Abstract
Copper is both an essential nutrient and a toxic element able to catalyze free radicals formation which damage lipids and proteins. Although the available copper redox species in aerobic environment is Cu(II), proteins that participate in metal homeostasis use Cu(I). With isolated Escherichia coli membranes, we have previously shown that electron flow through the respiratory chain promotes cupric ions reduction by NADH dehydrogenase-2 and quinones. Here, we determined Cu(II)-reductase activity by whole cells using strains deficient in these respiratory chain components. Measurements were done by the appearance of Cu(I) in the supernatants of cells exposed to sub-lethal Cu(II) concentrations. In the absence of quinones, the Cu(II)-reduction rate decreased ~70% in respect to the wild-type strain, while this diminution was about 85% in a strain lacking both NDH-2 and quinones. The decrease was ~10% in the absence of only NDH-2. In addition, we observed that quinone deficient strains failed to grow in media containing either excess or deficiency of copper, as we have described for NDH-2 deficient mutants. Thus, the Cu(II)-reduction by E. coli intact cells is mainly due to quinones and to a lesser extent to NDH-2, in a quinone-independent way. To our knowledge, this is the first in vivo demonstration of the involvement of E. coli respiratory components in the Cu(II)-reductase activity which contributes to the metal homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper is an essential nutrient required for many biochemical functions, mainly as a cofactor of several enzymes (Linder 1991). However, copper is a toxic element able to catalyze free radicals formation that produce the alteration of lipids and proteins without causing DNA damage in bacteria (Gutteridge and Halliwell 2000; Chillappagari et al. 2010; Macomber et al. 2007). For those reasons, the viability of the cells depends on a tight regulation of copper level. The mechanisms involved in copper homeostasis exist in all cells, but they are not totally elucidated (Rensing and Grass 2003; Thiele 2003; Solioz and Stoyanov 2003). Most of the proteins involved in copper metabolism bind Cu(I), the biological form of copper that is transported (Fan and Rosen 2002; Solioz and Stoyanov 2003). This may be the primary reason why cells are endowed with copper reductase activity (Solioz and Stoyanov 2003). For instance, copper uptake in Saccharomyces cerevisiae requires the plasma membrane reductases, Fre1 and Fre2, which are also involved in iron uptake (Georgatsou et al. 1997; Hassett and Kosman 1995).
In E. coli some relevant enzymes require a copper cofactor, like the membrane-bound cytochrome C oxidase, the terminal enzyme of the electron transport chain, or the copper zinc superoxide dismutase, localized in periplasm (Minagawa et al. 1992; Benov et al. 1995). It is likely that only a small amount of free copper exists in the cytoplasm but it is not necessarily the same for the periplasm (Macomber et al. 2007; Vulpe and Packman 1995; Bagai et al. 2008), where a great deal of copper homeostasis occurs. The efflux system CusCBA transports copper from periplasm to outside of the cell. Additionally, copper is bound to the small periplasmic protein CusF, and Cu(I) is oxidized by the multicopper oxidase CueO into the less toxic form Cu(II) (Singh et al. 2004; Rensing and Grass 2003). The Cu(I) is exported from cytoplasm by the ATPase CopA, which together with CueO is under the regulatory control of CueR (Petersen and Moller 2000; Rensing et al. 2000; Stoyanov et al. 2001; Outten et al. 2000). It is unclear how periplasmic copper enters the cytoplasm and when and where it is reduced to Cu(I) (Solioz and Stoyanov 2003; Helbig et al. 2008).
Respiratory chain components of E. coli are localized in the inner membrane (Ingledew and Poole 1984). The aerobic respiratory chain is formed by several dehydrogenases (NADH dehydrogenases, d-lactate dehydrogenase, succinate dehydrogenase, etc.), quinones (mainly ubiquinone-8), and two terminal oxidase complexes (cytochrome bo and cytochrome bd) (Ingledew and Poole 1984; Puustinen et al. 1991; Anraku and Gennis 1987). Two NADH dehydrogenases were described (Calhoun and Gennis 1993): NDH-1, a homologue of mitochondrial complex I, consisting of 14 different subunits encoded by the nuo operon, and the FAD-containing NDH-2, consisting of a single polypeptide chain of M r 47,200 encoded by the ndh gene. E. coli can synthesize two types of quinones, ubiquinone and menaquinone, whose ratio in membrane is variable and depends on the aeration and the different growth phases (Wallace and Young 1977b). They act as mobile electron and H+ carriers from the dehydrogenases to the terminal oxidases.
