Abstract
Nicotianamine (NA) is among the most studied plant metal chelators. A large body of evidence supports its crucial role for Fe distribution in plants and as a precursor of phytosiderophore synthesis in grasses. NA forms stable complexes in vitro not only with Fe(II) and Fe(III) but also with various other divalent metal cations including Zn(II). Early observations indicated a possible contribution of NA to Zn trafficking in plants. Numerous studies on transgenic monocot and dicot plants with modulated NA levels have since then reported Zn accumulation phenotypes. NAS genes were shown to represent promising targets for biofortification efforts. For instance, NA was found to bind Zn in rice grains in a form bioavailable for humans. Recently, additional strong support for the existence of Zn–NA complexes in planta has been obtained in rice, Arabidopsis thaliana and the Zn hyperaccumulating plant A. halleri. We review the evidence for a role of NA in the intercellular and long-distance transport of Zn in plants and discuss open questions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zn needs to be distributed to a myriad of Zn-requiring proteins. During evolution Zn has with increasing availability in an oxidizing atmosphere been recruited for more and more functions in biological systems (Dupont et al. 2006). This is reflected by the estimated rise in the proportion of Zn-binding proteins from 5–6 % of the proteome in bacteria and archaea to about 9 % in eukaryotes (Andreini et al. 2006). Zn is a cofactor for a large number of enzymes in all six enzyme commission (EC) classes, most prominently among hydrolases (EC3) (Andreini et al. 2008). More than 300 enzyme families are Zn-dependent (Coleman 1992). The increase in the relative number of Zn-proteins in eukaryotes is largely attributable to an expanded usage of Zn in regulatory proteins such as transcription factors. This inspired the hypothesis that the bioavailability of Zn was a limiting factor in the evolution of eukaryotes with their higher demand for DNA-binding proteins (Dupont et al. 2010).
Low-molecular-weight chelators are essential for the necessary intra- and inter-cellular trafficking of Zn. In accordance with the Irving–Williams series (Irving and Williams 1948) Zn is strongly preferred over other micronutrients except copper for interaction with N-, O- and S-containing organic ligands (Colvin et al. 2010). Thus, unwanted interactions of Zn with proteins and other components of biological systems need to be suppressed through tight control over the availability of free intracellular Zn. For eukaryotic cells, results from a multitude of experimental approaches converged in the estimate that cytosolic labile Zn lies in the picomolar range (Colvin et al. 2010), meaning essentially the absence of free Zn ions and a sharp contrast to the cellular Zn quota of 0.1–0.5 mM, which the large number of Zn-requiring sites translates into (Eide 2006). Effective buffering maintains free cytosolic Zn within the narrow range that ensures sufficient supply to Zn proteins while preventing unspecific interactions. Zn buffering as well as “Zn muffling”, i.e. the modulation of non-steady state changes in cytosolic free Zn ions (Colvin et al. 2010), are dependent not only on the function of Zn-binding proteins and Zn transporters but also on high-affinity low-molecular-weight chelators. In mammalian cells glutathione (GSH) takes part in cytosolic Zn buffering and muffling (Hogstrand et al. 2009).
A second function of low-molecular-weight chelators in Zn homeostasis is the facilitation of long-distance transport. In plants, Zn is taken up by roots from the surrounding soil. Following uptake, which is possibly assisted by chelator-dependent mobilization, Zn has to reach vascular tissues for the translocation to Zn sites in leaves, stems, flowers and seeds. Xylem and phloem have to be loaded and unloaded. Furthermore, there is exchange between these tissues. Besides GSH plant cells synthesize other Zn chelators including phytochelatins (PCs), GSH-derived metal binding peptides, and nicotianamine (NA) (Sinclair and Krämer 2012). The latter has been mostly investigated in the context of Fe nutrition (for reviews see Curie et al. 2009; Scholz et al. 1992; Schuler and Bauer 2011). Here we discuss the evidence for an important role of NA in plant Zn homeostasis, with emphasis on most recent findings.
