Abstract
Pseudomonas entomophila L48 is a recently identified entomopathogenic bacterium which, upon ingestion, kills Drosophila melanogaster, and is closely related to P. putida. The complete genome of this species has been sequenced and therefore a genomic, genetic and structural analysis of the siderophore-mediated iron acquisition was undertaken. P. entomophila produces two siderophores, a structurally new and unique pyoverdine and the secondary siderophore pseudomonine, already described in P. fluorescens species. Structural analysis of the pyoverdine produced by the closely related P. putida KT2440 showed that this strain produces an already characterised pyoverdine, but different from P. entomophila, and no evidence was found for the production of a second siderophore. Growth stimulation assays with heterologous pyoverdines demonstrated that P. entomophila is able to utilize a large variety of structurally distinct pyoverdines produced by other Pseudomonas species. In contrast, P. putida KT2440 is able to utilize only its own pyoverdine and the pyoverdine produced by P. syringae LMG 1247. Our data suggest that although closely related, P. entomophila is a more efficient competitor for iron than P. putida.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When fluorescent pseudomonads are grown under iron limiting conditions, they produce a yellow–green, fluorescent siderophore, called pyoverdine (Meyer and Abdallah 1978). The structures of pyoverdines produced by different strains of fluorescent pseudomonads have been characterised. They are made of three distinct structural parts: a small peptide chain of l- and d-amino acids (6–12 amino acids depending on the producing strain), linked to a yellow–green chromophore group and a small dicarboxylic acid or its monoamide connected amidically to the NH2-group of the chromophore (Teintze et al. 1981; Budzikiewicz 1993, 1997). Pyoverdines contain both catechol and hydroxamate groups that are participating in Fe(III) binding, the chromophore contains one catechol group, and the peptide chain two hydroxamate groups.
Besides pyoverdine, several other secondary siderophores, which have a relatively lower affinity for iron, have been identified in fluorescent pseudomonads. These include thioquinolobactin, pyochelin, pseudomonine, corrugatin and the recently identified ornicorrugatin (Cox and Graham 1979; Risse et al. 1998; Mercado-Blanco et al. 2001; Cornelis and Matthijs 2002; Matthijs et al. 2007, 2008).
The ability to produce the high affinity siderophore pyoverdine in addition to other siderophores of lower affinity makes fluorescent pseudomonads well equipped to compete with other micro-organisms for iron (Ravel and Cornelis 2003).
Another trait that makes fluorescent pseudomonads good competitors for iron is the ability to utilise iron complexes of a variety of different siderophores produced by other micro-organisms, including fungi and bacteria (Poole et al. 1990; Jurkevitch et al. 1992; Meyer 1992; Raaijmakers et al. 1995). Whereas fluorescent pseudomonads are able to utilise siderophores of members of other genera, the pyoverdines produced by fluorescent pseudomonads cannot be used by bacteria outside this group. In this study a genomic, genetic and structural analysis of pyoverdine-mediated iron acquisition in the entomopathogenic bacterium Pseudomonas entomophila L48 and P. putida KT2440 was undertaken. Genomic and mass analysis showed the presence of the secondary siderophore pseudomonine in P. entomophila. In addition the ability to utilize heterologous pyoverdines was investigated revealing a high capacity of P. entomophila L48 to utilize pyoverdines produced by other fluorescent pseudomonads. In contrast, the closely related P. putida KT2440 was only able to use one heterologous pyoverdine in addition to its own pyoverdine and did not produce any secondary siderophore.
Materials and methods
Pyoverdine typing and isolation from different Pseudomonas strains
A large collection of about 160 Pseudomonas strains isolated at the source (site W2) and the mouth (site W15) of the Woluwe River (Brussels, Belgium) was subjected to isoelectric focusing (IEF) analysis of culture supernatants according to Koedam et al. (1994) (S. Matthijs, unpublished data). The strains were grouped according to their IEF profile and each profile was assigned a type number. P. aeruginosa strains producing type I, II and III pyoverdine were given the same type number, the pyoverdines coming from other Pseudomonas were numbered starting from type 1, type 2, …. Strains producing a unique siderotype were subsequently selected (Table 1; Fig. 1) and the pyoverdine was re-isolated by a medium scale purification to confirm the siderotype and to obtain a salt-free sample for mass analysis. Therefore 100 ml cultures were grown for 42–48 h at 26°C in 1 l Erlenmeyers. Subsequently the cultures were centrifuged for 15 min at 10,000g, the supernatant was filtered with a 0.2 μm Minisart Satorius filter (Stedim Biotech) and applied to a C-18 column that was activated with methanol and washed with distilled water. Elution was done with acetonitrile/H2O (70/30%). The siderotypes were confirmed by IEF and the mass of the pyoverdine was determined by LC/MS or MS/MS.