It has been previously shown in our laboratory, using isolated membranes, that an electron transfer from respiratory chain components to Cu(II) takes place (Rodríguez-Montelongo et al. 1995). In addition, purified NDH-2 is able to reduce Cu(II) in the presence of either FAD or quinone (Rapisarda et al. 1999). Dehydrogenases and quinones are thermodynamically able to reduce Cu(II), consistent with the fact that reduction potential for NAD+/NADH and ubiquinone/ubiquinol are around −320 and +50 mV, respectively, and that for Cu(II/I) is +340 mV. Here, we measured in vivo the Cu(II)-reduction capacity of E. coli strains that are deficient in quinones and NDH-2. We found that the cellular Cu(II)-reduction is mainly attributed to quinones and to a lesser extent to NDH-2. The presence of these components enhances cellular growth in media with excess or deficiency of copper. The reduction by respiratory chain components would participate in the metal uptake/efflux through specific transporters which only recognize Cu(I). This is the first time in which the Cu(II) reduction activity of E. coli respiratory components is demonstrated in vivo.
Materials and methods
Bacterial strains and growth conditions
The E. coli strains used in this study are shown in Table 1. Mutations were transduced by a P1 vir lysate. Cells were grown aerobically at 37°C, in minimal salt medium M9 with 0.1% tryptone and supplemented with either 0.5% glycerol or glucose as indicated. Quinone mutants do not grow in media supplemented with glycerol as carbon source, but they can grow with glucose, although more slowly than the wild type (not shown). When required, the following antibiotics were used: 15 μg ml−1 tetracycline, 30 μg ml−1 chloramphenicol and 50 μg ml−1 kanamycin.
To test growth in media with low or high metal concentration, strains were streaked on M9 plates containing 0.1% tryptone, 0.5% glucose and different concentrations of sodium citrate, CuSO4 and FeCl3, as indicated. Citrate cannot be metabolized by the studied strains and it functions as an intermediate strength metal chelator. Plates were incubated at 37°C for 24 h.
Bacterial suspensions preparation
Bacteria were harvested in mid-exponential phase of growth by centrifugation at 4°C. Then, cells were washed and resuspended (A 560nm = 0.75) in 20 mM citrate–phosphate buffer pH 7.5.
Determination of Cu(II)-reductase activity by entire cells
Cellular cupric-reductase activity, as appearance of Cu(I) in the incubation media, was measured using bathocuproine disulfonate (Sigma–Aldrich). This chelator forms a colored complex with cuprous ions (A max 480 nm) (Poillon and Dawson 1963). For the determinations, bacterial suspensions were supplemented with 0.5% glycerol, glucose or d-lactate as reduced substrates. The incubations were performed in correspondence with the carbon sources used for cell growths, except when d-lactate was used while cells were grown in the presence of glucose. Supplemented suspensions were incubated aerobically at 37°C up to 120 min in the presence of CuSO4 at the concentrations indicated in each experiment and bathocuproine disulfonate 0.5 mM (except in the assay with 1 mM Cu(II) where 2.5 mM were used). At different times, aliquots were centrifuged and the A 480nm was determined in supernatants. In parallel, suspensions incubated without copper and bathocuproine disulfonate were performed as negative control. After each incubation time, both copper and bathocuproine disulfonate were added to control cell-free supernatants to allow color formation which is independent of the cells.
Cu(I) values were calculated by a standard curve obtained in the same buffer without cells, but with the addition of 25 mM sodium ascorbate as a reducer. Cu(II)-reduction rate values were calculated with the difference between the Cu(I) produced by samples and by the corresponding negative controls after 60 min. Experiments testing wild type and mutant strains were always run in parallel. After the incubations, viability was determined by counting CFU on LB-agar plates incubated at 37°C for 24 h.
