Abstract
The current solution to iron-mediated damage in transfusional iron overload disorders is decorporation of excess unmanaged metal, chelation therapy. The clinical development of the tridentate chelator deferitrin (1, Table 1) was halted due to nephrotoxicity. It was then shown by replacing the 4′-(HO) of 1 with a 3,6,9-trioxadecyloxy group, the nephrotoxicity could be ameliorated. Further structure–activity relationship studies have established that the length and the position of the polyether backbone controlled: (1) the ligand’s iron clearing efficiency (ICE), (2) chelator tissue distribution, (3) biliary ferrokinetics, and (4) tissue iron reduction. The current investigation compares the ICE and tissue distribution of a series of (S)-4,5-dihydro-2-[2-hydroxy-4-(polyether)phenyl]-4-methyl-4-thiazolecarboxylic acids (Table 1, 3–5) and the (S)-4,5-dihydro-2-[2-hydroxy-3-(polyether)phenyl]-4-methyl-4-thiazolecarboxylic acids (Table 1, 8–10). The three most effective polyether analogues, in terms of performance ratio (PR), defined as mean ICEprimate/ICErodent, are 3 (PR 1.1), 8, (PR 1.5), and 9, now in human trials, (PR 2.2). At the onset of the clinical trial on 9, no data were available for ligand 3 or 8. This is unfortunate, as 3 has many advantages over 9, e.g., the ICE of 3 in rats is 2.5-fold greater than that of 9 and analogue 3 achieves very high levels in the liver, pancreas, and heart, the organs most affected by iron overload. Finally, the impact of 3 on the urinary excretion of kidney injury molecule-1 (Kim-1), an early diagnostic biomarker for monitoring acute kidney toxicity, has been carried out in rats; no evidence of nephrotoxicity was found. Overall, the results suggest that 3 would be a far superior clinical candidate to 9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans have evolved a highly efficient iron management system in which we absorb and excrete about 1 mg of the metal daily; there is no mechanism for the excretion of excess iron. Thus, when iron accumulates beyond this point, as in transfusional iron overload (Olivieri and Brittenham 1997; Vichinsky 2001; Kersten et al. 1996), or from increased absorption of dietary iron, (Conrad et al. 1999; Lieu et al. 2001), without effective treatment, body iron progressively increases with deposition in the liver, pancreas, heart, and elsewhere. Without decorporation, the metal buildup eventually leads to liver disease that may progress to cirrhosis (Angelucci et al. 2000; Bonkovsky and Lambrecht 2000; Pietrangelo 2002), pancreatic damage, e.g., diabetes (Cario et al. 2004; Wojcik et al. 2002), and, ultimately, heart failure (Brittenham et al. 1994; Brittenham 2000). Iron-induced cardiac damage still remains the primary cause of death in transfusional iron overload (Zurlo et al. 1989).
Although iron is a critical micronutrient for all prokaryotes and eukaryotes, it is not easily accessible. The low solubility of Fe(III) hydroxide (K sp= 1 × 10−39) (Raymond and Carrano 1979), the predominant form of the metal in the biosphere, has led to the development of sophisticated iron storage and transport systems in nature. Microorganisms utilize low molecular weight, virtually ferric-ion-specific ligands, siderophores (Byers and Arceneaux 1998), that they secrete into the environment in order to sequester and incorporate the metal. Higher eukaryotes tend to employ proteins to transport and store iron, e.g., transferrin and ferritin, respectively (Bergeron 1986; Theil and Huynh 1997; Ponka et al. 1998).
In humans, plasma nontransferrin-bound iron (NTBI), a heterogeneous pool of iron in the circulation, seems to be the principal source of the abnormal tissue distribution of iron that develops with chronic iron overload. While plasma NTBI is rapidly taken up by hepatocytes in the liver, the major iron storage organ, this mechanism for NTBI management can become overwhelmed. The toxicity associated with excess iron, whether a systemic or a focal problem, derives from its interaction with reactive oxygen species, e.g., endogenous hydrogen peroxide (H2O2) (Graf et al. 1984; Halliwell 1994a; Halliwell 1994b; Koppenol 2000). In the presence of Fe(II), H2O2 is reduced to the hydroxyl radical (HO•), a very reactive species, and (HO−), a process known as the Fenton reaction. The liberated Fe(III) can be reduced back to Fe(II) via a variety of biological reductants (e.g., ascorbate), a problematic cycle. The hydroxyl radical reacts very quickly with a variety of cellular constituents and can initiate free radicals and radical-mediated chain processes that damage DNA and membranes, as well as produce carcinogens (Halliwell 1994a; Babbs 1992; Hazen et al. 1998). While iron-mediated damage can be focal, as in reperfusion damage (Millán et al. 2008), Parkinson’s (Zecca et al. 2008), and Friedreich’s ataxia (Pietrangelo 2007), or global, as in transfusional iron overload, e.g., thalassemia (Pippard 1987), sickle cell disease (Pippard 1987; Olivieri 2001), and myelodysplasia (Malcovati 2007), with multiple organ involvement, the solution in both scenarios is the same: chelate and promote the excretion of the excess unmanaged iron.
The iron-chelating agents now in use or that have been clinically evaluated (Fig. 1) are desferrioxamine B mesylate (DFO), 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone, L1), 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoic acid (deferasirox, Exjade), and the desferrithiocin [(S)-4,5-dihydro-2-(3-hydroxy-2-pyridinyl)-4-methyl-4-thiazolecarboxylic acid (DFT)] analogue (S)-4,5-dihydro-2-(2,4-dihydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid, deferitrin (1, Fig. 1). Each of these ligands presents with notable shortcomings. DFO must be given subcutaneously (s.c.) for protracted periods of time, e.g., 12 h a day, five or more days a week, resulting in a serious patient compliance issue (Pippard 1989; Giardina and Grady 2001; Olivieri and Brittenham 1997). Deferiprone, while orally active, simply does not remove enough iron to maintain patients in a negative iron balance (Hoffbrand et al. 1998; Olivieri 1996; Olivieri et al. 1998; Richardson 2001). Exjade did not show noninferiority to DFO (Piga et al. 2006) and is associated with numerous side effects, including some serious renal toxicity issues (Nick et al. 2002; Nisbet-Brown et al. 2003; Exjade Prescribing Information). Finally, the clinical development of deferitrin (1), although promising, was recently halted by Genzyme due to nephrotoxicity (Galanello et al. 2007).
Structures of iron chelators that are now in use or that have been clinically evaluated: desferrioxamine B mesylate (DFO), 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone, L1), 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoic acid (deferasirox, Exjade), and the desferrithiocin [(S)-4,5-dihydro-2-(3-hydroxy-2-pyridinyl)-4-methyl-4-thiazolecarboxylic acid (DFT)] analogue (S)-4,5-dihydro-2-(2,4-dihydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid, deferitrin (1)
Deferitrin has been reengineered, and extensive structure–activity relationship (SAR) studies led to the discovery of a number of polyether analogues with excellent iron clearing properties with little, if any, impact on renal function (Bergeron et al. 2006, 2007, 2008, 2010). One of these ligands, (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6,9-trioxadecyloxy)]-4-methyl-4-thiazolecarboxylic acid (9, Table 1), is now in clinical trials. Although the preclinical toxicity profile of 9 was benign, the clinical results remain to be elucidated.
In the course of the SAR studies, several issues became clear: the length and the position of the polyether backbone has a profound effect on (1) the ligand’s iron clearing efficiency (ICE) (Bergeron et al. 2007, 2010), (2) chelator tissue distribution (Bergeron et al. 2007, 2008), (3) biliary ferrokinetics (Bergeron et al. 2008, 2010), and (4) tissue iron reduction (Bergeron et al. 2010). It also became painfully obvious that a method that could provide information about ligand-induced renal damage early in the drug discovery process was sorely needed. Assessment of chelator-induced impaired renal function has traditionally relied on the detection of a rise in blood urea nitrogen (BUN) and/or serum creatinine (SCr). However, because of the functional reserve of the kidney, these parameters are often unreliable indicators of acute kidney injury; the ultimate answer requires histopathology. The Critical Path Institute’s Preventive Safety Testing Consortium (PSTC) has identified kidney injury molecule-1 (Kim-1, rat) or (KIM-1, human) as an early diagnostic biomarker for monitoring acute kidney tubular toxicity (Goodsaid et al. 2009; Hoffmann et al. 2010). Kim-1 is a type 1 transmembrane protein located in the epithelial cells of proximal tubules (Han et al. 2002; Bonventre 2009). After injury, e.g., exposure to a nephrotoxic agent or ischemia, the ectodomain of Kim-1 is shed from the proximal tubular kidney epithelial cells into the urine (Zhou et al. 2008; Vaidya et al. 2006). BioAssay Works has recently developed RenaStick, a direct lateral flow immunochromatographic assay, which allows for the rapid detection (less than 30 min) and quantitation of Kim-1 (rat) or KIM-1 (human) in urine (Vaidya et al. 2009).