Discovery and prevalence of the metal chelator nicotianamine
NA was first isolated as a new amino acid from leaves of tobacco (Noma et al. 1971). Its structure was determined following isolation from beech nuts (Kristensen and Larsen 1974). A little later NA was found to represent the “normalizing factor” that restores wild-type Fe distribution in leaves of the tomato mutant chloronerva (Budesinsky et al. 1980). A large body of literature has since then established NA as a major metal chelator in plants. Guided by the obvious intercostal chlorosis phenotype of the chloronerva mutant, which is due to the mislocalization of Fe, NA has been ascertained as a crucial factor for the trafficking of Fe in plants (Curie et al. 2009; Schuler and Bauer 2011; Stephan et al. 1996). Early investigations had indicated that NA is present not only in all vascular plants, but also in mosses and certain fungi such as basidiomycetes (Rudolph et al. 1985). Thus, it was hypothesized that NA function goes beyond long-distance transport of Fe. In grasses NA serves in addition as precursor for the synthesis of mugineic acid (MA) phytosiderophores (Shojima et al. 1990).
The enzyme responsible for the condensation of three molecules S-adenosyl-methionine (SAM) to form NA, nicotianamine synthase (NAS), was purified from barley (Herbik et al. 1999; Higuchi et al. 1999). In parallel, the gene defective in chloronerva was isolated and found to encode an NAS (Ling et al. 1999). With this it became possible to infer the distribution of NA in nature from genomic data. For instance, a functional NAS was found in the ascomycete Neurospora crassa (Trampczynska et al. 2006). Based on currently available sequence data we can see that NAS genes are apparently ubiquitous among higher plants. In addition they are found in mosses (Physcomitrella patens) but not in algae. Thus, one could speculate that NAS genes were important for the evolution of land plants. However, the Selaginella moellendorffii genome (www.phytozome.net/selaginella) apparently does not carry an NAS gene. Outside the plant kingdom NAS genes are found in archaea, bacteria and ascomycetes (Fig. 1). All these organisms appear to possess only a single copy while in higher plants small gene families arose. Interestingly, they remained intron-less.
Phylogenetic tree of NAS proteins in representative prokaryotes and eukaryotes. Phylogenetic analysis was performed by the distance-based neighbor-joining method with CLUSTAL W. The tree was visualized with NJPLOT software. Aligned sequences (amino acid residues 33–276 of AtNAS1) were used to generate the phylogenetic tree. The scale bar of 0.1 is equal to 10 % sequence divergence. The accession numbers of the sequences are shown in parenthesis: Methanobacterium sp. (KQ53444.1), Methanothermobacter thermautotrophicus (WP_010876313.1), delta proteobacterium NaphS2 (WP_006421039.1), Bacillus halodurans (WP_010898230.1), Xanthomonas albilineans (WP_012916945.1), Geobacillus thermoglucosidasius (YP_004588391.1), Chaetomium globosum (EAQ85681.1), Neurospora crassa (XP_958379.1), Gaeumannomyces graminis (EJT72278.1), Magnaporthe oryzae (XP_003719353.1), Physcomitrella patens (EDQ52909.1), OsNAS1 (BAF11809.1), OsNAS2 (BAF11808.1), OsNAS3 (BAF22621.1), SbNAS1 (C5WR27), SbNAS2 (C5X6C8), SbNAS3 (Sb01g037480), HvNAS1 (BAA74580.1), HvNAS2 (BAA74582.1), HvNAS3 (BAA74581.1), HvNAS4 (BAA74583.1), HvNAS5-1 (AB011267.1), HvNAS6 (BAA74586.1), HvNAS7 (BAA74587.1), HvNAS8 (AAD32650.1), HvNAS9 (AAD32651.1), AtNAS1 (AED90808.1), AtNAS2 (AED96719.1), AtNAS3 (AEE28417.1), AtNAS4 (AEE33394.1), AhNAS1 (AFH08365.1), AhNAS2 (AFH08366.1), AhNAS3 (CAE45015.1), AhNAS4 (CCD74500.1), LjNAS1 (BAH22562.1), LjNAS2 (BAH22563.1), LjNAS3 (chr3.LjT36L22.160.r2.a), VvNAS1 (F6I4S7), VvNAS2 (A5BHX9). Os: Oryza sativa (rice), Sb: Sorghum bicolor (sorghum), Hv: Hordeum vulgare ssp. vulgare (barley), At: Arabidopsis thaliana, Ah: Arabidopsis halleri, Lj: Lotus japonicus (a wild legume), Vv: Vitis vinifera (grape)
NA as a metal chelator