Determination of Fe(III)-reductase activity by entire cells
Cellular Fe(III)-reductase activity was determined in similar incubations as described above, but using bathophenantroline disulfonate (Sigma–Aldrich). This chelator forms a colored complex with Fe(II) (A max 540 nm) (Kingsley and Getehell 1956). The reaction mix contained bacterial suspensions, 0.5% glucose, 0.5 mM bathophenantroline disulfonate and 0.2 mM FeCl3. Negative controls without the metal and the chelator were run in parallel, as described for Cu(II)-reductase assay.
Fe(II) values were calculated by a standard curve obtained using the same buffer without cells, but with the addition of 25 mM sodium ascorbate as a reducer. Fe(III)-reduction rate values were calculated with the difference between the Fe(II) produced by samples and by the corresponding negative controls after 60 min. After incubations, viability was determined as described above.
Protein determination
Protein concentration was determined in aliquots of cell suspensions by the method of Lowry et al. 1951, using bovine serum albumin as standard.
Results
Cu(II)-reductase activity by bacterial suspensions
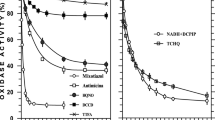
Cu(II)-reductase activity was measured using the chelator bathocuproine disulfonate by a procedure which senses the Cu(I) ions that are progressively accumulated in bacterial suspensions supernatants due to cell activities. To find appropriated analysis conditions, we tested the mentioned activity with the wild-type strain AN387, which was incubated for different periods of time with copper and the chelator in the presence of glycerol as a reduced substrate. The appearance of Cu(I) in the sample increased proportionally with time up to 80 min (Fig. 1a). The negative control, cell supernatant where metal and chelator were added after each incubation time, showed a lower formation of Cu(I) compared to the sample (Fig. 1a). The Cu(II)-reduction rates at different copper concentrations determined at 60 min incubation are shown in Fig. 1b. We observed a strong increase in the rate from 0.05 to 0.2 mM CuSO4, but no significant differences were observed when concentration was further raised to 1 mM. It should be noted that none of the tested copper concentrations affected the viability of cells incubated for 150 min (data not shown). The results were independent of the chelator concentration used (0.5 or 2.5 mM bathocuproine disulfonate). With 0.2 mM CuSO4, approximately 50% (0.1 mM) from the total copper added was reduced by the wild type strain (AN387) after 150 min, consuming around 0.2 mM bathocuproine disulfonate in the 2:1 complexes formation. For following experiments, Cu(II) and bathocuproine disulfonate concentrations were fixed at 0.2 and 0.5 mM, respectively. To corroborate that cells of the strain AN387 are able to reduce Cu(II) using several reduced substrates, glucose and d-lactate were also tested. There was a considerable reduction with all the substrates, but higher rate values were obtained with glycerol (Fig. 1c).
Cu(II)-reduction by AN387 E. coli cells. Cell suspensions (A 560nm = 0.75) were incubated at 37°C with 0.5% reduced substrate. a Time-course of Cu(I) formation by negative controls (open triangles) incubated only with glycerol, and by samples (closed squares) incubated with glycerol, 0.2 mM CuSO4, and 0.5 mM bathocuproine disulfonate. b Cu(II)-reduction rates by cell suspensions after 60 min of incubation in media with glycerol, bathocuproine disulfonate, and different copper concentrations, as indicated. c Cu(II)-reduction rates by suspensions incubated for 60 min in the presence of the indicated reduced substrates, 0.2 mM CuSO4, and 0.5 mM bathocuproine disulfonate. Reduction rate values are the means ± SD of four independent experiments
Cu(II)-reduction in respiratory chain deficient strains
It has been previously demonstrated, using isolated membranes, that respiratory chain electron transfer produces Cu(II)-reduction mainly through NDH-2 and quinones (Rodríguez-Montelongo et al. 1995). Here, we measured the metal reduction activity by whole cells in respiratory chain deficient mutants derived from AN387 strain, either with glucose or with d-lactate as reduced substrates (Fig. 2). The activity with glucose of strain SSK2 (ubi) diminished around 50% in respect to the wild-type. In SSK3 (ubi men), the cellular Cu(II)-reduction rate decreased even more. In addition, a very low cupric reduction activity (<15% in respect to the wild-type) was detected in strain SVT23408 (ubi men ndh) (Fig. 2). The NDH-2 contribution, independent of quinone, was evident when copper reduction activities in SSK3 and SVT23408 were compared. In line with this difference, the strain SVT208, only deficient in NDH-2, diminished the activity around a 10% in respect to the wild-type (Fig. 2). The strains SVT3408 (men ndh) and SVT2408 (ubi ndh) decreased the Cu(II)-reductase activity around 10–15% and 50–55% in respect to the wild type, respectively (not shown).