Isolectrofocusing patterns of pyoverdines produced by strains W15Aug26 (lane 1), W15Aug23 (lane 2), W15Aug24 (lane 3), W2Dec36A (lane 4), W2Aug1 (lane 5), P. putida KT2440 (lane 6), P. libanensis LMG 21606T (lane 7), P. fluorescens 5 (lane 8), W15Feb38 (lane 9), W2Aug36 (lane 10), W2Feb31B (lane 11), W15Ap2 (lane 12), P. fluorescens Pf0-1 (lane 13), P. syringae subsp. syringae LMG 1247T (lane 14), P. fluorescens ATCC 17400 (lane 15), W2Dec33 (lane 16), W2Jun14 (lane 17), P. brassicacearum LMG 21623T (lane 18), P. kilonensis LMG 21624T (lane 19), P. agarici LMG 2112T (lane 20), P. vancouverensis LMG 20222T (lane 21), P. brenneri LMG 23068T (lane 22), P. fluorescens LMG 14562 (lane 23), P. entomophila L48 (lane 24)
To identify the pyoverdines produced by the Woluwe isolates, pyoverdine was isolated from reference strains (producing an already characterized pyoverdine) and the siderotype and the mass of the pyoverdine was compared with the result of the reference strain pyoverdine. In case the IEF profile and the mass were identical they we considered as producing the same pyoverdine. These reference strains included: P. aeruginosa PAO1 (type I, Briskot et al. 1986), P. aeruginosa 7NSK2 (type II, de Chial et al. 2003), P. aeruginosa 59.20 (type III, de Chial et al. 2003), P. fluorescens SBW25 (type 1, Moon et al. 2008), P. putida G4R (type 3, Salah-el-Din et al. 1997), P. fluorescens CHA0 (type 6, Wong-Lun-Sang et al. 1996), PL7 (type 7, Barelmann et al. 2002), B10 (type 9, Teintze et al. 1981), P. fluorescens A6 (CFBP 2392; type 32, Beiderbeck et al. 1999), P. fluorescens W (type 37, Demange et al. 1990). To increase the number of unique pyoverdines, following reference strains were included in this study: P. fluorescens Pf0-1 (type 14, Meyer et al. 2008), P. syringae subsp. syringae (type 15, Jülich et al. 2001), and P. fluorescens ATCC 17400 (type 16, Demange et al. 1990).
Large scale pyoverdine purification
For the pyoverdine growth stimulation experiments a large scale pyoverdine purification was done from previously selected strains (Woluwe isolates and reference strains) producing a specific siderotype (Table 2). Therefore pyoverdine was purified from 42 to 48 h old culture supernatant of the Pseudomonas strains grown at 26°C in 5 l Erlenmeyer flasks containing 1 l of iron-poor casamino acids (CAA) medium at 150 rpm. Bacterial cells were removed by centrifugation at 10,000g during 15 min. After filtration the supernatant was passed on a C-18 column that was activated with methanol and washed with distilled water. Elution was done with acetonitrile/H2O (70/30%). Most of the acetonitrile was evaporated with a rotavapor and the samples were lyophilized. The pyoverdine was quantified spectrophotometrically by measuring the absorption at 405 nm (Höfte et al. 1993) and adjusted to a concentration of 8 mM. Aliquots of the pyoverdine were stored at −20°C.
Mass analysis
HRMS-data were obtained with a QT of Micro mass spectrometer (positive ion mode). Under standard measurement conditions the sample was dissolved in CH3CN/H2O (1:1) containing 0.1% formic acid. Reserpine was used as lock signal.