Starting with the discovery that NA rescues the chloronerva mutant phenotype, metal chelating capacities of NA were investigated in vitro. The first reports demonstrated the formation of stable complexes with Fe(III) and Cu(II) (Budesinsky et al. 1980). Subsequent more extensive studies determined the following log stability constants for complexes with divalent transition metal cations: Mn(II), 8.8; Fe(II), 12.1/12.8; Zn(II), 14.6/15.4; Ni(II), 16.1; Cu(II), 18.6 (Benes et al. 1983) (the second value for Fe(II) and Zn(II) was later reported by Anderegg and Ripperger 1989). At pH > 6 these complexes were calculated to exist practically undissociated in aqueous solution suggesting their possible occurrence in the cytosol and the phloem (Stephan et al. 1996). Unequivocal detection of NA-metal complexes by direct infusion electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) was more recently used to study the nature and stability of NA complexes with the physiologically relevant metal cations across a pH range of 2.5–8.5 (Rellan-Alvarez et al. 2008). Mn(II)–NA, Fe(II)–NA, Fe(III)–NA, Zn(II)–NA, Ni(II)–NA and Cu(II)–NA were all completely formed at alkaline pH. Differences were apparent around neutral and at acidic pH. For instance, while Mn(II)-NA only started to form at pH > 6.2, Fe(II)–NA and Zn(II)–NA were completely formed at pH 6.2. Ni(II)–NA and Cu(II)–NA were detected even at pH values of 4 and lower. Interestingly, in metal-exchange experiments at acidic (5.5) and slightly alkaline pH (7.5) to approximate conditions in xylem and phloem, respectively, addition of Zn(II) to Fe(II)–NA complexes resulted in effective competition yet a stronger formation of Zn(II)–NA under acidic conditions. Possibly this reflects pH effects on the kinetics of the exchange reaction. Taken together these speciation data suggest that in planta Zn(II)–NA complexes can exist in cytosol and phloem as well as in the xylem (Rellan-Alvarez et al. 2008).
Early observations indicating a role of NA in Zn chelation
Phytosiderophores of the MA type are derived from and structurally similar to NA. Therefore, observations implicating phytosiderophores in Zn chelation can be interpreted as early indirect evidence for an involvement also of NA in plant Zn trafficking. Indeed it was found that wheat and other grasses show enhanced release of phytosiderophores under Zn deficiency (Cakmak et al. 1994). Moreover, variation in this process correlated with Zn efficiency among wheat genotypes suggesting a contribution of phytosiderophores to Zn mobilization in the soil (Cakmak et al. 1996). Correspondingly, it was later found that transcript abundance of NAS and other MA biosynthesis genes is higher in barley roots under Zn-deplete conditions. The contribution of MA to Zn adsorption from soil particles could be confirmed by directly demonstrating Zn–MA uptake through barley roots (Suzuki et al. 2006). Zn–phytosiderophore uptake had previously been shown for maize (von Wiren et al. 1996). In contrast, the contribution of MAs to Zn nutrition in rice appears to lie rather in the facilitation of Zn translocation (Bashir et al. 2012). Secretion of MAs by roots of Zn deficient rice plants is reduced while MA synthesis is transcriptionally activated. Furthermore, Zn–deoxymugineic acid (DMA) enhances Zn root-to-shoot translocation rather than root Zn uptake (Suzuki et al. 2008).
When the accumulation of Zn and other micronutrients was studied in wild-type tomato and chloronerva, only small effects were seen with the exception of a strongly reduced root-to-shoot translocation of Cu (Pich et al. 1994). Shoot Zn levels appeared largely unaffected by the presence or absence of NA. However, roots of chloronerva plants accumulated more Zn than wild type.
Modulations of plant NA concentrations and their effects on Zn distribution
Cloning of NAS genes (and other MA biosynthesis genes) enabled the engineering of NA levels, i.e. the generation (i) of NA-deficient plants other than chloronerva, and (ii) of NA-overproducing plants. Tobacco plants free of detectable NA were obtained via the overexpression of a barley NA aminotransferase (NAAT), which catalyzes the conversion of NA to an intermediate in MA biosynthesis (Takahashi et al. 2003). NAAT overexpressors phenocopied the interveinal chlorosis of the chloronerva mutant concomitant with aberrant distribution of Fe in leaves. Leaf concentrations were significantly reduced for Fe, Cu and Zn. Conversely, HvNAS-overexpressing tobacco plants accumulated more Fe and Zn in leaves, flowers and seeds. These findings led the authors to speculate about a role of NA in intracellular Zn transfer (Takahashi et al. 2003). Enhanced leaf Zn accumulation was independently confirmed in 35::AtNAS plants (Douchkov et al. 2005). Additional experiments with HvNAS-overexpressing tobacco plants grown on serpentine soil revealed stronger Zn accumulation in stems and seeds (Kim et al. 2005).