Cu(II)-reduction by E. coli cells deficient in quinones and NDH-2. Cell suspensions of the indicated strains were incubated during 60 min in the presence of 0.2 mM CuSO4, 0.5 mM bathocuproine disulfonate, and 0.5% glucose (closed bars) or 0.5% d-lactate (open bars) as reduced substrates. Values are the means ± SD of three independent experiments
The remaining activity in SVT23408 would be independent of the electron flow through respiratory chain, maybe mediated by glutathione (GSH). For this reason, we measured the Cu(II)-reductase activity in SVT010 strain deficient in gshA, which encodes γ-glutamylcysteine synthetase (an enzyme involved in GSH synthesis). This strain decreased the activity near ~30% in respect to the wild type (data not shown).
The reduced substrate d-lactate can only be oxidized by the respiratory d-lactate dehydrogenase, providing pyruvate which is rapidly oxidized in the citric acid cycle and yielding large amounts of NADH. In order to function, this enzyme needs quinones as the oxidized acceptor in the inner membrane. Using d-lactate, the strains AN387 and SVT208 (ndh) were able to reduce Cu(II) to Cu(I), while the quinone deficient strains only reduced a low amount during the experiment (Fig. 2). This low reduction rate obtained with d-lactate in the mutants was in the same order of the activity value of SVT23408 (ubi men ndh) in the presence of glucose.
Fe(III) reduction by bacterial suspensions
Fe(III)-reduction rate was determined comparing the wild-type and the strain SVT23408 (ubi men ndh), that has shown the highest difference in Cu(II)-reductase activity. The results show that independently of the absence of NDH-2 and quinones, the Fe(III)-reduction rates were comparable to the wild-type. The obtained values were around 1 ηmol Fe(II) min−1 mg prot−1 for the tested strains (Table 2). It is important to emphasize that the Fe(III)-reduction rate was approximately four times lower than that for Cu(II) in the wild-type.
Growth of quinone mutants in media with excess or deficiency of copper
NDH-2 mutants did not grow in media containing high or low copper concentration (Rodríguez-Montelongo et al. 2006). For these previous experiments, we have used citrate as a suitable chelator to modulate the free metal concentration available for the cells, since it has a relative low affinity for aqueous Cu(II) ions [association constant ~105.9 M] (Dawson et al. 1986). Citrate plays two different roles: (a) at 100 mM, avoids copper precipitation in studies involving high metal concentrations; (b) at 300 mM, produces copper deficiency in media without metal addition. Considering that the deficiency on growth of ndh mutant maybe due to the diminution in Cu(II)-reduction activity and that the quinone mutants also have low copper reduction capacity, we analyzed the growth of quinone mutant strains in media with high or low metal concentrations. As shown in Fig. 3, the parental strain AN387 grew in all culture conditions. Although the quinone mutants grew more slowly, the plates were evaluated after 24 h, when all the strains were in stationary phase on the control plate (Fig. 3a). The addition of 100 mM citrate did not affect the growth of quinone mutants SSK2, SSK3 or SVT23408 (Fig. 3a, b), whereas the supplementation of this medium with 70 mM CuSO4 blocked the growth of all mutants (Fig. 3c, copper excess). On the other hand, the quinone mutants did not grow on M9 medium containing 300 mM citrate (Fig. 3d, copper deficiency). The addition of 2 mM CuSO4 but not of 2 mM FeCl3, enabled the mutants to recover a similar growth than in M9 (Fig. 3e, f). Mutants also recovered growth by the addition of 2.5 mM of sodium ascorbate to M9 plates containing 300 mM citrate (Fig. 3g).
