Abstract
Bacillus anthracis Ser/Thr protein kinase PrkC (BasPrkC) is important for virulence of the bacterium within the host. Homologs of PrkC and its cognate phosphatase PrpC (BasPrpC) are the most conserved mediators of signaling events in diverse bacteria. BasPrkC homolog in Bacillus subtilis regulates critical processes like spore germination and BasPrpC modulates the activity of BasPrkC by dephosphorylation. So far, biochemical and genetic studies have provided important insights into the roles of BasPrkC and BasPrpC; however, regulation of their activities is not known. We studied the regulation of BasPrkC/BasPrpC pair and observed that Zn2+ metal ions can alter their activities. Zn2+ promotes BasPrkC kinase activity while inhibits the BasPrpC phosphatase activity. Concentration of Zn2+ in growing B. anthracis cells was found to vary with growth phase. Zn2+ was found to be lowest in log phase cells while it was highest in spores. This variation in Zn2+ concentration is significant for understanding the antagonistic activities of BasPrkC/BasPrpC pair. Our results also show that BasPrkC activity is modulated by temperature changes and kinase inhibitors. Additionally, we identified Elongation Factor Tu (BasEf-Tu) as a substrate of BasPrkC/BasPrpC pair and assessed the impact of their regulation on BasEf-Tu phosphorylation. Based on these results, we propose Zn2+ as an important regulator of BasPrkC/BasPrpC mediated phosphorylation cascades. Thus, this study reveals additional means by which BasPrkC can be activated leading to autophosphorylation and substrate phosphorylation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ser/Thr and Tyr mediated phosphorylation is one of the most important post-translational events in signaling of bacteria. We had earlier reported that in Bacillus anthracis, the etiological agent of anthrax, loss of key Tyrosine kinase(s) may lead to gain of novel dual specificity protein kinases (phosphorylates Ser/Thr and Tyr) (Arora et al. 2012; Mattoo et al. 2008). In wake of these observations, it can be speculated that Ser/Thr protein kinases (STPKs) of B. anthracis are unique and may be regulated by novel mechanisms, uncommon to other Bacillus or bacterial species. Of the three characterized kinases, two (PrkD and PrkG) were shown to be dual specificity protein kinases (Arora et al. 2012). The third kinase BasPrkC (B. anthracis PrkC) was a bonafide STPK, speculated to be an “infection specific kinase” (Bryant-Hudson et al. 2011).
Bacillus species exhibit a particular bimodal lifestyle alternating between the vegetative and the spore phases (Pilo and Frey 2011). B. anthracis spores infect humans through the respiratory tract and germinate in phagosomes of alveolar macrophages, leading to inhalational anthrax, the most fatal manifestation of the disease (Alvarez et al. 2010). Germinated vegetative cells produce toxins and cause death of the host. Thus, spore germination is a crucial step for the initiation of pathogenesis (Alvarez et al. 2010; Baweja et al. 2008; Khanna and Singh 2001; Singh et al. 1989, 1999; Zaman et al. 2005). In Bacillus subtilis, PrkC senses ‘muropeptides’ as a signal for spore germination (Shah et al. 2008). B. anthracis mutant strains-Bas∆prkC (single gene mutant) and Bas∆prkC∆prpC (double gene mutant with Ser/Thr phosphatase PrpC) were found to be severely defective for growth and virulence in the host as compared to the parental strain (Bryant-Hudson et al. 2011; Shakir et al. 2010), indicating their role in spore germination during infection. BasPrpC inactivates BasPrkC by dephosphorylation, thus ceasing the signals emanating from BasPrkC (Shakir et al. 2010).Therefore, BasPrkC helps in establishing infection and BasPrpC regulates kinase activity and so the balance between the two is crucial to the process.
STPKs have evolved two properties essential for their function: the ability to recognize and phosphorylate their cognate substrates, and sensitive means of regulation (Biondi and Nebreda 2003). PrpC-homologs are usually co-transcribed with PrkC homologs in most bacterial species, and PrpC reverses the effects of PrkC-mediated phosphorylation (Beltramini et al. 2009; Faucher et al. 2008; Jin and Pancholi 2006; Novakova et al. 2005). If they both are co-transcribed and expressed together, then the question remains that how their opposing activities are regulated at the same time. In this study, we have identified Zn2+ metal ion as a common regulator of BasPrkC/BasPrpC, which can affect auto- and trans-activities of these proteins.
Materials and methods
In silico analysis
To generate a structural model, BLAST was performed against pdb structure database using BasPrkCc (1–282 aa) and PrpC (full length) sequences as queries. For BasPrkC, Staphylococcus aureus PknB (PDB id: 4EQM) and for BasPrpC, Streptococcus agalactiae Ser/Thr phosphatase (PDB id: 2PK0)—were found to possess maximum sequence homology and selected as templates (Rakette et al. 2012; Rantanen et al. 2007). Modeller version 9.10 was used to generate 50 models for each protein-BasPrkCc and BasPrpC. The models were corroborated by Verify_3D program (http://nihserver.mbi.ucla.edu/SAVES/).
Bacterial strains and growth conditions
Escherichia coli strain DH5α (Novagen) was used for cloning and BL21 (DE3) (Stratagene) was used for the expression of recombinant proteins. E. coli cells were grown and maintained with constant shaking (220 rpm) at 37 °C in LB broth (Difco) supplemented with 100 μg/ml ampicillin (Sigma) when needed. B. anthracis Sterne strain was grown in LB broth at 37 °C with shaking at 220 rpm. For solid media, LB-Agar (Difco) was used for both E. coli and B. anthracis.
Growth curve of B. anthracis Sterne strain in the presence of ZnCl2
B. anthracis primary culture was inoculated from a single colony and grown till OD600 of 1.0. Then secondary cultures were inoculated from primary culture (1:1,000 dilutions). To study the effect of Zn2+, ZnCl2 (Sigma) was added to the cultures at the concentrations of 1 mM, 5 mM and 10 mM, in duplicates. Absorbance was taken at 600 nm and plotted against time.
Inductively coupled plasma optical emission spectrometry (ICP-OES)
ICP-OES was performed with B. anthracis Sterne strain, starting with spore phase. Spores were prepared from 4 day old cultures, as discussed previously (Setlow and Setlow 1987). Purified spores were heated at 70 °C for 40 min to kill any residual vegetative cells and germination efficiency was estimated. Approximately 1.5 × 109 spores were used for analysis of Zn2+. For analysis of Zn2+ in cells, ~1 × 109 spores were inoculated in 500 ml LB medium, with an initial OD600 of 0.08. Cells were harvested at different phases of growth—early log (2 h old culture, OD600 = 0.2), log (4 h old culture, OD600 = 1.0), stationary (9 h old culture, OD600 = 2.9) and 24 h old culture (OD600 > 3). Harvested cells were washed with de-ionized, autoclaved MilliQ water and dried completely, followed by estimation of dry-weight. Dry cells were resuspended in 1 % HNO3 (Merck) and lysed by sonication for 5 min. Clarified samples were processed for ICP-OES in 6 replicates, as described previously (Francois et al. 2012; Liu et al. 2004). ICP-OES was performed at Shriram Institute for Industrial Research, Delhi, India. The ICP-OES system was calibrated with 1 % HNO3 and standard curve was prepared by different dilutions of Zn2+ standards within the limits of detection (ranging from 0.01 to 0.5 ppm). The emission line used for the Zn2+ analysis was 213.857 nm. Water sample containing 1 % HNO3 was taken as blank, which had very low intensity (approximately 20.813 counts/s) below the detection limit of ICP-OES of Zn2+ in water sample indicating very low amount of the metal.