The current investigation provides a comparison of the iron clearance properties and tissue distribution of a series of (S)-4,5-dihydro-2-[2-hydroxy-4-(polyether)phenyl]-4-methyl-4-thiazole-carboxylic acids (Table 1, 3–5) and (S)-4,5-dihydro-2-[2-hydroxy-3-(polyether)phenyl]-4-methyl-4-thiazolecarboxylic acids (Table 1, 8–10). The ICE of the ligands was assessed in rodents and in primates; chelator tissue distribution was evaluated in rodents. Additional rodent toxicity studies of the lead candidate, (S)-4,5-dihydro-2-[2-hydroxy-4-(3,6-dioxaheptyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (3, Table 1), including the impact of this ligand on urinary Kim-1 excretion, have also been carried out.
Experimental section
Materials and instrumentation
Reagents were purchased from Aldrich Chemical Co. (Milwaukee, WI), and Fisher Optima-grade solvents were routinely used. Reactions were run under a nitrogen atmosphere, and organic extracts were dried with sodium sulfate. Silica gel 40–63 from SiliCycle, Inc. (Quebec City, QC, Canada) was used for column chromatography. Glassware that was presoaked in 3 N HCl for 15 min, washed with distilled water and distilled ethanol, and oven-dried was used in the isolation of 8 and 10. Optical rotations were run at 589 nm (sodium D line) and at 20°C utilizing a Perkin-Elmer 341 polarimeter, with c being concentration in grams of compound per 100 ml of a CHCl3 solution. Chemical shifts (δ) for 1H NMR spectra at 400 MHz are given in parts per million downfield from tetramethylsilane for CDCl3. Chemical shifts (δ) for 13C NMR spectra at 100 MHz are given in parts per million referenced to the residual solvent resonance in CDCl3 (δ 77.16). Coupling constants (J) are in hertz. The base peaks are reported for the ESI-FTICR mass spectra.
Male Sprague–Dawley rats were procured from Harlan Sprague–Dawley (Indianapolis, IN). Male Cebus apella monkeys (3.5–4.0 kg) were obtained from World Wide Primates (Miami, FL). Ultrapure salts were obtained from Johnson Matthey Electronics (Royston, UK). Exjade was manufactured by Novartis (Basel, Switzerland). All hematological and biochemical studies were performed by Antech Diagnostics (Tampa, FL). Histopathological analysis was carried out by Florida Vet Path (Bushnell, FL). Atomic absorption (AA) measurements were made on a Perkin-Elmer model 5100 PC (Norwalk, CT). Techni Ice (Melbourne, Australia) was used to collect the rat urine chilled. A Rat Kim-1 Rapid Test R-Rena Strip Kit for the detection of Kim-1 in rat urine was obtained from BioAssay Works (Ijamsville, MD). A Chromatoreader ReaScan (Otsuka Electronics Co., Japan) was utilized to read the test strips and to allow for the quantitation of Kim-1 in rat urine.
Chemical synthesis
(S)-4,5-Dihydro-2-[2-hydroxy-3-(3,6-dioxaheptyloxy)phenyl]-4-methyl-4- thiazolecarboxylic acid (8)
Degassed phosphate buffer (0.1 M, 40 ml, pH 5.95) and 16 (3.45 g, 20.1 mmol) were added to 13 (3.67 g, 15.5 mmol) in degassed CH3OH (40 ml). Sodium bicarbonate (2.08 g, 24.8 mmol) was added in portions, and the reaction mixture (pH 6.2–6.6) was heated at 70°C for 24 h. After cooling to room temperature, solvent was removed by rotary evaporation. The residue was dissolved in 8% NaHCO3 (50 ml) and was extracted with CHCl3 (3 × 50 ml). The aqueous portion was cooled in an ice-water bath, acidified to pH ≈ 1 with 5 N HCl, and extracted with EtOAc (4 × 50 ml). The EtOAc extracts were washed with saturated NaCl and were concentrated in vacuo. Drying under high vacuum furnished 5.11 g of 8 (93%) as an orange oil: [α] +56.1° (c 0.70). 1H NMR δ: 1.72 (s, 3H), 3.26 (d, 1H, J = 11.2), 3.39 (s, 3H), 3.57–3.60 (m, 2H), 3.74–3.76 (m, 2H), 3.88 (d, 1H, J = 11.6), 3.89–3.92 (m, 2H), 4.20–4.23 (m, 2H), 6.80 (t, 1H, J = 8.0), 7.01–7.07 (m, 2H). 13C NMR δ 24.52, 39.92, 59.11, 69.07, 69.86, 70.79, 72.02, 83.23, 116.33, 117.87, 118.55, 122.80, 147.70, 150.20, 172.52, 176.71; HRMS m/z calcd for C16H22NO6S, 356.1162 (M + H); found, 356.1154.
(S)-4,5-Dihydro-2-[2-hydroxy-3-(3,6,9,12-tetraoxatridecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (10)
Degassed phosphate buffer (0.1 M, 40 ml, pH 5.95) and 16 (2.4 g, 14.0 mmol) were added to 15 (3.56 g, 10.9 mmol) in degassed CH3OH (40 ml). Sodium bicarbonate (1.47 g, 17.5 mmol) was added in portions, and the reaction mixture (pH 6.2–6.6) was heated at 75°C for 24 h. After cooling to room temperature, the bulk of the solvent was removed by rotary evaporation. The residue was dissolved in 8% NaHCO3 (50 ml) and was extracted with CHCl3 (3 × 100 ml). The aqueous fraction was cooled at 0°C, acidified to pH ≈ 1 with 5 N HCl, and extracted with EtOAc (4 × 40 ml). Combined EtOAc extracts were washed with saturated NaCl and concentrated in vacuo. Drying under high vacuum furnished 4.12 g of 10 (85%) as a yellow-orange oil: [α] +17.5° (c 0.40). 1H NMR δ: 1.72 (s, 3H), 3.24 (d, 1H, J = 11.6), 3.40 (s, 3H), 3.56–3.59 (m, 2H), 3.62–3.78 (m, 10H), 3.89 (m, 2H), 3.90 (d, 1H, J = 11.6), 4.16–4.25 (m, 2H), 6.79 (t, 1H, J = 8.0), 7.00–7.06 (m, 2H). 13C NMR δ 24.53, 39.94, 58.99, 68.90, 69.75, 70.35, 70.50, 70.52, 70.58, 70.82, 71.94, 83.24, 116.33, 117.57, 118.47, 122.69, 147.61, 150.10, 172.09, 175.18. HRMS m/z calcd for C20H30NO8S, 444.1687 (M + H); found, 444.1683.
2-Hydroxy-3-(3,6-dioxaheptyloxy)benzonitrile (13)
Compound 11 (3.2 g, 23.7 mmol) was added to a suspension of 60% NaH (1.9 g, 47.5 mmol) in DMSO (30 ml) using oven-dried glassware. After the reaction mixture was stirred at room temperature for 1 h, 12 (6.48 g, 23.7 mmol) in DMSO (25 ml) was added. The reaction mixture was stirred for 24 h at room temperature, poured with stirring into cold water (50 ml), and extracted with CHCl3 (3 × 50 ml). The aqueous phase was acidified to pH ≈ 1 with 5 N HCl and was extracted with CHCl3 (4 × 50 ml). The latter CHCl3 extracts were concentrated in vacuo. Purification using column chromatography, eluting with 9% CH3OH/CHCl3, gave 3.98 g of 13 (71%) as a colorless oil. 1H NMR δ: 3.41 (s, 3H), 3.60–3.63 (m, 2H), 3.74–3.77 (m, 2H), 3.85–3.87 (m, 2H), 4.16–4.19 (m, 2H), 6.83 (t, 1H, J = 8.0), 7.08 (dd, 1H, J = 7.8, 1.6), 7.14 (dd, 1H, J = 8.2, 1.4). 13C NMR δ: 58.99, 69.25, 69.53, 70.43, 71.67, 99.88, 116.48, 118.61, 119.88, 125.55, 146.67, 151.04. HRMS m/z calcd for C12H16NO4, 238.1072 (M + H); found, 238.1072.
2-Hydroxy-3-(3,6,9,12-tetraoxatridecyloxy)benzonitrile (15)
Compound 11 (3.20 g, 23.7 mmol) was added to a suspension of 60% NaH (1.90 g, 47.5 mmol) in DMSO (30 ml) and the reaction mixture was stirred at room temperature for 1 h. After addition of 14 (8.68 g, 24.0 mmol) in DMSO (25 ml), the reaction mixture was stirred for 24 h at room temperature and was poured into cold water (60 ml) with constant stirring and was extracted with CHCl3 (3 × 60 ml). The aqueous phase was acidified to pH ≈ 1 with 6 N HCl and was extracted with CHCl3 (5 × 40 ml). The latter CHCl3 extracts were concentrated in vacuo. Purification using column chromatography, eluting with 10% CH3OH/CHCl3, gave 5.40 g of 15 (70%) as a light brown oil. 1H NMR δ: 3.42 (s, 3H), 3.57–3.59 (m, 2H), 3.65–3.71 (m, 10H), 3.74–3.76 (m, 2H), 3.85–3.87 (m, 2H), 4.14–4.16 (m, 2H), 6.80 (t, 1H, J = 8.0), 7.03 (dd, 1H, J = 8.0, 1.6), 7.13 (dd, 1H, J = 8.0, 1.6), 9.39 (br s, 1H). 13C NMR δ: 59.13, 68.65, 69.30, 70.15, 70.34, 70.53, 70.56, 70.58, 71.64, 100.24, 116.71, 117.41, 119.26, 125.29, 146.95, 151.54. HRMS m/z calcd for C16H24NO6, 326.1598 (M + H); found, 326.1598.
