acid 470 to 679 forms a cytoplasmic domain. The putative Hcγ-GCS protein showed maximum homology with most of the basidiomycetes displaying 71%, 70%, 69% and 68% sequence similarity with the γGCS protein sequence form Coprinopsis cinerea (XP001830305), Armillaria gallica (PBK91420), Agaricus bisporus (XP006460756) and Laccaria bicolor (XP001877753), respectively. The Phylogenetic tree forms three different clusters of basidiomycetes, mucoromycetes and ascomycetes each sharing common evolutionary history (Fig. 5).
The maximum parsimony tree constructed using MEGA 7. γGCS enzymes from three different classes of fungi were clustered separately into basidiomycetes, mucoromycetes and ascomycetes, showing their common evolutionary history. The tree was constructed using 1000 bootstrap test. Accession number of each protein has been mentioned in parenthesis
The multiple sequence alignment of different homologous sequences revealed many domains that were found to be conserved in many fungi. Amino acids 249–649 were identified as a conserved glutamylcysteine synthetase catalytic subunit and amino acids 26–84 were identified as 30S ribosomal protein S1. Both the sequences were found to be highly conserved. Most of the predicted ATP binding domains in the primary sequence of Hcγ-GCS protein were found to be conserved. This includes amino acids 188–204 “HVSRPCTANIRRRRGSK”, amino acids 249–268 “IYLDAMGFGMGCCCLQLTFQ”, amino acids 299–316 “WRGYLADVDCRWNVIAGS” (Supplementary Fig. 1). N-glycosylation motifs were also predicted at site N163, N511 and N518. The secondary structure of the predicted Hcγ-GCS protein consists of about 37% α-helices, 15% β-strands and 49% coils.
For generating the tertiary structure, the structures homologous to Hcγ-GCS protein sequence were identified in PDB library. Four PBD hits were observed 3ig5, 3ig8, 3ivv and 3ivw. Since 3ig5 (Saccharomyces cerevisiae glutamate cysteine ligase) showed maximum identity of 42%, it was selected as a template for homology modeling (Fig. 6). Various ligand binding sites were identified on the generated 3D model by COACH. Most of the substrate binding sites like E52, E101, C260, C261, C262, Q264, R309, I313, R435 and ADP binding sites L46, W47, G48, D49, E50, H99, E108, P114, T266, F267, Q268, R431, E433 were found conserved throughout the selected basidiomycetes.
Tertiary structure of Hcγ-GCS enzyme generated by I-TASSER a constructed by homology modeling using PBD structure 3ig5 as template. b The putative substrate and ADP binding site in the active site have been highlighted in green and blue color respectively. The active site amino acids were highlighted in pink
Discussion
Ectomycorrhizas have been exploited for their potential role in alleviating the metal stress in their host plants but the molecular and cellular mechanisms underlying this substantial metal tolerance are largely unknown. The potential application of ectomycorrhizal fungi in bioremediation of metal polluted areas makes it necessary to have deep insight into their metal binding properties. The present study reports bioaccumulation of heavy metal Cd by ectomycorrhizal fungus H. cylindrosporum when exposed to its external stress. The concentration of accumulated Cd is directly proportional to the dose of external metal. However, the dry weight plummeted in response to the increasing concentrations of external Cd. The IC50 value observed in this study was at 8.0 µM of Cd. The IC50 for Cd reported for other ectomycorrhizal fungi, Pisolithus tinctorius, Cenococcum geophilum, Paxillus involutus, and Suillus luteus were 89.0, 9.0, 2.2 and 0.4 µM, respectively (Hartley et al. 1997). These results suggested that the ectomycorrhizal fungus H. cylindrosporum is sensitive to Cd.