Cloning and mutagenesis of B. anthracis genes and protein purification
For cloning the full length kinase basprkC (bas3713, 1–657aa), its catalytic domain basprkC c (1–337aa) and phosphatase basprpC (bas3714), the genes were PCR amplified from B. anthracis genomic DNA, using gene specific forward and reverse primers. The resulting PCR products were cloned into the BamHI and XhoI sites of pProEx-HTc vector and/or pGEX-5X-3. The details of primers and plasmids are provided in Table 1. Gene encoding BasEf-Tu (bas0108) was cloned similarly into the vectors pGEX-5X-3 and pProEx-HTc. The clones were confirmed with restriction digestion and DNA sequencing (Biolinkk).
To generate Lysine (Lys40) mutant of BasPrkC, site-directed mutagenesis was carried out using the QuikChange®XL site-directed mutagenesis kit (Stratagene) as per the manufacturer’s instructions, using HTc-BasPrkCc as template. The clones were confirmed with DNA sequencing.
The recombinant plasmids were transformed, overexpressed in E. coli BL-21 (DE3) and the proteins were purified by Ni2+-NTA or Glutathione Sepharose affinity columns (Qiagen) as described (Gupta et al. 2009). The purified proteins were visualized by coomassie stained SDS polyacrylamide gel and the concentrations were estimated by Bradford assay (Bio-Rad).
In vitro kinase assays
In vitro kinase assays of the full length kinase and its catalytic domain (1 μg) were carried out in kinase buffer (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES] [pH 7.2], 5 mM MgCl2 and 5 mM Dithiothreitol [DTT]) containing 2 μCi [γ-32P]ATP (BRIT, India) followed by incubation at 25 °C for 30 min or as indicated. Phosphorylation of BasEf-Tu (5 μg) was also carried out similarly. Reactions were terminated by 5X SDS sample buffer followed by boiling at 100 °C for 5 min. Proteins were separated by SDS-PAGE and analyzed by PhosphorImager (Personal Molecular Imager-PMI, Bio-Rad). For determining ionic requirements of BasPrkCc, in vitro kinase assays were performed as described (using 2 μM BasPrkC), except that kinase buffer contained only 20 mM HEPES [pH 7.2] and 5 mM DTT and various concentrations of divalent cations (MgCl2/MnCl2/ZnCl2/CaCl2/NiCl2) were included additionally. In the in vitro kinase inhibition assays, the inhibitors were added to the indicated concentrations and the reactions were carried out as described previously. The control reactions contained DMSO (solvent in which inhibitors were dissolved), which had no effect on kinase activity. The images were quantitated by PMI software (Bio-Rad).
Protein thermostability assays and calculation of melting temperature
BasPrkCc, BasPrkCfl (full length, 1–657aa) and PknB were desalted using PD10-desalting columns (GE healthcare) and re-dissolved in 20 mM Tris–Cl [pH 7.4]. Thermostability assays were performed in UV spectrophotometer (Cary 100 UV–Vis Spectrophotometer, Agilent Technologies) by measuring the absorbance of proteins (500 nM each) at 280 nm at increasing temperatures (40 °C–95 °C, 0.5 °C/min). Melting temperature was also calculated using CD spectroscopy (Jasco J-815 CD spectrometer) with increasing temperatures (40 °C–90 °C). The melting curve was plotted (absorbance versus temperature) and melting temperature (T m) was calculated at the temperature where 50 % denaturation was observed. Subsequently, fraction of unfolded protein (αunfolded) at any temperature was calculated as described earlier and plotted against temperature (Kumar et al. 2011; Owczarzy 2005). At any point, the fraction of melted peptide, θ is calculated from the standard formula, θ = (A − AL)/(AU − AL), where A, AL, and AU are sample absorbance, absorbance of the lower baseline, and absorbance of the upper baseline, respectively.
To assess the activity of kinases at higher temperatures, in vitro kinase reactions of BasPrkCc were performed at indicated temperatures between 25 °C and 60 °C for 30 min. In another assay, BasPrkCfl and PknB were first incubated at different temperatures—35 °C, 40 °C, 45 °C or 50 °C for 30 min and then the kinase assays were performed at same temperature.
Immunoblotting to identify phosphorylated residues
To detect the phosphorylated residues, immunoblotting with α-pSer and α-pThr antibodies was performed as described previously (Gupta et al. 2009; Arora et al. 2012; Sajid et al. 2011a). The blotting was carried out with primary antibodies α-pSer and α-pThr (Invitrogen) at 1:10,000 dilution and goat anti-rabbit IgG secondary antibodies (Bangalore Genei) (1:10,000) for 1 h each at room temperature. The blots were developed using SuperSignal® West Pico Chemiluminescent Substrate kit (Pierce Protein Research Products) according to manufacturer’s instructions.
Dephosphorylation assays of BasPrkCc and BasEf-Tu using BasPrpC
Phosphorylated BasPrkCc (1 μg) and BasEf-Tu (5 μg) were subjected to dephosphorylation by BasPrpC (1 μg). Dephosphorylation reaction was carried out at 25 °C for increasing time-points up to 30 min. Reactions were terminated by adding 5X SDS sample buffer followed by boiling for 5 min at 100 °C. The samples were resolved on SDS-PAGE and the signals were analyzed by PhosphorImager. The phosphatase activity of BasPrpC was determined by performing pNPP hydrolysis assay. BasPrpC (1 μM) was added to a reaction mixture containing phosphatase assay buffer (50 mM Tris pH 8.0, 5 mM DTT, 40 μM or 4 mM MnCl2, depending on the range of Zn2+) and 10 mM pNPP in a 96-well plate and incubated at 37 °C for indicated time points and absorbance was read at 405 nm (Microplate reader, Bio-Rad). ZnCl2 was added to the reactions, in lower range (1–100 μM) and in higher range (0.1–1 mM), as indicated.
The effect of Zn2+ was studied by first performing the kinase assay for 30 min. To this reaction, different concentrations of ZnCl2 and BasPrpC (1 μg) were added and incubated for additional 30 min (25 °C). Reactions were terminated by 5X SDS sample buffer followed by boiling at 100 °C for 5 min. Proteins were separated by SDS-PAGE and analyzed by PhosphorImager.
Results
Genomic organization and domain architecture of PrkC–PrpC pair in bacteria
BasPrkC and its homologs are known to regulate key processes in different bacteria: spore germination in Bacillus species, antimicrobial resistance and intestinal persistence in Enterococcus faecalis, physiology and cell growth in Mycobacterium and Corynebacterium species (Kristich et al. 2007; Shah et al. 2008; Molle and Kremer 2010; Fiuza et al. 2008). Genomic organization of orthologs of BasPrkC-BasPrpC pair is investigated in diverse gram positive bacteria such as B. subtilis, Clostridium botulinum, Listeria monocytogenes, Lactobacillus lactis, E. faecalis, Streptococcus pneumoniae, S. aureus etc. (Fig. 1a). The orthologs of BasPrkC–BasPrpC show strong conservation in various gram positive bacteria. Our results indicate that BasPrkC–BasPrpC pair is co-transcribed in B. anthracis (data not shown), which is also supported by similar observations of Bryant-Hudson et al. (2011). The homologs of this pair are also known to be co-transcribed in other bacterial species (Debarbouille et al. 2009; Jin and Pancholi 2006; Madec et al. 2002).