Biological methods
All animal experimental treatment protocols were reviewed and approved by the University of Florida’s Institutional Animal Care and Use Committee.
Cannulation of bile duct in non-iron-overloaded rats
The cannulation has been described previously (Bergeron et al. 1990, 1993). Bile samples were collected from male Sprague–Dawley rats (400–450 g) at 3 h intervals for up to 48 h. The urine sample(s) was taken at 24 h intervals. Sample collection and handling are as previously described (Bergeron et al. 1990, 1993).
Iron loading of C. apella monkeys
The monkeys were iron overloaded with intravenous iron dextran as specified in earlier publications to provide about 500 mg of iron per kg of body weight (Bergeron et al. 1990, 1992); the serum transferrin iron saturation rose to between 70 and 80%. At least 20 half-lives, 60 days (Wood et al. 1968), elapsed before any of the animals were used in experiments evaluating iron-chelating agents.
Primate fecal and urine samples
Fecal and urine samples were collected at 24 h intervals and processed as described previously (Bergeron et al. 1990, 1993, 1998). Briefly, the collections began 4 days prior to the administration of the test drug and continued for an additional 5 days after the drug was given. Iron concentrations were determined by flame absorption spectroscopy as presented in other publications (Bergeron et al. 1990, 1996).
Drug preparation and administration: iron clearance and chelator tissue distribution
In the iron clearing experiments, the rats were given 8 and 10 orally (p.o.) at a dose of 300 μmol/kg. An additional group of rats was given 3 p.o. at a dose of 50 μmol/kg. The primates were given 8 and 10 p.o. at a dose of 75 μmol/kg. In the chelator tissue distribution studies, the rats were given 3 and 8 s.c. at a dose of 300 μmol/kg. Ligand 3 was also given p.o. at the same dose. The drugs were administered to the rats and primates as their monosodium salts (prepared by the addition of 1 equiv of NaOH to a suspension of the free acid in distilled water). Drug preparation for the rodent urinary Kim-1 excretion studies involving 1, 3, and Exjade are described below.
Calculation of iron chelator efficiency
In the text below, the term “iron-clearing efficiency” (ICE) is used as a measure of the amount of iron excretion induced by a chelator. The ICE, expressed as a percent, is calculated as (ligand-induced iron excretion/theoretical iron excretion) × 100. To illustrate, the theoretical iron excretion after administration of one millimole of DFO (Fig. 1), a hexadentate chelator that forms a 1:1 complex with Fe(III), is one milli-g-atom of iron. Two millimoles of desferrithiocin (DFT, Fig. 1), a tridentate iron chelator that forms a 2:1 complex with Fe(III), are required for the theoretical excretion of one milli-g-atom of iron. The theoretical iron outputs of the chelators were generated on the basis of a 2:1 ligand:iron complex. The efficiencies in the rats and monkeys were calculated as set forth elsewhere (Bergeron et al. 1998, 1999). Data are presented as the mean ± the standard error of the mean; P-values were generated via a one-tailed Student’s t-test in which the inequality of variances was assumed; a P-value of <0.05 was considered significant.
Collection of chelator tissue distribution samples from rodents
Male Sprague–Dawley rats (250–350 g) were given a single s.c. injection of the monosodium salts of 3 and 8 prepared as described above at a dose of 300 μmol/kg; 3 was also given p.o. at the same dose. At times 0.5, 1, 2, 4 and 8 h after dosing (n = 3) rats per time point, the animals were euthanized by exposure to CO2 gas. Blood was obtained via cardiac puncture into vacutainers containing sodium citrate. The blood was centrifuged, and the plasma was separated for analysis. The liver, heart, pancreas, and kidneys were removed from the animals and frozen.
Tissue analytical methods
Tissue samples of animals treated with (S)-4′-(HO)-DADFT-norPE (3) and (S)-3′-(HO)-DADFT-norPE (8) were prepared for HPLC analysis by homogenizing them in 0.5 N HClO4 at a ratio of 1:3 (w/v). Then, as a rinse, CH3OH at a ratio of 1:3 (w/v) was added, and the mixture was stored at −20°C for 30 min. This homogenate was centrifuged. The supernatant was diluted with mobile phase A (95% buffer [25 mM KH2PO4, pH 3.0]/5% CH3CN), vortexed, and filtered with a 0.2 μm membrane. Analytical separation was performed on a Discovery RP Amide C16 HPLC system with UV detection at 310 nm as described previously (Bergeron et al. 1998, 2003). Mobile phase and chromatographic conditions were as follows: solvent A, 5% CH3CN/95% buffer; solvent B, 60% CH3CN/40% buffer. The concentrations were calculated from the peak area fitted to calibration curves by nonweighted least-squares linear regression with Rainin Dynamax HPLC Method Manager software (Rainin Instrument Co.). The method had a detection limit of 0.25 μM and was reproducible and linear over a range of 1–1000 μM. Tissue distribution data are presented as the mean; P-values were generated via a one-tailed student’s t-test, in which the inequality of variances was assumed; a P-value of <0.05 was considered significant.
28-day toxicity assessment of (S)-4′-(HO)-DADFT-norPE (3) in rats
Male Sprague–Dawley rats, 300–350 g, (n = 5) per group were given 3 p.o. once daily for 28 days at a dose of 56.9, 113.8 and 170.7 μmol/kg/d. Note that the doses chosen for 3 are approximately 1, 2, and 3 times the dose necessary to cause the excretion of 450 μg Fe/kg in the primates, the suggested iron clearance required to keep a thalassemia patient in negative iron balance (Brittenham 1990). Because of the protracted nature of the experiments (28 days), the animals were not fasted. To minimize food content in the stomach, the drug was not given until the afternoon. Additional animals served as age-matched controls. Urine was collected from the metabolic cages as described below to allow for the determination of Kim-1 levels. The rats were euthanized one day post drug (day 29), and extensive tissues (Bergeron et al. 1999) were collected for histopathological analysis.
Drug preparation and administration: rodent toxicity/urinary Kim-1 excretion studies
The impact of ligand 3 on urinary Kim-1 excretion was evaluated in rodents under three chelator dosing regimens. The drug was given p.o.: (1) once daily during the course of the 28-day toxicity trial, described above; (2) once daily at a dose of 384 μmol/kg/d × 10 days, and (3) twice daily at a dose of 237 μmol/kg/dose (474 μmol/kg/d) × 7 days. The studies were performed on rats with normal iron stores. Untreated age-matched rats were used as negative controls. Exjade was used as a positive control for the 384 μmol/kg/d × 10 days dosing regimen, while deferitrin (1) served as a positive control for the 237 μmol/kg/dose b.i.d. (474 μmol/kg/d) × 7 days drug exposure. The animals in the 10- and 7-day dosing protocols were fasted overnight and were given the first dose of the chelator first thing in the morning. The rats were fed ∼3 h post-drug and had access to food for ~5 h before being fasted overnight. Ligands 1 and 3 were administered to the rats p.o. as their monosodium salts, prepared as described above. Exjade was administered to the rats p.o. as a suspension in water.
Collection of urine for Kim-1 studies
The rats were housed in individual metabolic cages. Twenty-four hour urine samples were collected from the metabolic cages-once every 4 days for the animals in the 28-day toxicity study, and daily for the rats in the 10- and 7-day drug exposure trials. In all experiments, a baseline (day 0) urine sample was collected and assessed for its Kim-1 content; each animal served as its own control. The urine was collected chilled as follows: the urine collection tube was detached from the metabolic cage and inserted into a 473 ml specimen container containing approximately 240 g of gelled Techni Ice (the powder from 8 pouches/240 ml water). A styrofoam ring was placed around the collection tube and the container was frozen overnight at −80°C. The following morning, a 50-ml centrifuge tube was inserted into the frozen urine collection tube. The frozen specimen containers were placed into hollowed out styrofoam blocks that were 18 × 18 × 13 cm (l × w × h). The styrofoam blocks were positioned under the metabolic cages to allow for the collection of the urine.
Performance of rodent urinary Kim-1 studies
The chilled urine was collected, vortexed, and warmed to room temperature; any sediment in the samples was allowed to settle. Equal amounts of the sample diluent buffer and test urine (75 μl each) were added to a clean test tube. The test tube was vortexed, and 120 μl of the diluted urine was added to the sample well of an R-Rena Strip (Vaidya et al. 2009). The result was read 20 min later using a ReaScan Test Reader. The quantity of Kim-1 excreted in the urine per day was calculated by multiplying the concentration of Kim-1 (ng/ml urine) × 24-h urine volume, divided by the weight of the animal. The result is expressed as urinary Kim-1 (ng/kg/24 h). Data are presented as the mean ± the standard error of the mean; P-values were generated via a one-tailed Student’s t-test in which the inequality of variances was assumed; a P-value of <0.05 was considered significant.