Several studies were performed with rice plants overexpressing NAS genes. They were motivated by the concept of biofortification (Mayer et al. 2008; Palmgren et al. 2008; Zhao and McGrath 2009). The micronutrient intake of a large proportion of the world’s population is too low. Fe and Zn deficiency in humans are widespread and among the most prevalent deficiencies. According to WHO estimates about two billion people are affected by insufficient Zn intake and Zn deficiency accounts for ca. 2.9 % of worldwide loss of healthy life years (WHO 2002). Thus, the potential health benefits of increasing the micronutrient density of major crops such as cereals are huge.
Activation-tagged rice plants with elevated expression of OsNAS3 or OsNAS2 and correspondingly higher NA concentrations were found to accumulate more Zn in leaves and seeds (Lee et al. 2009, 2011). Increases were about twofold for OsNAS3 lines and nearly threefold for OsNAS2 lines specifically in the endosperm. These effects were attributed to higher NA levels and enhanced phytosiderophore secretion. Speciation analysis demonstrated the existence of a new pool of low-molecular-weight Zn species in seeds of OsNAS2-overexpressing rice plants with NA and DMA being the dominant ligands. Importantly, Zn chelated by NA and DMA is more readily bioavailable after consumption as was demonstrated in feeding studies with Zn-deficient mice (Lee et al. 2011). Thus, engineering NA synthesis in cereals has great Zn (and Fe) biofortification potential.
OsNAS3-overexpressing rice plants accumulated more Fe in their roots, leaves and seeds, too (Lee et al. 2009). Comparable observations were reported for rice lines overexpressing A. thaliana NAS1 plus the Fe storage protein ferritin. Zn and Fe brown grain concentrations were 1.5–2-fold elevated, respectively (Wirth et al. 2009). The combination of ferritin and NAS overexpression with that of a Fe–NA transporter (OsYSL12) yielded field-harvested rice grain with 1.6-fold higher Zn concentrations (Masuda et al. 2012). When the association between NA levels and Zn accumulation was analyzed in grains across large populations of wild-type and transgenic rice lines overexpressing one of the three rice NAS genes, a clear positive correlation was found (Johnson et al. 2011). Increases in polished grains due to elevated Fe and Zn accumulation in the endosperm again demonstrated considerable biofortification promise.
Recent evidence for the contribution of NA to Zn trafficking in plants
The findings for NAS-overexpressing rice plants strongly suggest a contribution of Zn–NA complexes to the mobility and storage of Zn. Indeed, a speciation analysis of rice phloem sap via size-exclusion and anion-exchange chromatography separated Zn and Fe fractions. The respective co-eluting ligands were identified by ESI-TOF-MS as NA for Zn and DMA for Fe (Nishiyama et al. 2012).
Using a similar approach, Zn–NA complexes were detected also in roots of the Zn hyperaccumulator A. halleri (Deinlein et al. 2012). About 15 plant taxa possess the remarkable ability to accumulate Zn in their aboveground tissues to levels >100-fold higher than normally found in plants—or in fact any other organism in nature (Krämer 2010). A series of comparative transcriptome studies showed that in A. halleri and the other hyperaccumulating model species Noccaea caerulescens several metal homeostasis genes are constitutively higher expressed when compared to the non-hyperaccumulating relative A. thaliana, leading to the hypothesis that hyperaccumulation evolved through alterations in the regulation of metal transporters and metal chelator synthesis (Hanikenne and Nouet 2011; Krämer 2010; Verbruggen et al. 2009). Among the genes showing the largest expression differences between A. halleri and A. thaliana are NAS genes (Becher et al. 2004; Talke et al. 2006; Weber et al. 2004). Similar results were reported for N. caerulescens (van de Mortel et al. 2006). Constitutively high AhNAS2 transcript levels in A. halleri roots are associated with elevated NA concentrations (Weber et al. 2004). This is a species-wide trait as shown by analyses of A. halleri populations from natural sites in Germany and Poland that exhibit different levels of soil Zn ranging from non-contaminated to heavily contaminated (Deinlein et al. 2012). The low-molecular-weight Zn fraction extractable from A. halleri roots co-elutes with NA, GSH and PCs. Suppression of AhNAS2 expression by RNAi resulted in reduced root NA concentrations which correlated with a significant reduction of root-to-shoot Zn translocation (Deinlein et al. 2012). Size exclusion chromatography coupled to ICP-MS indicated a shift in Zn buffering from NA to thiols in AhNAS2-suppressed plants. Cultivation on native A. halleri soils demonstrated a contribution of root NA to Zn mobility. Root NA concentrations correlated positively with leaf Zn accumulation on non-contaminated soil (Fig. 2). Thus, AhNAS2 is besides AhHMA4 (Hanikenne et al. 2008) the second Zn hyperaccumulation factor identified to date.