Genomic and domain organization of BasPrkC and BasPrpC. a Conservation of BasPrkC/BasPrpC pair in different bacterial species is shown. The kinase gene is indicated by red arrow. The genomic organization was adapted from NCBI. b Diagrammatic representation of domain organization of BasPrkC, showing N-terminal kinase domain and C-terminal PASTA domains, separated by transmembrane region. c Diagrammatic representation of BasPrpC domain organization, a PP2C-family phosphatase. The domain organization was adapted from SMART domain analysis. d PDB view of Ribbon model of BasPrkCc. The model indicates N- and C-terminals and the critical Lys40 residue (encircled). e PDB view of Ribbon model of BasPrpC. The model indicates N- and C-terminals and the critical residues (encircled) as—(1) Asp18 (2) Asp36 (3) Asp194 (4) Asp233. Both the tertiary structure models were derived from the primary sequences of the proteins, using Modeller version 9.10 and corroborated by Verify_3D program. (Color figure online)
BasPrkC and its homologs possess exclusive domain architecture: extracellular C-terminal Penicillin-binding protein And Serine/Threonine kinase Associated (PASTA) domain and intracellular N-terminal catalytic domain (Bryant-Hudson et al. 2011; Ruggiero et al. 2011). BasPrkC has three PASTA domains, oriented back-to-back in the same direction (Fig. 1b). These domains are separated from the intracellular kinase (catalytic) domain via a hydrophobic transmembrane region and a juxtamembrane region. BasPrpC is a cytosolic protein, unlike its Mycobacterium tuberculosis homolog-PstP, which is a membrane protein. BasPrpC belongs to the PP2C-class of metal dependent phosphatases (PPM family) and targets Ser/Thr phosphorylated proteins including PrkC (Chopra et al. 2003; Shakir et al. 2010; Sajid et al. 2011b) (Fig. 1c). To find structural details, we performed homology modeling of BasPrkCc and BasPrpC using primary amino acid sequences, as described in materials and methods. The critical residues of verified BasPrkCc and BasPrpC structures are labeled (Fig. 1d, e).
Regulation of BasPrkC activity
To elucidate the regulatory mechanisms of BasPrkC kinase activity, effects of few known and some unique cofactors were investigated. STPKs require various divalent cations as cofactors for their activity (Arora et al. 2010; Av-Gay et al. 1999; Udo et al. 1997). In line with other bacterial STPKs, BasPrkC was also shown to be active in the presence of Mg2+ and Mn2+ ions, although it was not clear if it requires both ions or either one is sufficient (Shakir et al. 2010). Activity of BasPrkCc (1–337aa, cytosolic domain) was assessed by in vitro kinase assays in presence of several metal ions, which indicated that it can also utilize Zn2+ ion as a cofactor in addition to Mg2+ or Mn2+ for its activity (Fig. 2a). To understand the specific effect of Zn2+ on BasPrkC activity, in vitro kinase assays were performed with increasing Zn2+ concentrations. An increase in phosphorylation was observed with increasing Zn2+ concentrations—from 0.01 to 0.5 mM, with as low as 0.01 mM Zn2+ (approx. 653 ppb) being able to activate BasPrkC in absence of other metal ions (Fig. 2b, Fig. 1 online supplemental resource). We could not observe any changes in BasPrkC activity below the given Zn2+ ion concentrations. This may be due to the lack of activation of BasPrkC at concentration of Zn2+ below 0.01 mM or technical limitation of in vitro kinase assays. BasPrkCc was not active in the presence of Ca2+ and Ni2+, thus confirming the specificity of BasPrkC for selected metal ions. To find the effect of Zn2+ on other B. anthracis kinases, PrkD and PrkG were also probed. There was no effect of Zn2+ on PrkD activity, while the activity of PrkG was reduced (data not shown).
Ionic regulation of BasPrkC. a Activity of BasPrkCc (1 μg each) in the presence of various metal ions (10 mM each). Maximum phosphorylation of BasPrkCc was observed in the presence of Mg2+, which was taken as 100 % and relative phosphorylation was calculated in the presence of other ions (using PMI image analysis software). It is evident from the figure that BasPrkCc can utilize Zn2+ in addition to Mg2+ and Mn2+ for its activation. b Activity of BasPrkCc (2 μM) was assessed with increasing concentrations of Zn2+ in the in vitro kinase assays. The representative coomassie stained SDS polyacrylamide gel images have been shown in Fig. 1 online supplemental resource. Maximum activity (at 0.5 mM Zn2+) was taken as 100 % and relative phosphorylation intensity was calculated. The molar ratio of BasPrkC: Zn2+ is indicated above the corresponding column. The experiments were repeated three times and the error bars represent the standard deviation (SD) of three individual values
Regulation of BasPrpC activity
Ser/Thr phosphatase BasPrpC dephosphorylates and thus inactivates BasPrkC (Shakir et al. 2010). In an earlier study on a homolog of BasPrpC in M. tuberculosis—PstP, we reported the regulation and inhibition of phosphatase activity by Zn2+ (Sajid et al. 2011b). Thus, the effect of Zn2+ on BasPrpC was also analyzed by a biochemical assay using para-nitrophenol phosphate (pNPP). Since BasPrpC requires Mn2+ for its activity, all the inhibition assays were performed in the presence of excessive Mn2+ ions. We found that Zn2+ inhibits BasPrpC activity in the concentration range of 1–100 μM (approx. 65.38–6,538 ppb), in presence of 40 μM Mn2+ (Fig. 3a). On performing assays at a higher concentration of Mn2+ (4 mM), Zn2+ was able to inhibit BasPrpC in range of 0.1 mM to 1.0 mM (Fig. 2 online supplemental resource). Thus, the effective inhibition of BasPrpC by Zn2+ also depends on relative concentration of Mn2+ ions, which is usually higher than Zn2+ in the B. anthracis cells (Tu et al. 2012). To further investigate the overall role of Zn2+ in regulating activity of both proteins—BasPrkC and BasPrpC, we studied the combined effect of Zn2+ and Mn2+ on their activities. Dephosphorylation of BasPrkCc by BasPrpC was proportionately inhibited in the presence of increasing Zn2+ concentrations (Fig. 3b). Thus, the equilibrium of kinase-phosphatase reaction shifts towards the kinase activity in the presence of Zn2+, leading to enhanced phosphorylation. Interestingly, Zn2+ also inhibited B. anthracis growth significantly (Fig. 3c), indicating that it plays an important role in B. anthracis physiology.