Results
Chemical synthesis
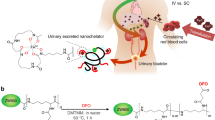
The synthesis of 3′-polyethers 8 and 10 (Table 1) was accomplished by modifying our efficient route to (S)-3′-(HO)-DADFT-PE (9) (Scheme 1) (Bergeron et al. 2008). In this instance, 2,3-dihydroxybenzonitrile (11) (Bergeron et al. 2008) was converted to its dianion with sodium hydride (2.0 equiv) in DMSO, and a molar equivalent of tosylate 12 (Brunner and Gruber 2004) or tosylate 14 (Brunner and Gruber 2004; Kitto et al. 2008) was added, resulting in 3-O-alkylated nitrile 13 (71% yield) or 15 (70% yield), respectively. Cyclocondensation of (S)-α-methyl cysteine (16) with precursor 13 at a pH of 6 generated (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6-dioxaheptyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (8), which has a shorter polyether chain than 9, in 93% yield (66% overall). In like manner, D-amino acid 16 and nitrile 15 gave (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6,9,12-tetraoxatridecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (10), which contains a longer polyether substituent than 9, in 85% yield (60% overall).
Synthesis of (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6-dioxaheptyloxy)phenyl]-4-methyl-4-thiazolecarboxylic Acid (8) and (S)-4,5-Dihydro-2-[2-hydroxy-3-(3,6,9,12-tetraoxatridecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic Acid (10)a. aReagents and conditions: (a) 60% NaH (2.0 equiv), DMSO, CH3[O(CH2)2]2OTs (12, 1.0 equiv), 71% (13) or CH3[O(CH2)2]4OTs (14, 1.0 equiv), 70% (15); (b) CH3OH (aq), pH 6, 70°C, 24 h, 93% (8) or 85% (10)]
Biological results
Chelator-induced iron clearance in non-iron-overloaded, bile duct-cannulated rodents
The 4′- and 3′-polyether families of desazadesferrithiocin (DADFT) ligands were chosen for evaluation because they best illustrate how the geometry and position of a polyether backbone, e.g., (S)-4,5-dihydro-2-[2-hydroxy-4-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (4, Table 1) versus (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid (9, Table 1), can influence a chelator’s behavior. We have included historical data in Table 1 to allow for comparative purposes (Bergeron et al. 2005, 2006, 2008, 2010).
In the rodents, the polyether analogues are more effective iron-clearing agents than their phenolic counterparts, e.g., 3–5 versus 1 and 8–10 versus 6 (Table 1). For example, the ICE of 1 in rodents is 1.1 ± 0.8% (Table 1). Introduction of a 3,6,9-trioxadecyloxy polyether fragment to yield analogue 4 increased the ICE to 5.5 ± 1.9% (P < 0.003), a five-fold increase (Bergeron et al. 2006). The shorter ether analogue, the 3,6-dioxaheptyloxy ligand (3), had an ICE of 26.7 ± 4.7% (Bergeron et al. 2010). Lengthening of the polyether chain to a 3,6,9,12-tetraoxatridecyloxy group, 5, resulted in a compound with an ICE of 12.0 ± 1.5% (Table 1). A similar trend was also observed with the 3′-polyether family of ligands (Table 1). The ICE of 6 in the rats is 4.6 ± 0.9% (Bergeron et al. 2005), while the ICE of the corresponding polyether analogue 9 is more than two-fold greater, 10.6 ± 4.4% (P < 0.04). The shorter ether ligand, the 3,6-dioxaheptoxy analogue, 8, had an ICE (15.1 ± 2.0) that was more than three-fold greater than that of its phenolic counterpart, 6 (P < 0.001). The ICE of the longer 3,6,9,12-tetraoxatridecyloxy polyether analogue, 10, was essentially the same as that of 9, 12.4 ± 1.7% versus 10.6 ± 4.4%, respectively (P > 0.05). Finally, the ICE in rats of the 4′-polyether parent, 4, is approximately half that of 3′-polyether parent, 9 (Table 1). However, the opposite is true when comparing the ICE of the shorter ether analogues, 3 versus 8: 3 is nearly twice as effective as 8 ( P < 0.02). In addition, the ICE of 3 is 2.5 times greater than that of the ligand currently in clinical trials, 9 (P < 0.005). Interestingly, the ICE of the 4′- and 3′-3,6,9,12-tetraoxa analogues 5 and 10 are virtually identical, 12.0 ± 1.5% and 12.4 ± 1.7%, respectively (Table 1).
Chelator-induced iron clearance in non-iron-overloaded, bile duct-cannulated rodents: dose response studies
In previous dose response studies with ligand 1, 4, and 9, given p.o. to bile duct-cannulated rats (Bergeron et al. 2008), we demonstrated that while the ICE of the parent drug 1 in rodents was uniformly poor at 300 and 150 μmol/kg (<2%), the polyether analogues 4 and 9 were significantly more efficient at all doses (Fig. 2). However, their deferration properties were shown to be saturable (Bergeron et al. 2008). The ICE for 4 increased nearly four-fold, from 5.5 ± 1.9% to 21.7 ± 3.5%, as the dose was decreased from 300 to 50 μmol/kg. The ICE for 9 also increased, nearly two-fold, on moving from 300 μmol/kg (10.6 ± 4.4%) to 50 μmol/kg (20.7 ± 4.4%). In the current study, 3 was given p.o. to the bile duct-cannulated rats at a dose of 300 or 50 μmol/kg (Fig. 2). The ICE of 3 was 26.7 ± 4.7% when it was given p.o. at 300 μmol/kg, and 22.8 ± 5.9% when the dose was reduced to 50 μmol/kg (P > 0.05). Therefore, in contrast to what was seen with 4 or 9, the deferration induced by analogue 3 was not saturable over this dose range. This suggests much greater flexibility in dosing with 3, which could be critical in managing patients with acute iron overload, e.g., iron poisoning.
Dose-response curves for compounds 1, 3, 4, and 9 administered p.o. to bile duct-cannulated rats. The ICE data, expressed as a percentage, (y-axis) for ligands 1, 4, and 9 are from Bergeron et al. (2008)
Chelator-induced iron clearance in iron-overloaded primates
The iron clearance data for ligands 1–10 in the primates are described in Table 1. Historical data have been included to allow for comparative purposes (Bergeron et al. 1999, 2003, 2005, 2006, 2008, 2010). Ligand 1 had an ICE of 16.8 ± 7.2%, while the ICE of the 4′-parent polyether (4) is 25.4 ± 7.4% (Bergeron et al. 2006). The shorter 3,6-dioxa analogue, 3, had an ICE of 26.3 ± 9.9% when it was given to the primates in capsules (Bergeron et al. 2010); the ICE was virtually identical when 3 was administered by gavage as its sodium salt, 28.7 ± 12.4% (P > 0.05). The ICE of the longer 3,6,9,12-tetraoxa analogue (5) was significantly less than that of either 3 or 4, 9.8 ± 1.9% (P < 0.001). The ICE of 6 and the 3′-polyether ligands, 7–9, were very uniform, 22–23% (Table 1). However, as with 5, the ICE the longer 3,6,9,12-tetraoxatridecyloxy 3′-polyether analogue (10) was significantly less than 8 or 9, 9.6 ± 4.9% (P < 0.001).
The ICE of the majority of the ligands in Table 1 are significantly greater in the iron-overloaded primates than in the non-iron-overloaded rats. In this regard, the performance ratio (PR), defined as the mean ICEprimate/ICErodent, provides an excellent decision-making tool for helping to determine which drugs to move forward. When iron chelators work well in both primates and rodents (PR close to 1.0), the probability of them also performing well in humans is higher. The three most effective polyether analogues, in terms of PR (Table 1), are 3 (PR 1.1), 8 (PR 1.5), and 9 (PR 2.2), suggesting that the ligands would be chosen to move forward in the following order 3 > 8 > 9. At the onset of the clinical trial focused on 9, no data were available for ligand 3 or 8. This is unfortunate, as 3 has many advantages over ligand 8 or 9. In addition to its superior ICE in rats, 3 also achieves much higher concentrations than 8 or 9 in the organs most adversely affected by excess iron: the liver, heart, and pancreas (discussed below). Finally, 3 is a crystalline solid, while 8 and 9 are oils.
Chelator tissue distribution in rodents
The tissue distribution description will focus on the 4′-DADFT ligands 1, 3, and 4 (Table 1) and on the 3′-DADFT analogues 8 and 9 (Table 1). In each instance, we will consider the liver, heart, plasma, pancreas, and kidney. In these studies, non-bile duct-cannulated (intact) rats were given a single 300-μmol/kg dose of the drug s.c. (Fig. 3) and/or p.o. (Fig. 4). The rats (n = 3/group) were sacrificed 0.5, 1, 2, 4 or 8 h post-drug. The historical data for ligands 1, 4, and 9 have been included for comparative purposes (Bergeron et al. 2006, 2007, 2008).