Bacterial growth in media with excess or deficiency of copper. Cells from stationary phase M9 cultures were streaked on M9 agar plates, with supplements as indicated. Plates were grown for 24 h. The experiments were repeated at least four times with similar results. I: AN387 (wt); II: SSK2 (ubi); III: SSK3 (ubi men); and IV: SVT23408 (ubi men ndh)
Discussion
Considering that Cu(II)-reduction is a central mechanism related to copper homeostasis (Solioz and Stoyanov 2003; Rensing and Grass 2003; Rodríguez-Montelongo et al. 2006), the in vivo studies about this topics gain importance. Here, we were able to measure the Cu(II)-reductase activity in living E. coli cells, using the sublethal copper concentration 0.2 mM, which is in agreement with the range applied in studies with whole cells of Lactococcus lactis (Rezaïki et al. 2008). Moreover, a similar approach was used to determine cellular cupric reductase activity in Streptomyces sp. using the chelator bicinchoninic acid reagent (Albarracín et al. 2008).
In our conditions, the sole absence of ubiquinone has drastically impaired cellular Cu(II)-reduction activity. Menaquinone and NDH-2 also contributed on the Cu(II)-reduction but in a minor extend. The reductase activity that we measured in vivo appears to be specific for copper, based on the results obtained when copper was replaced by iron. Indeed, similar values of ferric-iron reduction rates were obtained using cells without quinones and NDH-2 in respect to the wild-type. This is consistent with the fact that Fe(II) appearance in supernatants is mediated by other mechanisms. It was previously described that E. coli NDH-2 does not reduce Fe(III) to Fe(II) either in membranes or in purified preparations (Rapisarda et al. 1999). In addition, quinones were described to be more efficient to reduce Cu(II) than Fe(III) in Lactococcus lactis (Rezaïki et al. 2008).
In agreement with the results obtained with NDH-2 (Rodríguez-Montelongo et al. 2006), quinone absence avoided the cellular growth in medium containing either high or low copper concentrations. Thus, these respiratory chain components allow the resistance to high metal concentration maybe by their involvement in Cu(II)-reductase activity. On the other hand, the cellular reduction activity would be important when bacteria are grown in copper deficient medium (300 mM citrate minimal plates), allowing the uptake of the metal to the cytoplasm by a not yet identified importer. This event is also supported by the growth of cells in medium with limited copper availability, when an exogenous reducer as sodium ascorbate was added.
We have additional data that shows the copper exporters CopA and Cus system also modifies the amount of Cu(I) determined outside the cell. The Cu(II)-reduction activity of a strain lacking copA gene or Cus system decrease nearly 40% in respect to the wild-type strain (Volentini et al. 2008, personal communication). These evidence could indicate that part of the measured extracellular Cu(I) by our methods, must be reduced inside the cell and that the variation in the reduced metal outside these mutants may be due to the Cu(I) deficient transport toward the exterior. On the other hand, the absence of cueO in the cell did not modify the cellular copper reduction rate (Volentini et al., unpublished data).
The challenge for copper-dependent organisms is to obtain sufficient amounts of this metal ion to satisfy their needs, while tightly controlling cellular copper to avoid toxicity (Ridge et al. 2008). In this way and on the basis of our present results, we propose a general mechanism for Cu(II)-reduction mediated by quinones, which is linked to all dehydrogenases, NDH-2 inclusive, using electrons from different substrates, such as d-lactate, NADH, etc. (Fig. 4). This could allow the metal reduction both in the cytoplasmic and periplasmic space, due to the properties of quinones to diffuse laterally and to catalyze reactions across the bilayer (Futarni et al. 1979; Hackenbrock 1981). On the other hand, there would be a quinone independent mechanism, exclusive of NDH-2 and maybe related to the previously described FAD-dependent activity of the enzyme (Rapisarda et al. 1999). In this case, the cellular copper reduction should be performed on the cytoplasmic side because of the protein disposition. The Cu(II)-reduction events promoted by respiratory components would have possible relationships with the metal uptake/efflux systems.