Effect of Zn2+ ions and estimation of Zn2+ concentration in B. anthracis cells. a Effect of Zn2+ on the activity of BasPrpC (1 μM) was evaluated by pNPP assay. Zn2+ reduced the activity of phosphatase in a concentration dependent manner. The molar ratio of BasPrpC:Zn2+ is indicated above the corresponding column, with constant Mn2+ (40 μM). As clearly evident, as low as 1 μM Zn2+ inhibited the activity of BasPrpC by 10 %, even in the presence of excess of Mn2+. b Phosphorylation of BasPrkCc was measured in the presence of BasPrpC and increasing concentrations of Zn2+. The phosphorylation on BasPrkCc without BasPrpC and Zn2+ was taken as 100 % and relative phosphorylation was calculated. In all the histograms, each value is average ± SE of three observations. c Growth curve of B. anthracis Sterne strain cells was performed in the absence and presence of Zn2+ ions (1–10 mM). Each value is average ± SE of two observations. d The cellular concentration of total Zn2+ content was estimated in B. anthracis Sterne strain cells, growing at different stages, starting with spores. The estimates are provided in parts per billion of Zn2+ (ppb) per mg of dry weight of the cells. The experiment was performed once in six replicates and the error bars represent the mean SD. value of six replicates. The concentrations were calculated using the Zn2+ standard curve (Fig. 3 online supplemental resource)
Concentration of Zn2+ in B. anthracis cells
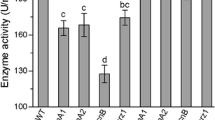
In prokaryotic cells, Zn2+ is present and required in minute quantities (McDevitt et al. 2011). As observed in Fig. 3c, even 1 mM additional Zn2+ in media is toxic to Bacillus cells. To find the role of Zinc in cellular development of B. anthracis, we tried to estimate the concentration of Zn2+ ions inside B. anthracis spores and germinating cells, during different growth phases. We expected Zn2+ to be present in very less quantity in the cells, since it was found to be toxic at higher concentrations. To determine Zn2+ content, we utilized the technique “Inductively Coupled Plasma Optical Emission Spectrometry” (ICP-OES), which is routinely used for determination of trace elements in biological samples (Graham et al. 2009; Tu et al. 2012). Samples were prepared in 1 % HNO3, diluted in de-ionized MilliQ water (although traces of Zn2+ may be present in MilliQ water, which are below the detection limit of ICP-OES, data not shown). In log phase cells (OD600 = 1.0), the concentration of Zn2+ was estimated to be 76.5 ppb/mg dry weight of cells. On comparing the various developmental phases of the cells, viz. spores, germinating spores, stationary phase and late stationary phase cells, the concentration of Zn2+ varied in all the samples (Fig. 3d, Fig. 3 online supplemental resource). Interestingly, spores contained highest amount of Zn2+ (>250 ppb/mg dry wt.). This concentration decreases as the spores started germinating and proceed to subsequent growth phases of culture, ultimately being lowest in log phase cells. Stationary phase cells also had higher Zn2+ content as compared to log phase cells, albeit less than that of spores. Thus, this analysis directly depicts that Zn2+ content of the B. anthracis cells vary according to the growth phase and physiological environments. This variation may proportionately alter the functioning of enzymes that are stimulated or inhibited by cellular Zn2+ concentration (like BasPrkC, BasPrpC, ribosomal proteins, superoxide dismutase or lethal factor).
Effect of temperature on stability and activity of BasPrkC
Bacillus spores are known to withstand high temperatures (Spotts Whitney et al. 2003). Since B. subtilis PrkC is shown to be involved in spore germination process (Shah et al. 2008), we sought to determine the thermostability of BasPrkC (in the temperature range of 40 °C–95 °C). With increase in temperature of the BasPrkC, hyperchromic effect was observed by CD spectroscopy and UV melting assays. BasPrkCc was observed to be very stable protein with a melting temperature (T m) of ~75 °C. The fraction of unfolded protein (αunfolded) at a given temperature was also calculated (Fig. 4a and Fig. 4 online supplemental resource). In addition to thermostability, the enzymatic activity of BasPrkCc at different temperatures was also measured to study its effect on kinase activity. As shown in Fig. 4b, BasPrkCc was active in the temperature range of 25 °C to 50 °C (Fig. 4b and Fig. 5 online supplemental resource). Similar results were observed when BasPrkCc was pre-incubated at 35 °C–50 °C for 30 min and then the kinase assays were performed (data not shown). Validation of these observations was carried out by performing similar assays with BasPrkCfl and PknB, the mycobacterial homolog of BasPrkC. While PknB does not exhibit any activity beyond 40 °C, BasPrkCfl was very active till 45 °C (Fig. 4c).
Effect of temperature and kinase inhibitors on autophosphorylation of BasPrkC a Thermal denaturation of BasPrkCc (500 nM) was measured by CD spectroscopy and the unfolded fraction (αunfolded) at a given temperature was calculated which was then plotted against temperature (40 °C–90 °C). b Autophosphorylation activity of BasPrkCc (1 μg) was determined at different temperatures (25 °C–60 °C) in the in vitro kinase assays. Maximum phosphorylation was observed at 45 °C, which was taken as 100 % and relative phosphorylation was calculated. The corresponding image representations have been shown in Fig. 5 online supplemental resource. c The activity of BasPrkCfl was compared with its close homolog PknB of M. tuberculosis, at various temperatures. The proteins were pre-incubated at indicated temperatures and then used for kinase assays at the same temperatures. Both kinases were active at 35 °C (taken as 100 %). While PknB lost its activity beyond 35 °C, BasPrkC-fl was active till 45 °C. d Activity of BasPrkCc was measured in the presence of kinase inhibitors (0–20 μM). The activity without any added inhibitor was taken as 100 % and relative phosphorylation was calculated. The corresponding representative gel images have been shown in Fig. 6 online supplemental resource. Each value is average ± SE of three observations
Inhibition of BasPrkC activity by protein kinase inhibitors
Activation of BasPrkC is dependent on its autokinase activity, as also known for several other STPKs (Johnson et al. 1996; Bryant-Hudson et al. 2011). In order to understand the mode of kinase regulation, molecules that can suppress BasPrkC autophosphorylation may be very useful. In this regard, the ability of four known kinase inhibitors was studied to assess BasPrkCc inhibition. The inhibitors used in this study were: staurosporine, which acts against Protein kinase C (Tamaoki et al. 1986); KN-93, an antagonist of Ca2+/CaM kinase II (Rokolya and Singer 2000); PKR inhibitor, an antagonist of RNA dependent protein kinases (Ruvolo et al. 2008); and Genistein, which suppresses the activities of Tyrosine kinases (Arora et al. 2012; Akiyama et al. 1987). Both staurosporine and PKR inhibitor decreased the autophosphorylation of BasPrkCc (Fig. 4d and Fig. 6 online supplemental resource), while no inhibition was noticed with KN-93 and Genistein (data not shown).
Reversible phosphorylation of BasEf-Tu, a common substrate of BasPrkC/BasPrpC
The effect of BasPrkC regulators was also investigated on substrate phosphorylation efficiency. In M. tuberculosis, PknB regulates protein synthesis by phosphorylating an essential protein, elongation factor Tu (Ef-Tu) (Sajid et al. 2011a). Ef-Tu homologs are also known to be phosphorylated by PrkC homologs in other bacterial pathogens (Sajid et al. 2011a). BasEf-Tu was indeed phosphorylated with BasPrkCc in a time-dependent manner for 30 min and relative phosphorylation was calculated (Fig. 5a, b and Fig. 7a online supplemental resource). For dephosphorylation assay, BasEf-Tu—previously phosphorylated by BasPrkCc, was incubated with BasPrpC. Time-dependent dephosphorylation of BasEf-Tu was observed, confirming the reversibility of phenomena (Fig. 5c and Fig. 7b online supplemental material). Immunoblot analysis of BasEf-Tu using α-pSer and α-pThr antibodies indicated that both Ser and Thr residues were phosphorylated (Fig. 8 online supplemental resource). M. tuberculosis STPK PknJ (Arora et al. 2010) and BasPrpC served as positive and negative controls, respectively. Thus, BasEf-Tu is phosphorylated by BasPrkCc on both Ser and Thr residues and is dephosphorylated by BasPrpC.