Root NA concentrations are positively correlated with leaf Zn accumulation in the Zn hyperaccumulator A. halleri. AhNAS2 is strongly overexpressed in A. halleri roots and concomitantly NA concentrations are higher relative to A. thaliana. A set of AhNAS2-RNAi lines differing in the degree of AhNAS2 suppression was generated and characterized. Shown here are data derived from experiments with these lines (Deinlein et al. 2012). NA concentrations of wild type and RNAi plants were determined in hydroponic culture. Plants were cultivated on native A. halleri soil from a non-contaminated site. Zn and Fe accumulation in leaves was determined. While Fe levels are not affected by root NA concentrations, Zn accumulation is reduced in AhNAS2-suppressed plants indicating a contribution of root NA to root-to-shoot translocation of Zn even under these near-natural conditions
Careful analysis of an A. thaliana nas mutant and the identification of a new component influencing cytosolic NA availability provided further insights. The genome of A. thaliana contains four NAS isoforms that originated from a single ancestral NAS gene. AtNAS1 and AtNAS4 are expressed both in roots and in shoots whereas AtNAS2 is exclusively expressed in root tissue and AtNAS3 only in leaves (Klatte et al. 2009). Through a series of crosses between T-DNA insertion lines and their offspring a collection of double, triple and finally quadruple mutants was generated. Line nas4x-2 is completely devoid of NA and showed severe symptoms such as leaf interveinal chlorosis and sterility (Schuler et al. 2012). Zn concentrations of leaves and flowers were reduced compared to wild type plants. This is consistent with the role of NA in root-to-shoot translocation of Zn in A. halleri. Transcript abundance of ZIP4, an established Zn deficiency marker, was higher in young and mature leaves as well as in flowers of nas4x-2 plants. Sterility was demonstrated to be caused by a lack of Fe and Zn, possibly because NA-mediated delivery of Fe and Zn to the growing pollen tube is defective (Schuler et al. 2012).
Zinc-induced Facilitator 1 (ZIF1) is a vacuolar membrane protein of the major facilitator superfamily. It was originally identified in a forward genetic screen as being important for A. thaliana basal Zn tolerance (Haydon and Cobbett 2007). More recently it was shown to mediate vacuolar sequestration of NA (Haydon et al. 2012). Yeast experiments indicated that ZIF is not able to transport Zn–NA complexes or Zn alone since no rescue of mutants defective in vacuolar sequestration of Zn was achieved by co-expressing ZIF1 and an NAS gene. In plants overexpressing ZIF1 the ratio between cytosolic and vacuolar NA was shifted towards compartmentalization in the vacuole. As a consequence, more Zn was trapped in root cell vacuoles resulting in reduced root-to-shoot transport of Zn and the induction of transcriptional Zn deficiency responses in shoots. Thus, cytosolic NA was again demonstrated to enhance Zn mobility in the root symplasm and translocation to the shoot.
Open questions
Taken together there is now convincing cumulative evidence for a major role of NA as a Zn ligand in plants. The findings derived from the modulation of NA levels in tobacco, rice, A. thaliana and the Zn hyperaccumulator A. halleri converge in the hypothesis that NA significantly contributes to intercellular and long-distance transport of Zn. However, the exact mechanisms of NA-mediated Zn trafficking and sequestration are far from understood.