Since Cd is a sulfhydryl reactive metal with high affinity for the thiols, its bioaccumulation inside the cell results in high demand for the thiol rich compounds. In H. cylindrosporum, the amount of glutathione produced was found to be directly dependent upon the external Cd stress. The amount of total GSH produced increased with increase in metals stress. About 0.85 nmol mg−1 of total GSH was produced per mg of mycelium in response to Cd stress, indicating that GSH biosynthesis is highly inducible by external Cd stress. Increase in GSH levels have also been reported in ectomycorrhizal fungus Paxillus involutus where approximately 1.2 nmol mg−1 GSH was produced in response to 50 ppm Cd stress (Courbot et al. 2004). Further, the burgeoning response of glutathione to heavy metal stress in H. cylindrosporum was justified by studying the expression level of rate limiting Hcγ-GCS gene when exposed to increasing metal concentrations. A significant increase in the expression of Hcγ-GCS gene was observed in response to Cd stress. Similar response has also been reported in various hyper accumulating plants (Pickering et al. 2000; Dhankher et al. 2002). These observations throw light on the fact that the process of GSH biosynthesis triggers immediately on exposure to Cd stress. The functional characterization of Hcγ-GCS gene in Cd hypersensitive S. cerevisiae mutant for γ-glutamylcysteine synthetase (gsh1Δ) further validates the role of this gene in protecting the organism under metal stress.
The sequence analysis of Hcγ-GCS protein showed homology with many reported γGCS proteins from different fungal species. Hcγ-GCS protein showed close relationship with γGCS protein from various basidiomycetes Laccaria bicolor, Agaricus bisporus and Gymnopus luxurians. Most of the conserved domains identified in these fungal γGCS proteins were found to be conserved even in plants, yeasts, parasites and invertebrates. The conserved glycine and cysteine rich motif “IYLDAMGFGMGCCCLQLTFQ” 249–268 amino acid from Lb-γGCS has also been reported to be conserved in invertebrate Ciona intestinalis, Chorispora bungeana, and S. cerevisiae (Ohtake and Yabuuchi 1991; Franchi et al. 2012; Nair et al. 2013). The aminoacid E52, E101, C260, Q264, R309, I319, R435 involved in substrate (l-glutamate) binding site were found to be conserved not only in fungi but also in invertebrates (Franchi et al. 2012). This shows that the catalytic unit of Hcγ-GCS is highly conserved. However the cysteine binding moiety has not been identified in the Hcγ-GCS tertiary structure. The same has also been reported in Leishmania donovani γGCS (Agnihotri et al. 2016).
Conclusion
This is the first report on cloning and characterization of γ-glutamylcysteine synthetase gene isolated from ectomycorrhizal fungus H. cylindrosporum. The gene has been successfully characterized using various molecular and bioinformatic approaches. It was observed that the exposure to Cd causes up-regulation of Hcγ-GCS gene resulting in increased glutathione production. The functional complementation of the Hcγ-GCS in S. cerevisiae mutant further justifies its role in heavy metal tolerance. This study provides a deep insight into the role of GSH biosynthesis in ameliorating heavy metal accumulation and detoxification in ectomycorrhizal fungus H. cylindrosporum, which may hold a potential application in bioremediation of contaminated sites.
References
Agnihotri P, Singh SP, Shakya AK, Pratap JV (2016) Biochemical and biophysical characterization of Leishmania donovani gamma-glutamylcysteine synthetase. Biochem Biophys Rep 8:127–138
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Bellion M, Courbot M, Jacob C, Blaudez D, Chalot M (2006) Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol Lett 254:173–181
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Courbot M, Diez L, Ruotolo R, Chalot M, Leroy P (2004) Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl Environ Microbiol 70:7413–7417
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat Biotechnol 20:1140–1145
Dietz R, Riget F, Cleemann M, Aarkrog A, Johansen P, Hansen JC (2000) Comparison of contaminants from different trophic levels and ecosystems. Sci Total Environ 245:221–231
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Franchi N, Ferro D, Ballarin L, Santovito G (2012) Transcription of genes involved in glutathione biosynthesis in the solitary tunicate Ciona intestinalis exposed to metals. Aquat Toxicol 114:14–22