Reversible phosphorylation of BasEf-Tu. a In vitro kinase assays illustrating BasPrkCc-mediated phosphorylation of BasEf-Tu. As shown in the autoradiogram, no phosphorylation was observed when kinase dead mutant (BasPrkC K40Mc ) or substrate alone was used. b BasEf-Tu (5 μg) was phosphorylated by BasPrkCc (1 μg) in a time-dependent manner, up to 30 min. The phosphorylation signal after 30 min was taken as 100 % and relative phosphorylation was estimated using PMI image analysis software. c BasEf-Tu (5 μg) was phosphorylated by BasPrkCc (1 μg) and then dephosphorylated by BasPrpC (1 μg) in a time-dependent manner. Dephosphorylation was evaluated by measuring signal intensity (zero minute taken as 100 % phosphorylation). Relative phosphorylation was measured after incubating with BasPrpC for various time-points. The representative gel images have been shown in Fig. 7 online supplemental resource. Each value is average ± SE of three observations
Regulation of BasEf-Tu phosphorylation by BasPrkC
We assessed the roles of several factors that can affect kinase activity such as Zn2+, temperature and kinase inhibitors on BasPrkC mediated reversible phosphorylation of BasEf-Tu. BasEf-Tu was first phosphorylated by BasPrkCc using standard in vitro kinase assay and then dephosphorylated by BasPrpC in the presence of Zn2+. As observed in Fig. 6a, Zn2+ partially protected the dephosphorylation of BasEf-Tu from BasPrpC, ultimately enhancing the extent of phosphorylation (Fig. 6a).
Effect of BasPrkC regulators on its phosphotransfer potential. a Effect of Zn2+ on the phosphorylation of BasEf-Tu was studied. BasEf-Tu phosphorylated by BasPrkCc without BasPrpC and Zn2+ was taken as 100 % and relative phosphorylation was calculated when BasPrpC was added, with or without Zn2+. b Relative phosphorylation of BasEf-Tu by BasPrkCc was studied in the presence of staurosporine and PKR inhibitor. The values are average ± SE of three individual observations
Furthermore, BasEf-Tu was phosphorylated by BasPrkCc at different temperatures. Phosphorylation of BasEf-Tu was observed till 45 °C, but a comprehensible increase in phosphorylation levels could not be detected due to precipitation of BasEf-Tu at higher temperature. Subsequently, the effect of BasPrkC antagonists was also assessed on its phosphotransfer potential and relative phosphorylation of BasEf-Tu was calculated. The phosphorylation of BasEf-Tu was reduced by almost 70 % in the presence of 20 μM staurosporine and PKR inhibitor (Fig. 6b).
Discussion
Phosphorylation is a proficient mechanism for regulation of many proteins, catalyzed by their cognate kinases. Phosphoproteomic studies of diverse bacterial species have revealed that in each of the proteome, more than 70 proteins are phosphorylated on Ser/Thr residues (Macek et al. 2007; Macek et al. 2008; Sun et al. 2010; Prisic et al. 2010). In B. subtilis, muropeptide-activated PrkC was shown to phosphorylate translation elongation factor Ef-G that acts as a germination signal for spores (Shah et al. 2008; Shah and Dworkin 2010), thus highlighting the importance of phosphorylation in physiology of Bacillus. While B. subtilis PrkC has broad role in other cellular processes, its B. anthracis homolog is important for virulence (Bryant-Hudson et al. 2011; Shakir et al. 2010); however, regulation of its activity is not well understood. The present study reports various ways by which BasPrkC can be activated and can phosphorylate its substrates.
STPKs are widely known to utilize metal ions as cofactors for their activity and metal-ion binding can induce conformational changes in STPKs, resulting in altered activity profiles (Newton 2001). Structural data on BasPrkC homologs—PknB of M. tuberculosis and Protein Kinase A of eukaryotes, indicates the presence of two binding sites of divalent ions coordinated to ATP (Ortiz-Lombardia et al. 2003). Amongst the three divalent cations tested in this study (Mg2+, Mn2+ and Zn2+), Mg2+ is thought to be the preferred cofactor of STPKs while Mn2+ is a coveted cofactor of Tyrosine kinases (Herberg et al. 1999; Swarup et al. 1984; Waas and Dalby 2003).
BasPrkC and BasPrpC homologs are found to be conserved in several other pathogenic and non-pathogenic bacteria. Autophosphorylation of kinase and extent of phosphotransfer depends on the relative activities of both BasPrkC and BasPrpC. Both BasPrkC and BasPrpC require Mg2+/Mn2+ for their activities, whereas Zn2+ has alternate effects on both these proteins and overall favors the phosphorylation events. Thus, variation in Zn2+ concentration can regulate the phosphorylation levels of substrates. Zn2+ mediated regulation of phosphorylation in B. anthracis could be very important as the BasPrkC/BasPrpC pair is co-expressed in the cell (Bryant-Hudson et al. 2011) and no regulatory mechanism is known, which could control their antagonistic activities simultaneously. In absence of such control, BasPrpC would inactivate BasPrkC and dephosphorylate its substrates. Our results indicate that presence of Zn2+ can inhibit dephosphorylation by BasPrpC while promoting the BasPrkC kinase activity. Interestingly in B. subtilis, PrkC is thought to regulate protein translation by phosphorylating Ef-G and CpgA during spore germination while Zn2+ is required by multiple ribosomal proteins (Gabriel and Helmann 2009; Nanamiya et al. 2004; Nanamiya et al. 2006; Pompeo et al. 2012). The inverse regulation of kinase/phosphatase pair, coupled by Zn2+ and the BasPrkC dependent phosphorylation of BasEf-Tu may correlate the regulation of translational efficiency to Zn2+ availability. Interestingly, the role of Zn2+ in eukaryotes is well known in extracellular signal transduction, secondary messenger metabolism and protein phosphorylation (Beyersmann and Haase 2001). Increase in extracellular Zn2+ enhances Tyrosine phosphorylation and Mitogen activated protein kinase activity in murine fibroblasts (Beyersmann and Haase 2001). In a recent study, activity of receptor Tyrosine phosphatase-β is shown to be inhibited by picomolar concentrations of free Zn2+ ions using a more sensitive fluorescence based Tyrosine phosphatase assay (Wilson et al. 2012). The role of Zn2+ is most crucial for eukaryotic Protein kinase C where it not only modulates the kinase activity but also its localization (Beyersmann and Haase 2001; Lehel et al. 1995; Zalewski et al. 1991).