Tissue distribution in the liver, heart, plasma, pancreas, and kidney of rats treated with DADFT analogues 1, 3, 4, 8, and 9 given s.c. at a dose of 300 μmol/kg. The concentrations (y-axis) are reported as nmol compound per g wet weight of tissue, or as μM (plasma). For all time points, n = 3. The data for ligands 1, 4 and 9 are from Bergeron et al. (2006, 2007, 2008)
Tissue distribution in liver, heart, plasma, pancreas, and kidney of rats treated with DADFT analogues 1, 3, 4, and 9 given p.o. at a dose of 300 μmol/kg. The concentrations (y-axis) are reported as nmol compound per g wet weight of tissue, or as μM (plasma). For all time points, n = 3. The data for ligands 1, 4, and 9 are from Bergeron et al. (2008)
The 4′-norpolyether (3) achieved the highest level in all of the tissues evaluated. This was the case with 3 regardless of the route of administration. In the liver at 0.5 h, the relative concentrations of the ligands given to the rats s.c. were 3 > 4 > 9 > 8 > 1 (Fig. 3). A similar trend was also observed at the 1 h time point, except that the concentrations of 4 and 9 were virtually identical. Although all of the ligands were still detected in the liver at 8 h, the concentrations had decreased to less than 50 nmol/g wet weight (Fig. 3). When the chelators were given p.o., the relative concentration of the drugs in the liver at 0.5 and 1 h were 3 > 9 > 4 > 1 (Fig. 4). Once again, by 8 h, the concentration of the drugs in the liver had decreased to less than 50 nmol/g wet weight (Fig. 4). Note that the p.o. tissue distribution of analogue 8 has not been assessed, simply because of the low chelator tissue levels observed after s.c. dosing.
In the heart, at 0.5 h, ligand 3 given s.c. achieved a concentration of 178 ± 26 nmol/g wet weight (Fig. 3). This concentration is almost four-fold higher than that of 4 (47 ± 15 nmol/g wet weight, P < 0.002), and more than eight-fold greater than that of the ligand currently undergoing clinical trials, 9 (22 ± 13 nmol/g wet weight, P < 0.002). At 1 h, the order of concentration in the heart is 3 ≫ 4 > 9 > 8 ≈ 1. By 2 h post-drug, the chelator concentrations had decreased to less than 20 nmol/g wet weight, and were undetectable at the 8 h time point. A similar trend was observed when the drugs were given p.o. (Fig. 4). At 0.5 h, the concentration of ligand 3 in the heart was 186 ± 54 nmol/g wet weight, while that of 4 was 36 ± 8 nmol/g wet weight (P < 0.02 versus 3) and that of 9 was 59 ± 11 nmol/g wet weight (P < 0.03 versus 3). By 2 h post-drug, the chelator concentrations of all of the drugs had decreased to less than 20 nmol/g wet weight, and were virtually undetectable at the 4 and 8 h time points (Fig. 4).
The plasma chelator concentration data are consistent with the idea that the ligands are cleared quickly whether they are given s.c (Fig. 3) or p.o. (Fig. 4). The highest relative concentration of the ligands in the plasma are seen at the 0.5 h time point: 3 > 4 > 1 ≈ 8 > 9 when the chelators were given s.c. (Fig. 3), and (3 ≫ 4 > 9 > 1) when the drugs were given p.o. (Fig. 4). By 2 h post-drug, the drug concentrations have decreased to less than 50 μM when dosed s.c., and less than 20 μM when they were administered p.o. No drug was detectable at the 8 h time point (s.c. or p.o.).
In the pancreas, at 0.5 h, ligand 3 achieves a concentration of 98 ± 6 nmol/g wet weight when the drug is given s.c. (Fig. 3), and 110 ± 15 nmol/g wet weight when it is dosed p.o. (Fig. 4). At 1 h, the concentration of 3 is still elevated, 92 ± 9 nmol/g wet weight and 67 ± 7 nmol/g wet weight when dosed s.c. and p.o., respectively. The pancreatic chelator content of 3 at 0.5 h is significantly greater than that of 9, the ligand currently undergoing clinical trials: six-fold greater (P < 0.001) when the drugs are given s.c. (Fig. 3) and nearly three-fold greater (P < 0.005) when they are dosed p.o. (Fig. 4). By 4 h, ligand 1 is no longer detectable when the ligands are dosed s.c., while the concentration of the other chelators have decreased to 11–15 nmol/g wet weight. When the chelators are given p.o., ligand 4 is no longer detectable at 4 h, while the concentration of the other chelators are less than 6 nmol/g wet weight. Very little if any of the ligands are detected at the 8 h time point (s.c. or p.o.).
In the kidney, we previously demonstrated that the 4′-polyether (4) was much less nephrotoxic than deferitrin (1), which, at the time, was consistent with the relative tissue levels at the 2 h time point: the concentration of 4 was significantly less than that of 1 (Bergeron et al. 2006). However, renal concentration data from samples collected earlier than 2 h, e.g., 0.5 and 1 h, revealed that the tissue chelator concentrations of the two drugs were virtually identical to each other (Bergeron et al. 2007). Thus, the reduced nephrotoxicity of 4 versus 1 could not be explained simply by lower kidney chelator levels. In the current study, at 0.5 h, the relative concentration in the kidney of the drugs given s.c. (Fig. 3) is 3 ≫ 4 ≈ 1 > 8 > 9. At 1 h, the order is 3 > 9 ≈ 4 > 8 > 1; at 2 h, the order is 8 ≈ 9 > 3 > 1 > 4. Finally, at the 8 h time point, the concentration of all of the chelators are less than 15 nmol/g wet weight. A similar trend was observed when the drugs were given p.o. (Fig. 4). At 0.5 h, the relative concentration of the chelators in the kidney is 3 > 9 > 1 > 4. At 1 h, the order is 3 > 1 > 9 > 4, while at 2 h, the concentration order is 1 > 3 > 4 > 9. At 8 h, the renal concentration of 1 is 31 nmol/g wet weight, while those of the other chelators are less than 10 nmol/g wet weight (Fig. 4).
Toxicity evaluation of ligand 3 in rodents: impact on urinary Kim-1 excretion
The high concentration of ligand 3 found in the kidney during the course of the tissue distribution studies after s.c. or p.o. administration caused some concern as to how this could affect the ligand’s toxicity profile. In a previous experiment, 3 was given p.o. to rats with normal iron stores once daily at a dose of 384 μmol/kg/d for 10 d (Bergeron et al. 2010). The drug was found to be well tolerated. Extensive tissues (Bergeron et al. 1999) were submitted for histological analysis; no drug-related abnormalities were found. In addition, the rats’ BUN and SCr levels were within the normal range. However, it is well documented that BUN and SCr are poor predictors of acute kidney injury. Therefore, in the current study, we elected to assess the affect of ligand 3 on Kim-1, a novel urinary biomarker that allows for the detection of very subtle changes in renal tubule integrity (Han et al. 2002; Bonventre 2009). Kim-1 appears in the urine very early in response to exposure to a nephrotoxic agent, far sooner than increases in BUN or SCr can be detected (Zhou et al. 2008; Vaidya et al. 2006). Accordingly, the impact of ligand 3 on urinary Kim-1 excretion was evaluated in rodents under three chelator dosing regimens. The drug was given p.o.: (1) once daily during the course of the 28 d toxicity trial described above; (2) once daily at a dose of 384 μmol/kg/d × 10 d, and (3) twice daily at a dose of 237 μmol/kg/dose (474 μmol/kg/d) × 7 d. The studies were performed on rats with normal iron stores. Untreated age-matched rats were used as negative controls. Exjade was used as a positive control for the 384 μmol/kg/d × 10 days dosing regimen, while deferitrin (1) served as a positive control for the 237 μmol/kg/dose b.i.d. (474 μmol/kg/d) × 7 d drug exposure.
The urinary Kim-1 content of the untreated, age-matched negative control rats (n = 5) from the 28-day toxicity trial of 3 was monitored for 24-h every 4 days, on day 0, 4, 8, 12, 16, 20, 24, and 28. The data are expressed as urinary Kim-1 (ng/kg/24 h). Very little Kim-1 was found in the urine of the untreated animals at any time (Fig. 5a). The rats were euthanized on day 29; their BUN at that time was 19 ± 3 mg/dl, while their SCr was 0.5 ± 0 mg/dl. Note that these values are well within the normal range for this species: 9–30 mg/dl for BUN, and 0.4–1.0 mg/dl for SCr (Antech Diagnostics).