Simplified model of the proposed general mechanism of Cu(II)-reduction by quinones in E. coli cells. Q: quinones; dehydrogenases: NDH-2, d-LDH, etc.; CopA and CusCBA: copper exporters; CusF: periplasmic copper chaperone; ?: putative copper importer; SH: reduced substrate; S: oxidized substrate; Cu(I) within a circle: mainly Cu+-monoglutathionate complex; IM inner membrane, OM outer membrane
The scenario in copper deficiency or excess would be much more complex due to transcriptional regulation of several genes related to copper homeostasis (Yamamoto and Ishihama 2005; Kershaw et al. 2005). We do not discard the participation of FMN, thiols, FeS moieties and/or GSH on the Cu(I) formation. It was reported that under aerobic growth conditions, GSH is essential to maintain a reduced environment in the cytosol of E. coli cells (Helbig et al. 2008; Carmel-Harel and Storz 2000). Indeed, the reduction rate decreased in the gshA mutant SVT010 and it may be possible that the low reduction percentage observed in the SVT23408 (ubi men ndh) mutant is conducted by one of these cellular reducers.
For the first time, the Cu(II)-reduction activity by E. coli respiratory components is demonstrated in vivo. This activity was mainly due to quinones, at the cytoplasmic or the periplasmic side, and to a lesser extent to NDH-2, only at the cytoplasm. The presence of these components enhanced cellular growth in media with excess or deficiency of copper, mediating the metal reduction process that contributes in its uptake/efflux through specific transporters which only recognize Cu(I).
References
Albarracín VH, Ávila AL, Amoroso MJ, Abate CM (2008) Copper removal ability by Streptomyces strains with dissimilar growth patterns and endowed with cupric reductase activity. FEMS Microbiol Lett 288:141–148
Anraku Y, Gennis RB (1987) The aerobic respiratory chain of Escherichia coli. Trends Biochem Sci 12:262–266
Bagai I, Rensing C, Blackburn NJ, McEvoy MM (2008) Direct metal transfer between periplasmic proteins identifies a bacterial copper chaperone. Biochemistry 47:11408–11414
Benov L, Chang LY, Day B, Fridovich I (1995) Copper, zinc superoxide dismutase in Escherichia coli: periplasmic localization. Arch Biochem Biophys 319:508–511
Calhoun MW, Gennis RB (1993) Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J Bacteriol 175:3013–3019
Carmel-Harel O, Storz G (2000) Roles of glutathione- and thioredoxindependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae response to oxidative stress. Ann Rev Microbiol 54:439–461
Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M (2010) Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J Bacteriol 192:2512–2524
Dawson RMC, Elliott DC, Elliott WH, Jones KM (1986) Data for Biochemical Research, 3rd edn. Oxford Science Publications, Oxford University Press, NY
Fan B, Rosen BP (2002) Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. J Biol Chem 277:46987–46992
Futarni AE, Hunt E, Hanska GG (1979) Vectorial redox reactions of physiological quinones, requirement of a minimum length of the isoprenoid side chain. Biochim Biophys Acta 547:583–596
Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem 272:13786–13792
Grabbe R, Schmitz R (2003) Oxygen control of nif gene expression in Klebsiella pneumoniae depends on NifL reduction at the cytoplasmic membrane by electrons derived from the reduced quinone pool. Eur J Biochem 270:1555–1566
Gutteridge JM, Halliwell B (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 899:136–147
Hackenbrock CR (1981) Lateral diffusion and electron transfer in the mitochondrial inner membrane. Trends Biochem Sci 6:151–154
Hassett R, Kosman DJ (1995) Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem 270:128–134
Helbig K, Bleuel C, Krauss GJ, Nies DH (2008) Glutathione and transition-metal homeostasis in Escherichia coli. J Bacteriol 190:5431–5438
Ingledew WJ, Poole RK (1984) The respiratory chains of Escherichia coli. Microbiol Rev 48:222–271
Kershaw CJ, Brown NL, Constantinidou C, Patel MD, Hobman JL (2005) The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology 151:1187–1198
Kingsley GB, Getehell O (1956) Serum iron determination. Clin Chem 2:175–183
Korshunov S, Imlay JA (2006) Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J Bacteriol 188:6326–6334
Linder MC (1991) Biochemistry of copper. Plenum, New York