The in planta existence of Zn–NA complexes at the neutral to slightly alkaline pH of the cytosol and the phloem is now well-documented. Do these complexes exist at the acidic pH of the vacuole and the xylem as well? In vitro data (Rellan-Alvarez et al. 2008) and the sequestration of NA and Zn in root vacuoles of ZIF-overexpressing A. thaliana plants (Haydon et al. 2012) support this assumption.
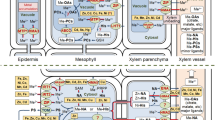
NA was demonstrated to mediate root-to-shoot translocation of Zn. Most likely this effect is associated with the facilitation of xylem loading. Zn buffering by cytosolic NA can suppress sequestration in root vacuoles and thereby enhance symplastic mobility of NA. Unknown is whether this occurs in all cells of the root or only in specific cell types (Fig. 3). In fact it appears highly likely that NA availability for cytosolic Zn buffering is tightly controlled in a cell-specific manner. For instance, ZIF1 expression—and thereby NA sequestration—is controlled by the transcription factor POPEYE, which is thought to mediate a pericycle-specific Fe deficiency response (Long et al. 2010). Cellular resolution of the analyses will be required to dissect the network of metal chelator and transporter activities that ensures homeostasis of metals such as Zn, Fe, Cu, or Mn, whose trafficking pathways are at least partly shared.
Possible mechanisms underlying the facilitation of Zn root-to-shoot translocation by NA. Shown is a schematic cross-section drawn after a micrograph of an A. halleri root (Hanikenne et al. 2008). Three different scenarios are illustrated. The black arrows indicate NA-dependent symplastic mobility of Zn either across the whole root (Hanikenne and Nouet 2011) or only in specific cell types such as the endodermis and pericycle. The grey symbol represents loading of either Zn–NA complexes or NA into the xylem
Does NA play in addition a direct role in the loading of the xylem, for instance via the interaction with Zn efflux systems such as HMA4, the main component of xylem loading in A. halleri (Hanikenne et al. 2008)? Is NA loaded into the xylem as a binding partner for Zn (Fig. 3)? To date, only Ni–NA complexes have been detected in xylem sap (Mari et al. 2006). Connected with these questions is a possible crosstalk between low-molecular-weight metal chelators. Compensatory up-regulation of synthesis was clearly demonstrated for citrate and NA to maintain Fe transport when deficiency of one of the two chelators occurs (Schuler et al. 2012). Data for A. halleri plants with reduced root NA indicated an up-regulation of thiol Zn chelation, suggesting the possibility of a similar crosstalk between Zn ligands (Deinlein et al. 2012).
Transport of NA and Zn–NA across the plasma membrane and organellar membranes is only partially understood. Transporters with homology to ZIF1 are present in rice. ENA1 mediates NA efflux when expressed in Xenopus oocytes (Nozoye et al. 2011) and has recently been depicted as an NA efflux transporter with biofortification potential in rice (Schroeder et al. 2013). NA efflux from xylem parenchyma cells into the xylem could be important if NA has a direct role in xylem translocation of Zn.
Candidates for the transport of Zn–NA complexes are proteins of the YSL family (Curie et al. 2009). However, it is not yet clear if they accept Zn–NA complexes as substrates. In rice, YSL proteins are considered likely candidates for Zn–NA and Zn–DMA transport but direct evidence is lacking (Bashir et al. 2012). The same applies to dicot plants (Sinclair and Krämer 2012).
With respect to the role of NA in Zn hyperaccumulation it will be important to ask whether stronger NAS expression is sufficient to explain elevated NA accumulation. Alternatively, the synthesis of the precursor SAM might be regulated differently, too. SAM synthetases are among the hyperaccumulation candidate genes in A. halleri (Talke et al. 2006). Also, it will be interesting to investigate the evolutionary history of NAS genes. Many metal homeostasis genes in hyperaccumulators show copy number expansion (Talke et al. 2006).