Pyoverdine-isoelectrofocusing
Isoelectrofocusing was done according to Koedam et al. (1994). Therefore 1.5 μl of an 8 mM pyoverdine stock (obtained by large scale pyoverdine purification) was loaded on the IEF gel. The digital photograph presented (Fig. 1) consists out of 2 IEF gels, which were combined with the aid of Adobe Photoshop Elements.
Purification and structure determination of pyoverdine of P. entomophila
Pyoverdine purified during the medium scale pyoverdine purification was subjected to mass analysis. Mass spectral data were obtained with a MAT 900 ST instrument providing an electrostatic/magnetic analyzer (EB) geometry connected to an octapole collision cell and a quadrupole ion trap (QIT), and equipped with an ESI II ion source (Finnigan MAT, Bremen, Germany); spray voltage 3.4–3.6 kV, capillary temperature 230°C. Source conditions were set to minimize fragmentation, resolution ca. 5,000 (10% valley). The samples were dissolved in water, methanol, and trifluoroacetic acid 50:50:0.1 (v/v). Fragmentation induced by low energy collision activation (CA) was effected in the octapole unit and in the QIT (~2 × 10−3 Pa He as bath gas diffusing in the collision octapole).
Screening for pyoverdine-negative mutants
The plasposon mutagenesis method (Dennis and Zylstra 1998) was used to generate transposon insertions in the chromosome of P. entomophila L48 and P. putida KT2440. Mid-log phase cultures of E. coli SM10 (λpir), the host of the plasposon pTnmod-OTc, was mixed with strain P. entomophila in a 1:1 ratio. P. entomophila was kept at 45°C for 20 min just before mixing of both strains in order to inactive its restriction system. After over night incubation on LB at 26°C, transposon insertions in strain P. entomophila were selected on CAA supplemented with 150 μg ml−1 tetracycline and 25 μg ml−1 chloramphenicol. A bank of 2,000 transconjugants was screened for mutants unable to produce pyoverdine as detected by loss of fluorescence. Pyoverdine-negative mutants were obtained in P. putida using the same conditions but the transconjugants were plated on CAA + 100 μg ml−1 tetracycline and 25 μg ml−1 chloramphenicol. To molecularly characterize the mutants the chromosomal DNA was isolated using the Puregene genomic DNA purification kit (Gentra systems), digested with PstI or SalI (Fermentas) and self-ligated. After transformation in E. coli DH5α, the plasmid was isolated with the invisorb spin plasmid mini two kit (Invitek—Westburg) and sequenced using primer pTnTc-F (5′-CGATGAGCGCATTGTTAGAT-3′) and pTnTc-R (5′-TCCCAGTCACGACGTTGTA-3′).
Construction of pmsCEAB in-frame deletion mutant of P. entomophila L48
For construction of the ΔpmsCEAB mutant the four genes were deleted in-frame of strain L48. A 1,017-bp fragment, including the first 16-bp of pmsC and the neighboring upstream region, was amplified by PCR with primers pmsC-AF-XbI (GTGTCTAGAGCGTACTCGCTCTCATCT) and pmsC-AR-HIII (GTGAAGCTTGAGGTCGACATCAACGATCA). A 784-bp fragment including the last 11 bp of pmsB and the adjacent downstream region was amplified by PCR with primers pmsB-BF-XbI (GTGTCTAGAAGAAGGCCTGATCGGTAG) and pmsB-BR-EI (GTGGAATTCGTCACAACTGGCTTCGACTT). The resulting upstream and downstream fragments were cut with the restriction enzymes HindIII and XbaI and with XbaI and EcoRI, respectively, and cloned by triple ligation into EcoRI and HindIII-digested pUK21. The 1,807-bp EcoRI–HindIII insert was checked by sequencing and re-cloned into the suicide plasmid pME3087. The resulting plasmid was then integrated into the chromosome of strain L48 by triparental mating using E. coli HB101/pME497 as the mobilizing strain, with selection for tetracycline- and chloramphenicol-resistant recombinants. Excision of the vector by a second crossing-over occurred after enrichment for tetracycline-sensitive cells (Schnider-Keel et al. 2000). The obtained mutant was verified by PCR.