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macomber L, Rensing C, Imlay JA (2007) Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189:1616–1626
Miller S, Douglas RM, Carter P, Booth IR (1997) Mutations in the glutathione-gated KefC K+ efflux system of Escherichia coli that cause constitutive activation. J Biol Chem 272:24942–24947
Minagawa J, Mogi T, Gennis RB, Anraku Y (1992) Identification of heme and copper ligands in subunit I of the cytochrome bo complex in Escherichia coli. J Biol Chem 267:2096–2104
Outten FW, Outten CE, Hale J, O’Halloran TV (2000) Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J Biol Chem 275:31024–31029
Petersen C, Moller LB (2000) Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene 261:289–298
Poillon WN, Dawson CR (1963) On the nature of copper in ascorbate oxidase: the valence state of copper in the denatured and native enzyme. Biochim Biophys Acta 77:27–36
Puustinen A, Finel M, Haltia T, Gennis RB, Wikström M (1991) Properties of the two terminal oxidases of Escherichia coli. Biochemistry 30:3936–3942
Rapisarda VA, Rodríguez-Montelongo L, Farías RN, Massa EM (1999) Characterization of an NADH-linked cupric reductase activity from the Escherichia coli respiratory chain. Arch Biochem Biophys 370:143–150
Rensing C, Grass G (2003) Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213
Rensing C, Fan B, Sharma R, Mitra B, Rosen BP (2000) CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci USA 97:652–656
Rezaïki L, Lamberet G, Derré A, Gruss A, Gaudu P (2008) Lactococcus lactis produces short-chain quinones that cross-feed Group B Streptococcus to activate respiration growth. Mol Microbiol 67:947–957
Ridge PG, Zhang Y, Gladyshev VN (2008) Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE 3:e1378
Rodríguez-Montelongo L, Farías RN, Massa EM (1995) Sites of electron transfer to membrane-bound copper and hydroperoxide-induced damage in the respiratory chain of Escherichia coli. Arch Biochem Biophys 323:19–26
Rodríguez-Montelongo L, Volentini SI, Farías RN, Massa EM, Rapisarda VA (2006) The Cu(II)-reductase NADH dehydrogenase-2 of Escherichia coli improves the bacterial growth in extreme copper concentrations and increases the resistance to the damage caused by copper and hydroperoxide. Arch Biochem Biophys 451:1–7
Singh SK, Grass G, Rensing C, Montfort WR (2004) Cuprous oxidase activity of CueO from Escherichia coli. J Bacteriol 186:7815–7817
Solioz M, Stoyanov JV (2003) Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev 27:183–195
Stoyanov JV, Hobman JL, Brown NL (2001) CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol Microbiol 39:502–511
Thiele DJ (2003) Integrating trace element metabolism from the cell to the whole organism. J Nutr 133:1579S–1580S
Volentini SI, Schurig-Briccio LA, Rintoul MR, Farías RN, Rodríguez-Montelongo L, Rapisarda VA (2008) Cellular Cu(II)-reduction by Escherichia coli respiratory chain in copper exporters deficient strains. Abstract in: Biocell Suppl 32:117. ISSN 0327–9545
Vulpe CD, Packman S (1995) Cellular copper transport. Ann Rev Nutr 15:293–322
Wallace BJ, Young IG (1977a) Aerobic respiration in mutants of Escherichia coli accumulating quinone analogues of ubiquinone. Biochim Biophys Acta 461:75–83
Wallace BJ, Young IG (1977b) Role of quinones in electron transport to oxygen and nitrate in Escherichia coli: studies with ubiA − and menA − double quinone mutant. Biochim Biophys Acta 461:84–100
Yamamoto K, Ishihama A (2005) Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56:215–227
Acknowledgments
We specially thank Dr E. M. Massa, who has been the mentor of our investigations in the subject, and the lab colleagues M. R. Rintoul, L. A. Schurig-Briccio, J. M. Villegas and L. Cerioni for helpful discussions. We also thank Dr B. J. Wallace for providing strains AN387, Dr R. A. Schmitz for strain RAS50, Dr C. Rensing for strain GR6, Dr J. Imlay for providing strains SSK1, SSK2 and SSK3 and Dr I. R. Booth for providing strain MJF335. This research was supported by grants from CIUNT, CONICET, and ANPCyT (Argentine).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volentini, S.I., Farías, R.N., Rodríguez-Montelongo, L. et al. Cu(II)-reduction by Escherichia coli cells is dependent on respiratory chain components. Biometals 24, 827–835 (2011). https://doi.org/10.1007/s10534-011-9436-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9436-3