A number of studies have highlighted the importance of Zn2+ in Bacillus physiology (Hantke 2005; Natori et al. 2007). The anthrax lethal toxin contains Zinc-dependent metalloprotease lethal factor and Zinc is crucial for its biological role and toxicity (Klimpel et al. 1994; Singh et al. 1999). For B. subtilis, even 5 mM (0.004 %) colloidal suspension of ZnO nanoparticles could inhibit more than 95 % of growth (Jones et al. 2008). In a comparative study of various bacilli, vegetative cells of B. anthracis Sterne strain were inactivated by AgION (a silver- and Zinc-containing zeolite) after 2 h of contact with antimicrobial-coated stainless steel (Galeano et al. 2003). In our analysis, we observed that B. anthracis growth is inhibited in the presence of ZnCl2. The concentration of Zn2+ in the Bacillus cells varies with developmental phases. Spores have high Zn2+ content, which start to decrease as spores start germinating and continue till cells reach log phase. This indicates that actively growing cells in log phase do not require high Zn2+, rather presence of additional 1 mM Zn2+ in culture inhibited growth (Fig. 3c). The cellular levels of Zn2+ again start rising as cells reach stationary phase. Taken together, these studies further substantiate the importance of BasPrkC and BasPrpC co-regulation, which respond to Zn2+ reciprocally and may help in maintaining cellular development. In our previous study, we showed that the expression of prkC is highest in late log phase, indicating its presence in stationary and sporulation phases (Arora et al. 2012). Since the role of BasPrkC has been previously indicated in sporulation and spore germination (Madec et al. 2002; Shah et al. 2008), the high content of Zn2+ in spores may have a correlation with BasPrkC activation.
However, certain precaution is needed while extrapolating these results as the Zinc concentration tested for in vitro assays was quite high as compared to the concentration observed in vivo. Additionally, our results depict the total concentration of Zinc in the cell, rather than free Zn2+ ions, which might actually be lesser. The term “free” here refers to specify the labile Zinc that is freely available for binding by newly synthesized Zinc metalloproteins. Attempts to measure free Zinc in bacteria have indicated a wide range of concentrations (10−9–10−15 M) (Eide 2006; Outten and O’Halloran 2001; Maret 2013; Haase et al. 2013; Wang nd Fierke 2013). Since living cells are exposed to variations from its surrounding environment, the levels of free Zn2+ may also vary in different environmental conditions—in culture or inside the host cells. This level is usually maintained by Zinc transporters and Zinc uptake regulators, which are responsible for balancing the Zinc content from being limiting or being too high to be toxic (Eide 2006). Thus, further studies are needed to validate how the oscillating levels of free Zn2+ in the cells may help in precise regulation of BasPrkC and BasPrpC activities.
Bacteria are known to survive in harsh conditions like extreme temperatures. In such conditions, the ability of macromolecules such as proteins to adapt and remain functional is critical for bacterial survival (Miller et al. 2010). In case of B. anthracis, spores are known to withstand high temperatures (Spotts Whitney et al. 2003). Thus, it is interesting to know that BasPrkC is the first bacterial STPK to exhibit thermostability at 75 °C, and is able to phosphorylate itself and its substrates even at 45 °C. The factors enhancing the thermostable nature of BasPrkC, as suggested for other thermostable proteins, could be greater hydrophobicity, better packing, increased hydrogen bonding and salt bridges (Kumar et al. 2000). Hence, further structural analyses are required to substantiate the thermostable nature of BasPrkC.
In the case of eukaryotic cellular signaling, it has been observed that pharmacological approaches could be helpful in identifying the substrates and their role in cellular responses (Lizcano and Alessi 2002). In view of the role of BasPrkC in spore germination, compounds that can retard BasPrkC activation may be projected as the basic class of compounds against anthrax. We evaluated several classes of inhibitors known to be active against different protein kinases. Staurosporine was previously shown to reduce spore germination in B. subtilis at a very low concentration (10 pM) by interfering with BasPrkC (Shah et al. 2008). Staurosporine and PKR inhibitors are diverse compounds that target ATP binding site in STPKs (Jammi et al. 2003; Ruegg and Burgess 1989; Li et al. 2009). We observed that 20 μM of staurosporine and PKR inhibitor substantially reduced the autophosphorylation ability of BasPrkCc in vitro, although phosphorylation of BasEf-Tu by BasPrkCc was not inhibited completely.
Ef-Tu was found to be phosphorylated by PrkC-homologs in B. subtilis (Levine et al. 2006), E. coli (Lippmann et al. 1993), Thermus thermophilus (Lippmann et al. 1993), L. monocytogenes (Archambaud et al. 2005) and M. tuberculosis (Sajid et al. 2011a), and thus could be proposed as a conserved substrate. Therefore, we performed phosphorylation and dephosphorylation assays to validate that it is also a common substrate of BasPrkC/BasPrpC in B. anthracis. Further, immunoblotting experiments were also carried out to confirm the phosphorylation of BasEf-Tu on Ser and Thr residues.
After identification of BasEf-Tu as BasPrkC substrate, we tried to find the factors that can affect the kinase activity and substrate phosphorylation. Zn2+ modulates the catalytic efficiencies of BasPrkC and BasPrpC, which in turn affects phosphorylation of BasEf-Tu, ultimately leading to enhanced phosphorylation with increasing Zn2+ concentrations. Additionally, the modulators of BasPrkC—temperature and kinase inhibitors—also affect the BasEf-Tu phosphorylation.
Thus, although the role of these two co-expressing and yet antagonistic enzymes has been discussed in different bacterial species, it was still not clear that how both enzymes work together efficiently. It appears that phosphorylation level of BasPrkC may be controlled through Zn2+ ions as it inhibits the activity of BasPrpC and activates BasPrkC.