References
Anderegg G, Ripperger H (1989) The normalizing factor for the tomato mutant chloronerva. 29. Correlation between metal–complex formation and biological activity of nicotianamine analogs. J Chem Soc Chem Commun 10:647–650
Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the three domains of life. J Proteome Res 5:3173–3178
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Bashir K, Ishimaru Y, Nishizawa NK (2012) Molecular mechanisms of zinc uptake and translocation in rice. Plant Soil 361:189–201
Becher M, Talke IN, Krall L, Krämer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37:251–268
Benes I, Schreiber K, Ripperger H, Kircheiss A (1983) Metal complex formation by nicotianamine, a possible phytosiderophore. Experientia 39:261–262
Budesinsky M, Budzikiewicz H, Prochazka Z, Ripperger H, Romer A, Scholz G, Schreiber K (1980) The normalizing factor for the tomato mutant chloroerva. 10. Nicotianamine, a possible phytosiderophore of general occurrence. Phytochemistry 19:2295–2297
Cakmak I, Gulut K, Marschner H, Graham R (1994) Effect of zinc and iron-deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency. J Plant Nutr 17:1–17
Cakmak I, Sari N, Marschner H, Ekiz H, Kalayci M, Yilmaz A, Braun HJ (1996) Phytosiderophore release in bread and durum wheat genotypes differing in zinc efficiency. Plant Soil 180:183–189
Coleman JE (1992) Zinc proteins–enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem 61:897–946
Colvin RA, Holmes WR, Fontaine CP, Maret W (2010) Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2:306–317
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Jean M, Misson J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Deinlein U, Weber M, Schmidt H, Rensch S, Trampczynska A, Hansen TH, Husted S, Schjoerring JK, Talke IN, Krämer U, Clemens S (2012) Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 24:708–723
Douchkov D, Gryczka C, Stephan UW, Hell R, Bäumlein H (2005) Ectopic expression of nicotianamine synthase genes results in improved iron accumulation and increased nickel tolerance in transgenic tobacco. Plant Cell Environ 28:365–374
Dupont C, Yang S, Palenik B, Bourne P (2006) Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc Natl Acad Sci USA 103:17822–17827
Dupont C, Butcher A, Valas R, Bourne P, Caetano-Anolles G (2010) History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc Natl Acad Sci USA 107:10567–10572
Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763:711–722
Hanikenne M, Nouet C (2011) Metal hyperaccumulation and hypertolerance: a model for plant evolutionary genomics. Curr Opin Plant Biol 14:252–259
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U (2008) Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453:391–395
Haydon MJ, Cobbett CS (2007) A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol 143:1705–1719
Haydon MJ, Kawachi M, Wirtz M, Hillmer S, Hell R, Krämer U (2012) Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 24:724–737
Herbik A, Koch G, Mock HP, Douchkov D, Czihal A, Thielmann J, Stephan UW, Bäumlein H (1999) Isolation, characterization and cDNA cloning of nicotianamine synthase from barley. A key enzyme for iron homeostasis in plants. Eur J Biochem 265:231–239
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Hogstrand C, Kille P, Nicholson RI, Taylor KM (2009) Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med 15:101–111
Irving H, Williams R (1948) Order of stability of metal complexes. Nature 162:746–747
Johnson AAT, Kyriacou B, Callahan DL, Carruthers L, Stangoulis J, Lombi E, Tester M (2011) Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 6:e24476
Kim S, Takahashi M, Higuchi K, Tsunoda K, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2005) Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol 46:1809–1818
Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P (2009) The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol 150:257–271
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Kristensen I, Larsen PO (1974) Azetidine-2-carboxylic acid derivatives from seeds of Fagus silvatica L. and a revised structure for nicotianamine. Phytochemistry 13:2791–2798
Lee S, Jeon US, Lee SJ, Kim YK, Persson DP, Husted S, Schjorring JK, Kakei Y, Masuda H, Nishizawa NK, An G (2009) Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci USA 106:22014–22019
Lee S, Persson DP, Hansen TH, Husted S, Schjoerring JK, Kim Y-S, Jeon US, Kim Y-K, Kakei Y, Masuda H, Nishizawa NK, An G (2011) Bio-available zinc in rice seeds is increased by activation tagging of nicotianamine synthase. Plant Biotechnol J 9:865–873
Ling HQ, Koch G, Bäumlein H, Ganal MW (1999) Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proc Natl Acad Sci USA 96:7098–7103
Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN (2010) The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22:2219–2236