Urinary Kim-1 excretion, expressed as Kim-1 (ng/kg/24 h), for group: a untreated age-matched control rats; b rats treated with 3 p.o. once daily at a dose of 170.7 μmol/kg/d × 28 d; c rats given Exjade p.o. once daily at a dose of 384 μmol/kg/d; d rats given 3 p.o. once daily at a dose of 384 μmol/kg/d × 10 d; e rats given deferitrin (1) p.o. twice daily at a dose of 237 μmol/kg/dose (474 μmol/kg/d) × 7 d, and f rats given 3 p.o. twice daily at a dose of 237 μmol/kg/dose (474 μmol/kg/d) × 7 d. Note that none of the rats survived the planned 10-day exposure to Exjade. For groups (a–d, f), n = 5; group (e), n = 3
Comparable results were found when rats (n = 5/dose) were given ligand 3 p.o. once daily for 28 days at a dose of 56.9, 113.8, or 170.7 μmol/kg/day. These doses are approximately 1, 2, and 3 times the dose necessary to excrete 450 μg Fe/kg in the primates, the suggested iron clearance required to keep a thalassemia patient in negative iron balance (Brittenham 1990). All of the rats survived the exposure to the drug. Urine was collected as described above for the control rats. The urinary Kim-1 data for the animals in the 170.7 μmol/kg/d group are presented in Fig. 5b. Their baseline urinary Kim-1 content was 8 ± 2 ng/kg/24 h and did not increase above this value throughout the course of the study. The rats were euthanized one day post drug (day 29); their BUN and SCr at that time were in the normal range. In fact, their BUN (20 ± 1 mg/dl) and SCr (0.5 ± 0 mg/dl) were virtually identical to those of the age-matched control rats. Similar results for urinary Kim-1 excretion, BUN, and SCr, were also observed in the rats given 3 at the two lower doses (data not shown). Extensive tissues (Bergeron et al. 1999) were collected from all of the rats. The tissues from the untreated control animals and the rats treated with 3 at 170.7 μmol/kg/d × 28 d were sent out for histopathology; no drug-related abnormalities were found.
In a second study, we chose to assess the impact of Exjade and 3 on urinary Kim-1 excretion when the chelators were given to the rats p.o. once daily at a dose of 384 μmol/kg/d × 10 d. Note that this is our “standard” 10-day toxicity dosing regimen (Bergeron et al. 1999); the dose is equivalent to 100 mg/kg/d of the DFT sodium salt, and corresponds to a dose of 143 mg/kg/d of Exjade. Exjade was selected to serve as a positive control for this study due to its well-known nephrotoxicity issues (Nick et al. 2002; Nisbet-Brown et al. 2003). Urinary Kim-1 levels were assessed at 24-h intervals. None of the rats (n = 5) survived the planned 10-day exposure to Exjade. One rat was found dead the morning of day 5. He had received four doses of the drug. The remaining rats became moribund and were sacrificed after being given the drug for 7 days (two rats) or 8 days (two rats). This is consistent with what had been reported during the rodent preclinical toxicity assessments of Exjade (Nick et al. 2002). The rats’ baseline (day 0) urinary Kim-1 value was 10 ± 8 ng/kg/24 h (Fig. 5c). After 2 days of Exjade, the Kim-1 had increased to 27 ± 15 ng/kg/24 h (P < 0.04). After 4 days of Exjade, the Kim-1 was 76 ± 39 ng/kg/24 h, and by day 8 the urinary Kim-1 of the two surviving rats was 1010 ± 173 ng/kg/24 h. Blood was taken from the moribund animals immediately prior to sacrifice; the serum was assessed for its BUN and creatinine content. The rats’ BUN was 139 ± 71 mg/dl, while their SCr was 2.5 ± 0.7 mg/dl. These values are well outside of the normal range. In fact, the BUN and SCr of the Exjade treated rats are seven-fold (P < 0.03) and five-fold (P < 0.01) greater, respectively, than those of the age-matched control rats. In addition, as no blood was obtained from the animal that was found dead, these values likely underestimate the actual impact of Exjade on these parameters.
Conversely, a separate group of rats (n = 5) was given analogue 3 p.o. under an identical dosing regimen, 384 μmol/kg/d for 10 d. Although this study had been performed previously (Bergeron et al. 2010), it was repeated to allow for the determination of urinary Kim-1 levels. All of the rats survived the chelator dosing period. Urinary Kim-1 levels were assessed at 24-h intervals. The baseline (day 0) urinary Kim-1 content of the 3-treated rats was 9 ± 9 ng/kg/24 h and remained within error of this value throughout the chelator dosing regimen (Fig. 5d). The rats were sacrificed on day 11 (one day post exposure). Their BUN and SCr at that time were 19 ± 1 mg/dl and 0.4 ± 0.1 mg/dl, respectively, well within the normal range, and virtually identical to those of the age-matched control rats.
In a final experiment, we chose to replicate our renal perfusion chelator dosing protocol (Bergeron et al. 2006) with deferitrin (1) and analogue 3, in which rats are given a chelator b.i.d at a dose of 237 μmol/kg/dose (474 μmol/kg/d, equivalent to 120 mg/kg/d of 1) × 7 d, to determine their impact on urinary Kim-1 excretion. Deferitrin (1) served as a positive control. Recall that we had previously demonstrated that the proximal tubules of rats treated with 1 p.o. b.i.d. × 7 d showed regional, moderate to severe vacuolization, a loss of brush border, and extrusion toward the lumen (Bergeron et al. 2006). In the current study, all of the 1-treated rats (n = 3) survived the 7-day chelator dosing period. Urine samples were collected at 24-h intervals. The rats’ baseline (day 0) urinary Kim-1 content was 13 ± 8 ng/kg/24 h (Fig. 5e). After 3 days of exposure to 1, the urinary Kim-1 had increased to 69 ± 47 ng/kg/24 h. At the end of the 7-day dosing period, the urinary Kim-1 had further increased to 189 ± 187 ng/kg/24 h (Fig. 5e). The rats were euthanized on day 8; their BUN at that time was 32 ± 13 mg/dl, while their SCr was 1.3 ± 1.0 mg/dl. Note that although the rats’ BUN and SCr levels are only slightly outside of the normal range for this species, these values are 1.6 and 2.6 times greater than the BUN and SCr, respectively, of the age-matched control rats.
A separate group of rats (n = 5) were given analogue 3 p.o. under an identical dosing regimen, 237 μmol/kg/dose b.i.d. (474 μmol/kg/d) × 7 d. All of the rats survived the dosing period. Urinary Kim-1 levels were assessed at 24-h intervals. The rats’ baseline (day 0) urinary Kim-1 content was 18 ± 9 ng/kg/24 h and stayed within error of this value for the duration of the drug exposure (Fig. 5f). The rats were euthanized on day 8; their BUN at that time was 16 ± 3 mg/dl, while their SCr was 0.5 ± 0.1 mg/dl. Both values are well within the normal range for this species, and are virtually identical to those of the age-matched control rats. Thus, despite the high levels of 3 observed in the kidney during the chelator tissue distribution studies, ligand 3 does not have an adverse affect on urinary Kim-1 excretion, even when the drug is administered to the rats for up to 28 d.
Discussion
Deferitrin (1, Fig. 1) was abandoned in clinical trials as an iron chelator for the treatment of iron overload disease because of its nephrotoxicity (Galanello et al. 2007). However, subsequent investigations revealed that replacing the 4′-(HO) of 1 with a 3,6,9-trioxadecyloxy group, ligand 4 (Table 1), increased iron clearing efficiency (ICE) and ameliorated the renal toxicity in rats of 1 (Bergeron et al. 2006). The ICE in both rodents and primates and the absence of toxicity seen with the 4′-polyether (4) compelled us to investigate what the impact of fixing the 3,6,9-polyether chain to the aromatic ring of DADFT at positions other than the 4′-carbon had on ICE and ligand tissue distribution (Bergeron et al. 2007). One of these ligands, in which the polyether chain has been fixed to the 3′-carbon, 9 (Table 1), is currently undergoing clinical trials. Although the preclinical toxicity profile of 9 was benign, the clinical results remain to be elucidated.
These findings encouraged an assessment of how altering the length of the polyether chain would affect a ligand’s ICE, lipophilicity, and physiochemical properties. In a previous study, the 3,6,9-trioxadecyloxy substituent at the 4′-position of ligand 4 was both shortened to a 3,6-dioxaheptyloxy moiety, providing 3, and lengthened to a 3,6,9,12-tetraoxatridecyloxy group, providing 5 (Bergeron et al. 2010). These same manipulations have now been carried out with the corresponding 3′-polyether parent (9), resulting in the shorter 3,6-dioxaheptyloxy polyether analogue (8), and the longer 3,6,9,12-tetraoxatridecyloxy ligand (10). Note that while the 4′- and 3′-polyether parents, 4, and 9, respectively, and analogues 5, 8, and 10 are oils, ligand 3 is a crystalline solid (Bergeron et al. 2010). The iron clearance properties of 3 and 5 in rodents and primates were reported in a previous publication (Bergeron et al. 2010). These measurements have now been made with the corresponding 3′-polyether analogues, 8 and 10, in rodents and primates (Table 1); a dose–response study with 3 in the rats has also been carried out (Fig. 2). In addition, the chelator tissue distribution of ligand 3 and 8 in rats after s.c. (Fig. 3) and/or p.o. (Fig. 4) dosing has been assessed. Finally, the impact of the lead candidate, 3, on rat urinary Kim-1 excretion has been evaluated under three chelator dosing regimens (Fig. 5).