References
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5595
Alvarez Z, Lee K, bel-Santos E (2010) Testing nucleoside analogues as inhibitors of Bacillus anthracis spore germination in vitro and in macrophage cell culture. Antimicrob Agents Chemother 54:5329–5336
Archambaud C, Gouin E, Pizarro-Cerda J, Cossart P, Dussurget O (2005) Translation elongation factor EF-Tu is a target for Stp, a serine-threonine phosphatase involved in virulence of Listeria monocytogenes. Mol Microbiol 56:383–396
Arora G, Sajid A, Gupta M, Bhaduri A, Kumar P, Basu-Modak S, Singh Y (2010) Understanding the role of PknJ in Mycobacterium tuberculosis: biochemical characterization and identification of novel substrate pyruvate kinase A. PLoS ONE 5:e10772
Arora G, Sajid A, Arulanandh MD, Singhal A, Mattoo AR, Pomerantsev AP, Leppla SH, Maiti S, Singh Y (2012) Unveiling the novel dual-specificity protein kinases in Bacillus anthracis: identification of the first prokaryotic DYRK-like kinase. J Biol Chem 287:26749–26763
Av-Gay Y, Jamil S, Drews SJ (1999) Expression and characterization of the Mycobacterium tuberculosis serine/threonine protein kinase PknB. Infect Immun 67:5676–5682
Baweja RB, Zaman MS, Mattoo AR, Sharma K, Tripathi V, Aggarwal A, Dubey GP, Kurupati RK, Ganguli M, Chaudhury NK, Sen S, Das TK, Gade WN, Singh Y (2008) Properties of Bacillus anthracis spores prepared under various environmental conditions. Arch Microbiol 189:71–79
Beltramini AM, Mukhopadhyay CD, Pancholi V (2009) Modulation of cell wall structure and antimicrobial susceptibility by a Staphylococcus aureus eukaryote-like serine/threonine kinase and phosphatase. Infect Immun 77:1406–1416
Beyersmann D, Haase H (2001) Functions of Zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14:331–341
Biondi RM, Nebreda AR (2003) Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J 372:1–13
Bryant-Hudson KM, Shakir SM, Ballard JD (2011) Autoregulatory characteristics of a Bacillus anthracis serine/threonine kinase. J Bacteriol 193:1833–1842
Chopra P, Singh B, Singh R, Vohra R, Koul A, Meena LS, Koduri H, Ghildiyal M, Deol P, Das TK, Tyagi AK, Singh Y (2003) Phosphoprotein phosphatase of Mycobacterium tuberculosis dephosphorylates serine-threonine kinases PknA and PknB. Biochem Biophys Res Commun 311:112–120
Debarbouille M, Dramsi S, Dussurget O, Nahori MA, Vaganay E, Jouvion G, Cozzone A, Msadek T, Duclos B (2009) Characterization of a serine/threonine kinase involved in virulence of Staphylococcus aureus. J Bacteriol 191:4070–4081
Eide DJ (2006) Zinc transporters and the cellular trafficking of Zinc. Biochim Biophys Acta 1763:711–722
Faucher SP, Viau C, Gros PP, Daigle F, Le MH (2008) The prpZ gene cluster encoding eukaryotic-type Ser/Thr protein kinases and phosphatases is repressed by oxidative stress and involved in Salmonella enterica serovar Typhi survival in human macrophages. FEMS Microbiol Lett 281:160–166
Fiuza M, Canova MJ, Zanella-Cleon I, Becchi M, Cozzone AJ, Mateos LM, Kremer L, Gil JA, Molle V (2008) From the characterization of the four serine/threonine protein kinases (PknA/B/G/L) of Corynebacterium glutamicum toward the role of PknA and PknB in cell division. J Biol Chem 283:18099–18112
Francois F, Lombard C, Guigner JM, Soreau P, Brian-Jaisson F, Martino G, Vandervennet M, Garcia D, Molinier AL, Pignol D, Peduzzi J, Zirah S, Rebuffat S (2012) Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl Environ Microbiol 78:1097–1106
Gabriel SE, Helmann JD (2009) Contributions of Zur-controlled ribosomal proteins to growth under Zinc starvation conditions. J Bacteriol 191:6116–6122
Galeano B, Korff E, Nicholson WL (2003) Inactivation of vegetative cells, but not spores, of Bacillus anthracis, B. cereus, and B. subtilis on stainless steel surfaces coated with an antimicrobial silver- and Zinc-containing zeolite formulation. Appl Environ Microbiol 69:4329–4331
Graham AI, Hunt S, Stokes SL, Bramall N, Bunch J, Cox AG, McLeod CW, Poole RK (2009) Severe Zinc depletion of Escherichia coli: roles for high affinity Zinc binding by ZinT, Zinc transport and Zinc-independent proteins. J Biol Chem 284:18377–18389
Gupta M, Sajid A, Arora G, Tandon V, Singh Y (2009) Forkhead-associated domain-containing protein Rv0019c and polyketide-associated protein PapA5, from substrates of serine/threonine protein kinase PknB to interacting proteins of Mycobacterium tuberculosis. J Biol Chem 284:34723–34734
Haase H, Hebel S, Engelhardt G, Rink L (2013) Application of Zinpyr-1 for the investigation of zinc signals in Escherichia coli. Biometals 26:167–177
Hantke K (2005) Bacterial Zinc uptake and regulators. Curr Opin Microbiol 8:196–202
Herberg FW, Doyle ML, Cox S, Taylor SS (1999) Dissection of the nucleotide and metal-phosphate binding sites in cAMP-dependent protein kinase. Biochemistry 38:6352–6360
Jammi NV, Whitby LR, Beal PA (2003) Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun 308:50–57
Jin H, Pancholi V (2006) Identification and biochemical characterization of a eukaryotic-type serine/threonine kinase and its cognate phosphatase in Streptococcus pyogenes: their biological functions and substrate identification. J Mol Biol 357:1351–1372
Johnson LN, Noble ME, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85:149–158
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Khanna H, Singh Y (2001) War against anthrax. Mol Med 7:795–796
Klimpel KR, Arora N, Leppla SH (1994) Anthrax toxin lethal factor contains a Zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol Microbiol 13:1093–1100
Kristich CJ, Wells CL, Dunny GM (2007) A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci USA 104:3508–3513
Kumar S, Tsai CJ, Nussinov R (2000) Factors enhancing protein thermostability. Protein Eng 13:179–191
Kumar S, Bose D, Suryawanshi H, Sabharwal H, Mapa K, Maiti S (2011) Specificity of RSG-1.2 peptide binding to RRE-IIB RNA element of HIV-1 over Rev peptide is mainly enthalpic in origin. PLoS ONE 6:e23300
Lehel C, Olah Z, Jakab G, Anderson WB (1995) Protein kinase C epsilon is localized to the Golgi via its Zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci USA 92:1406–1410
Levine A, Vannier F, Absalon C, Kuhn L, Jackson P, Scrivener E, Labas V, Vinh J, Courtney P, Garin J, Seror SJ (2006) Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes. Proteomics 6:2157–2173
Li S, Duan P, You G (2009) Regulation of human organic anion transporter 1 by ANG II: involvement of protein kinase Calpha. Am J Physiol Endocrinol Metab 296:E378–E383
Lippmann C, Lindschau C, Vijgenboom E, Schroder W, Bosch L, Erdmann VA (1993) Prokaryotic elongation factor Tu is phosphorylated in vivo. J Biol Chem 268:601–607
Liu H, Bergman NH, Thomason B, Shallom S, Hazen A, Crossno J, Rasko DA, Ravel J, Read TD, Peterson SN, Yates J III, Hanna PC (2004) Formation and composition of the Bacillus anthracis endospore. J Bacteriol 186:164–178
Lizcano JM, Alessi DR (2002) The insulin signalling pathway. Curr Biol 12:R236–R238
Macek B, Mijakovic I, Olsen JV, Gnad F, Kumar C, Jensen PR, Mann M (2007) The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol Cell Proteomics 6:697–707
Macek B, Gnad F, Soufi B, Kumar C, Olsen JV, Mijakovic I, Mann M (2008) Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol Cell Proteomics 7:299–307
Madec E, Laszkiewicz A, Iwanicki A, Obuchowski M, Seror S (2002) Characterization of a membrane-linked Ser/Thr protein kinase in Bacillus subtilis, implicated in developmental processes. Mol Microbiol 46:571–586
Maret W (2013) Inhibitory zinc sites in enzymes. Biometals 26:197–204