Mari S, Gendre D, Pianelli K, Ouerdane L, Lobinski R, Briat JF, Lebrun M, Czernic P (2006) Root-to-shoot long-distance circulation of nicotianamine and nicotianamine-nickel chelates in the metal hyperaccumulator Thlaspi caerulescens. J Exp Bot 57:4111–4122
Masuda H, Ishimaru Y, Aung MS, Kobayashi T, Kakei Y, Takahashi M, Higuchi K, Nakanishi H, Nishizawa NK (2012) Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci Rep 2:543
Mayer JE, Pfeiffer WH, Beyer P (2008) Biofortified crops to alleviate micronutrient malnutrition. Curr Opin Plant Biol 11:166–170
Nishiyama R, Kato M, Nagata S, Yanagisawa S, Yoneyama T (2012) Identification of Zn-nicotianamine and Fe-2′-deoxymugineic acid in the phloem saps from rice plants (Oryza sativa L.). Plant Cell Physiol 53:381–390
Noma M, Noguchi M, Tamaki E (1971) New amino acid, nicotianamine, from tobacco leaves. Tetrahedron Lett 12:2017–2020
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011) Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 286:5446–5454
Palmgren MG, Clemens S, Williams LE, Krämer U, Borg S, Schjoerring JK, Sanders D (2008) Zinc biofortification of cereals: problems and solutions. Trends Plant Sci 13:464–473
Pich A, Scholz G, Stephan UW (1994) Iron-dependent changes of heavy-metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of 2 tomato genotypes—nicotianamine as possible copper translocator. Plant Soil 165:189–196
Rellan-Alvarez R, Abadia J, Alvarez-Fernandez A (2008) Formation of metal-nicotianamine complexes as affected by pH, ligand exchange with citrate and metal exchange. A study by electrospray ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 22:1553–1562
Rudolph A, Becker R, Scholz G, Prochazka Z, Toman J, Macek T, Herout V (1985) The occurrence of the amino-acid nicotianamine in plants and microorganisms—a reinvestigation. Biochem Physiol Pflanz 180:557–563
Scholz G, Becker R, Pich A, Stephan UW (1992) Nicotianamine—a common constituent of strategy-I and strategy-II of iron acquisition by plants—a review. J Plant Nutr 15:1647–1665
Schroeder JI, Delhaize E, Frommer WB, Guerinot ML, Harrison MJ, Herrera-Estrella L, Horie T, Kochian LV, Munns R, Nishizawa NK et al (2013) Using membrane transporters to improve crops for sustainable food production. Nature 497:60–66
Schuler M, Bauer P (2011) Heavy metals need assistance: the contribution of nicotianamine to metal circulation throughout the plant and the Arabidopsis NAS gene family. Front Plant Sci 2:69
Schuler M, Rellán-Álvarez R, Fink-Straube C, Abadía J, Bauer P (2012) Nicotianamine functions in the phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. Plant Cell 24:2380–2400
Shojima S, Nishizawa N, Fushiya S, Nozoe S, Irifune T, Mori S (1990) Biosynthesis of phytosiderophores—in vitro biosynthesis of 2′-deoxymugineic acid from l-methionine and nicotianamine. Plant Physiol 93:1497–1503
Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823:1553–1567
Stephan UW, Schmidke I, Stephan VW, Scholz G (1996) The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. Biometals 9:84–90
Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S et al (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48:85–97
Suzuki M, Tsukamoto T, Inoue H, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2008) Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol Biol 66:609–617
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003) Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15:1263–1280
Talke IN, Hanikenne M, Krämer U (2006) Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol 142:148–167
Trampczynska A, Böttcher C, Clemens S (2006) The transition metal chelator nicotianamine is synthesized by filamentous fungi. FEBS Lett 580:3173–3178
van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MGM (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142:1127–1147
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
von Wiren N, Marschner H, Römheld V (1996) Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol 111:1119–1125
Weber M, Harada E, Vess C, von Roepenack-Lahaye E, Clemens S (2004) Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J 37:269–281
WHO (2002) The world health report 2002. World Health Organization, Geneva (http://www.who.int/whr/2002/)
Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S, Tohge T, Fernie AR, Gunther D, Gruissem W et al (2009) Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J 7:631–644
Zhao FJ, McGrath SP (2009) Biofortification and phytoremediation. Curr Opin Plant Biol 12:373–380
Acknowledgments
Work in the authors’ laboratory is supported by the Deutsche Forschungsgemeinschaft. U.D. and S.U. gratefully acknowledge postdoctoral fellowships from the Deutsche Akademische Austauschdienst and the Japanese Society for the Promotion of Science, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clemens, S., Deinlein, U., Ahmadi, H. et al. Nicotianamine is a major player in plant Zn homeostasis. Biometals 26, 623–632 (2013). https://doi.org/10.1007/s10534-013-9643-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9643-1