The 4′-3,6-dioxaheptyloxy analogue, ligand 3, had an ICE in rats that was nearly five-fold greater than that of its parent, 4; it was also very effective (ICE > 26%) in the iron-loaded primates (Bergeron et al. 2010). The longer 3,6,9,12-tetraoxatridecyloxy analogue of 4, chelator 5, had twice the ICE in rodents of 4, but its ICE in primates was reduced relative to that of 4 (Bergeron et al. 2010). In the current study, the ICE in rats of the shorter 3′-polyether ligand, 8, was 1.4-fold greater than that of its parent, 9. The ICE of the longer 3,6,9,12-tetraoxatridecyloxy polyether analogue, 10, in rats was essentially the same as that of 9. Interestingly, while the ICE in rats of the 4′-polyether parent, 4, is approximately half that of 3′-polyether parent, 9, the opposite is true when comparing the ICE of the shorter ether analogues, 3 versus 8: 3 is nearly twice as effective as 8. In addition, the ICE of 3 in rats is 2.5-fold greater than that of the ligand currently in clinical trials, 9. Finally, in rodent chelator dose–response studies, while the deferration induced by 4 and 9 was shown to be saturable as the dose was decreased from 300 to 50 μmol/kg (Bergeron et al. 2008), this was not the case with ligand 3 (Fig. 2). This suggests much greater flexibility in dosing with 3, which could be critical in managing patients with acute iron overload, e.g., iron poisoning. In the primates, the ICE of 8 was within error of that of the 3′-polyether parent, 9. However, the ICE of the tetraoxatridecyloxy-substituted analogue, 10, in primates was significantly less than 8 or 9. The performance ratio, ICEprimate/ICErodent, suggests that the ligands would be chosen to move forward in the following order 3 > 8 > 9. However, at the onset of the clinical trial focused on 9, no data were available for ligand 3 or 8.
The effect of altering the length and position of the polyether chain on ligand-tissue concentrations is significant. The 4′-norpolyether (3) achieved the highest level of all of the ligands in the liver, heart and pancreas, the organs most affected by iron overload. This was the case whether the chelators were given s.c. (Fig. 3) or p.o. (Fig. 4). For example, in the heart, at 0.5, the concentration of 3 given s.c. was almost four-fold higher than that of 4, and greater than eight-fold that of 9. In the pancreas, at 0.5 h, the concentration of 3 was six-fold greater than 9 when the drugs were given s.c., and nearly three-fold greater than 9 when they were given to the rats p.o. However, high levels of ligand 3 were also found in the kidney after s.c. or p.o. dosing. This caused some concern as to how this might affect the ligand’s toxicity, particularly nephrotoxicity, profile.
Recall that the major hurdle in exploiting the DFT pharmacophore, e.g., deferitrin (1), as an orally active iron chelator was its nephrotoxicity (Galanello et al. 2007). Iron chelator-induced proximal tubule damage is not uncommon. In fact, one of the problems associated with a currently accepted iron overload treatment, Exjade, is proximal tubule-derived renal damage (Nick et al. 2002; Yacobovich et al. 2010; Exjade Prescribing Information). Unfortunately, biomarkers, e.g., BUN and SCr levels, often do not increase until a serious loss of renal function has unfolded. This results in the performance of long-term, expensive exposure studies until sufficient evidence of nephrotoxicity presents to warrant terminating the trial. However, this problem has now been overcome with the discovery of kidney injury molecule-1 (Kim-1, rodent) and (KIM-1, human).
Kim-1 is a type 1 transmembrane protein located in the epithelial cells of proximal tubules (Han et al. 2002). The ectodomain of the Kim-1 proximal tubule protein is released into the urine very early after exposure to a nephrotoxic agent or ischemia (Bailly et al. 2002); it appears far sooner than increases in BUN or SCr are detected. Although urinary Kim-1 measurements have been performed for several years using enzyme-linked immunosorbent assays (Bailly et al. 2002) or a Luminex assay (van Timmeren et al. 2006) involving multi-analyte profiling beads (xMAP) technologies, these assessments required 3–4 h of assay time and were dependent on a large analyzer. However, to circumvent these issues, BioAssay Works has recently developed RenaStick, a direct lateral flow immunochromatographic assay, which allows for the rapid detection and quantitation of rat urinary Kim-1 (Vaidya et al. 2009). A similar test kit is also available to determine the KIM-1 content of human urine. This now makes it possible in both rodents and patients to uncover potential chelator-induced renal toxicity early on.
The impact of ligand 3 on urinary Kim-1 excretion has been evaluated in rodents when the drug was given p.o.-once daily for 28 d (56.9, 113.8, or 170.7 μmol/kg/day); once daily for 10 d (384 μmol/kg/d), and twice daily at 237 μmol/kg/dose (474 μmol/kg/d) × 7 d. The studies were performed on rats with normal iron stores. Untreated age-matched rats were used as negative controls. Exjade was used as a positive control for the 384 μmol/kg/d × 10 d dosing regimen, while deferitrin (1) served as a positive control for the 237 μmol/kg/dose b.i.d. (474 μmol/kg/d) × 7 d drug exposure. Very little Kim-1 was found in the urine of the age-matched negative control rats at any time (Fig. 5a). However, considerable quantities of Kim-1 were found in the urine of the Exjade-treated rats (Fig. 5c); the rats’ BUN and SCr were also significantly increased. In fact, none of the Exjade-treated rats survived the planned 10-day exposure to the drug. The b.i.d. × 7 d dosing of deferitrin (1) was also associated with an increase in urinary Kim-1 excretion (Fig. 5e). Although the BUN and SCr of the 1-treated rats were slightly elevated, all of animals survived the exposure to the drug. In sharp contrast, very little Kim-1 was found at any time in the urine of any of the rats exposed to 3 (Fig. 5b, d, f). Finally, the BUN and SCr of the 3-treated groups of rats were virtually identical to those of the age-matched control animals.
Our results have provided additional evidence for the efficacy, safety, and tolerability of the 3,6-dioxaheptyloxy polyether ligand (3). The ICE of 3 in rats is 2.5-fold that of 9, the drug currently undergoing clinical trials. In addition, while the deferration in rats induced by 4 and 9 was shown to be saturable as the dose was decreased from 300 to 50 μmol/kg (Bergeron et al. 2008), this was not the case with 3. In rodent chelator tissue distribution studies, analogue 3 was shown to achieve very high levels in the liver, pancreas and heart, the organs most affected by iron overload. Although significant quantities of 3 were also found in the kidney, no evidence of nephrotoxicity was found. Overall, these results indicate the need for prompt completion of the preclinical evaluation of 3 in preparation for studies in human volunteers. Analogue 3 may provide an orally effective alternative to s.c. DFO for the treatment of both chronic transfusional iron overload and of acute iron poisoning.
References
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G (2000) Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 343:327–331
Antech Diagnostics (2010) http://www.antechdiagnostics.com/. Accessed October 2010
Babbs CF (1992) Oxygen radicals in ulcerative colitis. Free Radic Biol Med 13:169–181
Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV (2002) Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem 277:39739–39748
Bergeron RJ (1986) Iron: a controlling nutrient in proliferative processes. Trends Biochem Sci 11:133–136
Bergeron RJ, Streiff RR, Wiegand J, Vinson JRT, Luchetta G, Evans KM, Peter H, Jenny H-B (1990) A comparative evaluation of iron clearance models. Ann NY Acad Sci 612:378–393
Bergeron RJ, Streiff RR, Wiegand J, Luchetta G, Creary EA, Peter HH (1992) A comparison of the iron-clearing properties of 1,2-dimethyl-3-hydroxypyrid-4-one, 1,2-diethyl-3-hydroxypyrid-4-one, and deferoxamine. Blood 79:1882–1890
Bergeron RJ, Streiff RR, Creary EA, Daniels RD Jr, King W, Luchetta G, Wiegand J, Moerker T, Peter HH (1993) A comparative study of the iron-clearing properties of desferrithiocin analogues with desferrioxamine B in a Cebus monkey model. Blood 81:2166–2173
Bergeron RJ, Wiegand J, Wollenweber M, McManis JS, Algee SE, Ratliff-Thompson K (1996) Synthesis and biological evaluation of naphthyldesferrithiocin iron chelators. J Med Chem 39:1575–1581
Bergeron RJ, Wiegand J, Brittenham GM (1998) HBED: a potential alternative to deferoxamine for iron-chelating therapy. Blood 91:1446–1452
Bergeron RJ, Wiegand J, McManis JS, McCosar BH, Weimar WR, Brittenham GM, Smith RE (1999) Effects of C-4 stereochemistry and C-4′ hydroxylation on the iron clearing efficiency and toxicity of desferrithiocin analogues. J Med Chem 42:2432–2440
Bergeron RJ, Wiegand J, McManis JS, Bussenius J, Smith RE, Weimar WR (2003) Methoxylation of desazadesferrithiocin analogues: enhanced iron clearing efficiency. J Med Chem 46:1470–1477
Bergeron RJ, Wiegand J, McManis JS, Weimar WR, Park JH, Eiler-McManis E, Bergeron J, Brittenham GM (2005) Partition-variant desferrithiocin analogues: organ targeting and increased iron clearance. J Med Chem 48:821–831