Mattoo AR, Arora A, Maiti S, Singh Y (2008) Identification, characterization and activation mechanism of a tyrosine kinase of Bacillus anthracis. FEBS J 275:6237–6247
McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC (2011) A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357
Miller C, Davlieva M, Wilson C, White KI, Counago R, Wu G, Myers JC, Wittung-Stafshede P, Shamoo Y (2010) Experimental evolution of adenylate kinase reveals contrasting strategies toward protein thermostability. Biophys J 99:887–896
Molle V, Kremer L (2010) Division and cell envelope regulation by Ser/Thr phosphorylation: Mycobacterium shows the way. Mol Microbiol 75:1064–1077
Nanamiya H, Akanuma G, Natori Y, Murayama R, Kosono S, Kudo T, Kobayashi K, Ogasawara N, Park SM, Ochi K, Kawamura F (2004) Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol Microbiol 52:273–283
Nanamiya H, Kawamura F, Kosono S (2006) Proteomic study of the Bacillus subtilis ribosome: finding of zinc-dependent replacement for ribosomal protein L31 paralogues. J Gen Appl Microbiol 52:249–258
Natori Y, Nanamiya H, Akanuma G, Kosono S, Kudo T, Ochi K, Kawamura F (2007) A fail-safe system for the ribosome under Zinc-limiting conditions in Bacillus subtilis. Mol Microbiol 63:294–307
Newton AC (2001) Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101:2353–2364
Novakova L, Saskova L, Pallova P, Janecek J, Novotna J, Ulrych A, Echenique J, Trombe MC, Branny P (2005) Characterization of a eukaryotic type serine/threonine protein kinase and protein phosphatase of Streptococcus pneumoniae and identification of kinase substrates. FEBS J 272:1243–1254
Ortiz-Lombardia M, Pompeo F, Boitel B, Alzari PM (2003) Crystal structure of the catalytic domain of the PknB serine/threonine kinase from Mycobacterium tuberculosis. J Biol Chem 278:13094–13100
Outten CE, O’Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492
Owczarzy R (2005) Melting temperatures of nucleic acids: discrepancies in analysis. Biophys Chem 117:207–215
Pilo P, Frey J (2011) Bacillus anthracis: molecular taxonomy, population genetics, phylogeny and patho-evolution. Infect Genet Evol 11:1218–1224
Pompeo F, Freton C, Wicker-Planquart C, Grangeasse C, Jault JM, Galinier A (2012) Phosphorylation of CpgA protein enhances both its GTPase activity and its affinity for ribosome and is crucial for Bacillus subtilis growth and morphology. J Biol Chem 287:20830–20838
Prisic S, Dankwa S, Schwartz D, Chou MF, Locasale JW, Kang CM, Bemis G, Church GM, Steen H, Husson RN (2010) Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc Natl Acad Sci USA 107:7521–7526
Rakette S, Donat S, Ohlsen K, Stehle T (2012) Structural analysis of Staphylococcus aureus serine/threonine kinase PknB. PLoS ONE 7:e39136
Rantanen MK, Lehtio L, Rajagopal L, Rubens CE, Goldman A (2007) Structure of Streptococcus agalactiae serine/threonine phosphatase. The subdomain conformation is coupled to the binding of a third metal ion. FEBS J 274:3128–3137
Rokolya A, Singer HA (2000) Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278:C537–C545
Ruegg UT, Burgess GM (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 10:218–220
Ruggiero A, Squeglia F, Marasco D, Marchetti R, Molinaro A, Berisio R (2011) X-ray structural studies of the entire extracellular region of the serine/threonine kinase PrkC from Staphylococcus aureus. Biochem J 435:33–41
Ruvolo VR, Kurinna SM, Karanjeet KB, Schuster TF, Martelli AM, McCubrey JA, Ruvolo PP (2008) PKR regulates B56(alpha)-mediated BCL2 phosphatase activity in acute lymphoblastic leukemia-derived REH cells. J Biol Chem 283:35474–35485
Sajid A, Arora G, Gupta M, Singhal A, Chakraborty K, Nandicoori VK, Singh Y (2011a) Interaction of Mycobacterium tuberculosis elongation factor Tu with GTP is regulated by phosphorylation. J Bacteriol 193:5347–5358
Sajid A, Arora G, Gupta M, Upadhyay S, Nandicoori VK, Singh Y (2011b) Phosphorylation of Mycobacterium tuberculosis Ser/Thr phosphatase by PknA and PknB. PLoS ONE 6:e17871
Setlow B, Setlow P (1987) Thymine-containing dimers as well as spore photoproducts are found in ultraviolet-irradiated Bacillus subtilis spores that lack small acid-soluble proteins. Proc Natl Acad Sci USA 84:421–423
Shah IM, Dworkin J (2010) Induction and regulation of a secreted peptidoglycan hydrolase by a membrane Ser/Thr kinase that detects muropeptides. Mol Microbiol 75:1232–1243
Shah IM, Laaberki MH, Popham DL, Dworkin J (2008) A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486–496
Shakir SM, Bryant KM, Larabee JL, Hamm EE, Lovchik J, Lyons CR, Ballard JD (2010) Regulatory interactions of a virulence-associated serine/threonine phosphatase-kinase pair in Bacillus anthracis. J Bacteriol 192:400–409
Singh Y, Leppla SH, Bhatnagar R, Friedlander AM (1989) Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J Biol Chem 264:11099–11102
Singh Y, Klimpel KR, Goel S, Swain PK, Leppla SH (1999) Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect Immun 67:1853–1859
Spotts Whitney EA, Beatty ME, Taylor TH Jr, Weyant R, Sobel J, Arduino MJ, Ashford DA (2003) Inactivation of Bacillus anthracis spores. Emerg Infect Dis 9:623–627
Sun X, Ge F, Xiao CL, Yin XF, Ge R, Zhang LH, He QY (2010) Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. J Proteome Res 9:275–282
Swarup G, Dasgupta JD, Garbers DL (1984) Tyrosine-specific protein kinases of normal tissues. Adv Enzyme Regul 22:267–288
Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F (1986) Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun 135:397–402
Tu WY, Pohl S, Gray J, Robinson NJ, Harwood CR, Waldron KJ (2012) Cellular iron distribution in Bacillus anthracis. J Bacteriol 194:932–940
Udo H, Inouye M, Inouye S (1997) Biochemical characterization of Pkn2, a protein Ser/Thr kinase from Myxococcus xanthus, a Gram-negative developmental bacterium. FEBS Lett 400:188–192
Waas WF, Dalby KN (2003) Physiological concentrations of divalent magnesium ion activate the serine/threonine specific protein kinase ERK2. Biochemistry 42:2960–2970
Wang D, Fierke CA (2013) The BaeSR regulon is involved in defense against zinc toxicity in E. coli. Metallomics 5:372–383
Wilson M, Hogstrand C, Maret W (2012) Picomolar concentrations of free Zinc(II) ions regulate receptor protein tyrosine phosphatase beta activity. J Biol Chem 287:9322–9326
Zalewski PD, Forbes IJ, Giannakis C, Betts WH (1991) Regulation of protein kinase C by Zn(2+)-dependent interaction with actin. Biochem Int 24:1103–1110
Zaman MS, Goyal A, Dubey GP, Gupta PK, Chandra H, Das TK, Ganguli M, Singh Y (2005) Imaging and analysis of Bacillus anthracis spore germination. Microsc Res Tech 66:307–311
Acknowledgments
Financial support to the work was provided by Council of Scientific and Industrial Research (CSIR)—funded project BSC-0104. We thank Dr. V. C. Kalia (CSIR—Institute of Genomics and Integrative Biology, Delhi) for critical reading of the manuscript. We also thank Jayadev Joshi and Mritunjay Saxena for help in protein modeling and docking studies. We are grateful to Dr. Vinay Kumar Jain, Shriram Institute for Industrial Research, New Delhi, India, for help in performing ICP-OES experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gunjan Arora and Andaleeb Sajid have contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arora, G., Sajid, A., Arulanandh, M.D. et al. Zinc regulates the activity of kinase-phosphatase pair (BasPrkC/BasPrpC) in Bacillus anthracis . Biometals 26, 715–730 (2013). https://doi.org/10.1007/s10534-013-9646-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9646-y