Galant A, Preuss ML, Cameron J, Jez JM (2011) Plant glutathione biosynthesis: diversity in biochemical regulation and reaction products. Front Plant Sci 2:45
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gay G (1990) Effect of the ectomycorrhizal fungus Hebeloma hiemale on adventitious root formation in derooted Pinus halepensis shoot hypocotyls. Can J Bot 68:1265–1270
Gutiérrez-Escobedo G, Orta-Zavalza E, Castaño I, De Las Peñas A (2013) Role of glutathione in the oxidative stress response in the fungal pathogen Candida glabrata. Curr Genet 59:91–106
Hartley J, Cairney JW, Meharg AA (1997) Do ectomycorrhizal fungi exhibit adaptive tolerance to potentially toxic metals in the environment? Plant Soil 189:303–319
He J, Li H, Ma C, Zhang Y, Polle A, Rennenberg H, Cheng X, Luo ZB (2015) Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in popular. New Phytol 205:240–254
Jentschke G, Goldbold DL (2000) Metal toxicity and ectomycorrhizas. Physiol Plant 109:107–116
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Kalsotra T, Khullar S, Agnihotri R, Reddy MS (2018) Metal induction of two metallothionein genes in the ectomycorrhizal fungus Suillus himalayensis and their role in metal tolerance. Microbiol 64:868–876
Khullar S, Reddy MS (2016) Ectomycorrhizal fungi and its role in metal homeostasis through metallothionein and glutathione mechanisms. Curr Biotechnol 5:1–11
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30:42–59
Melin ELIAS (1953) Physiology of mycorrhizal relations in plants. Ann Rev Plant Physiol 4:325–346
Minet M, Dufour ME, Lacroute F (1992) Complementation of mutants by Arabidopsis thaliana cDNA. Plant J 32:417–422
Nair PM, Park SY, Chung JW, Choi J (2013) Transcriptional regulation of glutathione biosynthesis genes, γ-glutamyl-cysteine ligase and glutathione synthetase in response to cadmium and nonylphenol in Chironomus riparius. Environ Toxicol Pharmacol 36:265–273
Ohtake Y, Yabuuchi S (1991) Molecular cloning of the γ-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast 7:953–961
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122:1171–1178
Pócsi I, Prade RA, Penninckx MJ (2004) Glutathione, altruistic metabolite in fungi. Adv Microb Physiol 49:1–76
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Reddy MS, Prasanna L, Marmeisse R, Fraissinet-Tachet L (2014) Differential expression of metallothioneins in response to heavy metals and their involvement in metal tolerance in the symbiotic basidiomycete Laccaria bicolor. Microbiol 160:2235–2242
Reddy MS, Kour M, Aggarwal S, Ahuja S, Marmeisse R, Fraissinet-Tachet L (2016) Metal induction of a Pisolithus albus metallothionein and its potential involvement in heavy metal tolerance during mycorrhizal symbiosis. Environ Microbiol 18:2446–2454
Ruiz JM, Rivero RM, Romero L (2003) Preliminary studies on the involvement of biosynthesis of cysteine and glutathione concentration in the resistance to B toxicity in sunflower plants. Plant Sci 165:811–817
Sengupta D, Ramesh G, Mudalkar S, Kumar KRR, Kirti PB, Reddy AR (2012) Molecular cloning and characterization of γ-glutamylcysteine synthetase (VrγECS) from roots of Vigna radiata (L.) Wilczek under progressive drought stress and recovery. Plant Mol Biol 30:894–903
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377:369–381
Stearns T, Ma H, Botstein D (1990) Manipulating yeast genome using plasmid vectors. Methods Enzymol 185:280–297
Thorsen M, Lagniel G, Kristiansson E, Junot C, Nerman O, Labarre J, Tamás MJ (2007) Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol Genom 30:35–43
Vido K, Spector D, Lagniel G, Lopez S, Toledano MB, Labarre J (2001) A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J Biol Chem 276:8469–8474
Acknowledgements
Authors are thankful to Department of Biotechnology, Govt. of India for sponsoring the research Project (BT/PR8339/BCE/8/1045/2013) to carry out the present work.
Funding
The authors report no financial interest or benefit arising from the direct application of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khullar, S., Reddy, M.S. Cadmium induced glutathione bioaccumulation mediated by γ-glutamylcysteine synthetase in ectomycorrhizal fungus Hebeloma cylindrosporum. Biometals 32, 101–110 (2019). https://doi.org/10.1007/s10534-018-00164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-00164-2