Bergeron RJ, Wiegand J, McManis JS, Vinson JRT, Yao H, Bharti N, Rocca JR (2006) (S)-4,5-Dihydro-2-(2-hydroxy-4-hydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid polyethers: a solution to nephrotoxicity. J Med Chem 49:2772–2783
Bergeron RJ, Wiegand J, Bharti N, Singh S, Rocca JR (2007) Impact of the 3,6,9-trioxadecyloxy group on desazadesferrithiocin analogue iron clearance and organ distribution. J Med Chem 50:3302–3313
Bergeron RJ, Wiegand J, McManis JS, Bharti N, Singh S (2008) Design, synthesis, and testing of non-nephrotoxic desazadesferrithiocin polyether analogues. J Med Chem 51:3913–3923
Bergeron RJ, Bharti N, Wiegand J, McManis JS, Singh S, Abboud KA (2010) The impact of polyether chain length on the iron clearing efficiency and physiochemical properties of desferrithiocin analogues. J Med Chem 53:2843–2853
Bonkovsky HL, Lambrecht RW (2000) Iron-induced liver injury. Clin Liver Dis 4:409–429 vi–vii
Bonventre JV (2009) Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transpl 24:3265–3268
Brittenham GM (1990) Pyridoxal isonicotinoyl hydrazone (PIH): effective iron chelation after oral administration. Ann NY Acad Sci 612:315–326
Brittenham GM (2000) Disorders of iron metabolism: iron deficiency and overload. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ (eds) Hematology: basic principles and practice, 3rd edn. Churchill Livingstone, New York, pp 397–428
Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 331:567–573
Brunner H, Gruber N (2004) Carboplatin-containing porphyrin-platinum complexes as cytotoxic and phototoxic antitumor agents. Inorg Chim Acta 357:4423–4451
Byers BR, Arceneaux JE (1998) Microbial iron transport: iron acquisition by pathogenic microorganisms. Met Ions Biol Syst 35:37–66
Cario H, Holl RW, Debatin KM, Kohne E (2004) Insulin sensitivity and β-cell secretion in thalassemia major with secondary haemochromatosis: assessment by oral glucose tolerance test. Eur J Pediatr 162:139–146
Conrad ME, Umbreit JN, Moore EG (1999) Iron absorption and transport. Am J Med Sci 318:213–229
Exjade Prescribing Information (2010) http://www.pharma.us.novartis.com/product/pi/pdf/exjade.pdf. Accessed October 2010
Galanello R, Forni G, Jones A, Kelly A, Willemsen A, He X, Johnston A, Fuller D, Donovan J, Piga A (2007) A dose escalation study of the pharmacokinetics, safety & efficacy of deferitrin, an oral iron chelator in beta thalassaemia patients. ASH Annu Meet Abstr 110:2669
Giardina PJ, Grady RW (2001) Chelation therapy in ß-thalassemia: an optimistic update. Semin Hematol 38:360–366
Goodsaid FM, Blank M, Dieterle F, Hausner E, Sistare F, Thompson A, Vonderscher J (2009) Novel biomarkers of acute kidney toxicity. Clin Pharmacol Ther 86:490–496
Graf E, Mahoney JR, Bryant RG, Eaton JW (1984) Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem 259:3620–3624
Halliwell B (1994a) Free radicals and antioxidants: a personal view. Nutr Rev 52:253–265
Halliwell B (1994b) Iron, oxidative damage, and chelating agents. In: Bergeron RJ, Brittenham GM (eds) The development of iron chelators for clinical use. CRC, Boca Raton, pp 33–56
Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV (2002) Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62:237–244
Hazen SL, d’Avignon A, Anderson MM, Hsu FF, Heinecke JW (1998) Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to oxidize α-amino acids to a family of reactive aldehydes. Mechanistic studies identifying labile intermediates along the reaction pathway. J Biol Chem 273:4997–5005
Hoffbrand AV, Al-Refaie F, Davis B, Siritanakatkul N, Jackson BFA, Cochrane J, Prescott E, Wonke B (1998) Long-term trial of deferiprone in 51 transfusion-dependent iron overloaded patients. Blood 91:295–300
Hoffmann D, Fuchs TC, Henzler T, Matheis KA, Herget T, Dekant W, Hewitt P, Mally A (2010) Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology 277:49–58
Kersten MJ, Lange R, Smeets ME, Vreugdenhil G, Roozendaal KJ, Lameijer W, Goudsmit R (1996) Long-term treatment of transfusional iron overload with the oral iron chelator deferiprone (L1): a Dutch multicenter trial. Ann Hematol 73:247–252
Kitto HJ, Schwartz E, Nijemeisland M, Koepf M, Cornelissen JJLM, Rowan AE, Nolte RJM (2008) Post-modification of helical dipeptido polyisocyanides using the ‘click’ reaction. J Mater Chem 18:5615–5624
Koppenol W (2000) Kinetics and mechanism of the Fenton reaction: implications for iron toxicity. In: Badman DG, Bergeron RJ, Brittenham GM (eds) Iron chelators: new development strategies. Saratoga, Ponte Vedra Beach, pp 3–10
Lieu PT, Heiskala M, Peterson PA, Yang Y (2001) The roles of iron in health and disease. Mol Aspects Med 22:1–87
Malcovati L (2007) Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leukemia Res 31:S2–S6
Millán M, Sobrino T, Arenillas JF, Rodríguez-Yáñez M, García M, Nombela F, Castellanos M, de la Ossa NP, Cuadras P, Serena J, Castillo J, Dávalos A (2008) Biological signatures of brain damage associated with high serum ferritin levels in patients with acute ischemic stroke and thrombolytic treatment. Dis Markers 25:181–188
Nick H, Wong A, Acklin P, Faller B, Jin Y, Lattmann R, Sergejew T, Hauffe S, Thomas H, Schnebli HP (2002) ICL670A: preclinical profile. Adv Exp Med Biol 509:185–203
Nisbet-Brown E, Olivieri NF, Giardina PJ, Grady RW, Neufeld EJ, Sechaud R, Krebs-Brown AJ, Anderson JR, Alberti D, Sizer KC, Nathan DG (2003) Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 361:1597–1602
Olivieri NF (1996) Long-term therapy with deferiprone. Acta Haematol 95:37–48
Olivieri NF (2001) Progression of iron overload in sickle cell disease. Semin Hematol 38:57–62
Olivieri NF, Brittenham GM (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761
Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, Burt AD, Fleming KA (1998) Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med 339:417–423
Pietrangelo A (2002) Mechanism of iron toxicity. Adv Exp Med Biol 509:19–43
Pietrangelo A (2007) Iron chelation beyond transfusion iron overload. Am J Hematol 82:1142–1146
Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, Donato G, Bordone E, Lavagetto A, Zanaboni L, Sechaud R, Hewson N, Ford JM, Opitz H, Alberti D (2006) Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica 91:873–880
Pippard MJ (1987) Iron overload and iron chelation therapy in thalassaemia and sickle cell haemoglobinopathies. Acta Haematol 78:206–211
Pippard MJ (1989) Desferrioxamine-induced iron excretion in humans. Bailliere’s Clin Haematol 2:323–343
Ponka P, Beaumont C, Richardson DR (1998) Function and regulation of transferrin and ferritin. Semin Hematol 35:35–54
Raymond KN, Carrano CJ (1979) Coordination chemistry and microbial iron transport. Acc Chem Res 12:183–190
Richardson DR (2001) The controversial role of deferiprone in the treatment of thalassemia. J Lab Clin Med 137:324–329
Theil EC, Huynh BH (1997) Ferritin mineralization: ferroxidation and beyond. J Inorg Biochem 67:30
Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV (2006) Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290:F517–F529
Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, Edwards JR, Bobadilla NA, Mefferd SC, Bonventre JV (2009) A rapid urine test for early detection of kidney injury. Kidney Int 76:108–114
van Timmeren MM, Bakker SJL, Vaidya VS, Bailly V, Schuurs TA, Damman J, Stegeman CA, Bonventre JV, van Goor H (2006) Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol 291:F456–F464
Vichinsky EP (2001) Current issues with blood transfusions in sickle cell disease. Semin Hematol 38:14–22
Wojcik JP, Speechley MR, Kertesz AE, Chakrabarti S, Adams PC (2002) Natural history of C282Y homozygotes for haemochromatosis. Can J Gastroenterol 16:297–302
Wood JK, Milner PF, Pathak UN (1968) The metabolism of iron-dextran given as a total-dose infusion to iron deficient Jamaican subjects. Br J Hamaetol 14:119–129
Yacobovich J, Stark P, Barzilai-Birenbaum S, Krause I, Yaniv I, Tamary H (2010) Acquired proximal renal tubular dysfunction in beta-thalassemia patients treated with deferasirox. J Pediatr Hematol Oncol 32:564–567
Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106:1866–1875
Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL (2008) Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci 101:159–170
Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, Di Gregorio F, Burattini MG, Terzoli S (1989) Survival and causes of death in thalassemia major. Lancet 2:27–30
Acknowledgments
The project described was supported by grant number R37DK049108 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. We thank Elizabeth M. Nelson and Katie Ratliff-Thompson for their technical assistance, and Miranda E. Coger for her editorial and organizational support. We acknowledge the spectroscopy services in the Chemistry Department, University of Florida, for the mass spectrometry analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergeron, R.J., Wiegand, J., Bharti, N. et al. Desferrithiocin analogue iron chelators: iron clearing efficiency, tissue distribution, and renal toxicity. Biometals 24, 239–258 (2011). https://doi.org/10.1007/s10534-010-9389-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9389-y