In silico analyses
Gene(s)/operon(s) for pyoverdine and pseudomonine biosynthesis, transport and regulation were found by BLAST homology and by searching the P. entomophila L48 and P. putida KT2440 genome (http://www.pseudomonas.com). The program NRPS predictor (http://www-ab.informatik.uni-tuebingen.de/toolbox/index.php?view=domainpred) which uses the methods of Stachelhaus et al. (1999) and Rausch et al. (2005) was used to predict the peptide backbone of the pyoverdine.
Utilization of exogenous pyoverdines by P. entomophila L48 and P. putida KT2440
Twenty milliliter CAA agar plates containing 0.5 mg ml−1 EDDHA were overlaid with 5 × 106 cells of the pyoverdine-negative mutant 1E10 (of P. entomophila) or 5A12 (of P. putida), and filter-paper disks impregnated with 6 μl of 8 mM purified pyoverdine were placed on the agar. The plates were incubated at 26°C and scored for the presence of detectable growth of the pyoverdine-negative mutant after 2 days.
Chrome azurol S assay
Siderophore production was detected by the chrome-azurol S (CAS) assay (Schwyn and Neilands 1987). On CAS-agar, siderophores remove iron from CAS, resulting in a blue to yellow–orange color change in zones surrounding the colonies.
EDDHA synthesis
Ethylenediamine bis(2-hydroxyphenyl)acetic acid (EDDHA) was synthesized according to Yunta et al. (2003). The synthesized product was verified by 1H-NMR.
Results
Genomic, genetic and structural analysis of pyoverdine of P. entomophila L48
The pyoverdine of P. entomophila was separated into three isoelectrophoresed bands (Fig. 1, lane 24), a major one characterized by a pHi value of 5.0 and two others, less pronounced in intensity, at pHi of 4.3 and 4.5, respectively. Thus, pyoverdine of P. entomophila L48 was classified into the acidic group of pyoverdines. Therefore, to determine its pyoverdine-mediated iron uptake capacity, strain L48 was tested towards its own pyoverdine and also towards the 22 pyoverdines of different bacterial origin already recognized as belonging to that group. None among the 22 heterologous pyoverdines was able to promote efficiently iron incorporation in strain L48 as did pyoverdine of strain L48 (J. -M. Meyer, unpublished results). Therefore, it was concluded that P. entomophila was characterized by an original pyoverdine structurally different from each of the more than 60 different compounds presently identified (Meyer et al. 2008).
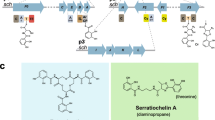
A search of the complete genome sequence of P. entomophila revealed 28 genes involved in pyoverdine-mediated iron acquisition, transport and regulation which are distributed across three different loci of the genome. The results are summarized in Fig. 2. As observed for other Pseudomonas species, including P. aeruginosa PAO1, P. putida KT2440, P. fluorescens Pf0-1 and P. fluorescens SBW25, the chromophore NRPS gene pvsA (PSEEN1815) is located at a separate locus (generally together with 1 enzyme involved of the modification of the peptide chain, and the sigma factor regulator PvdS) while the receptor gene fpvA and pvdE gene are found at the same locus as the peptide chain biosynthesis genes (Moon et al. 2008).
The organization of the pyoverdine genes in P. entomophila L48 and P. putida KT2440. Gene names, if given, are shown beneath genes, PSEEN and PP numbers are shown above. Tn5 insertions are indicated by a triangle. Genes are not drawn to linear scale, and double vertical lines represent intervening DNA with the size shown above
Based on in silico analysis of non-ribosomal peptide synthetases (NRPS) genes PSEEN3229 till PSEEN3232, and PSEEN3234 a ten amino acid long peptide backbone was predicted. To determine the structure the purified pyoverdine was subjected to mass analysis. Assuming that m/z 668.5 corresponds to [M + H + K]++ it may lose K+ and yield m/z 1298 being [M + H]+. Quadrupole CA yields m/z 204 typical for the pyoverdine chromophore and 400 (A1 Suca-Chr-Ala) which loses NH3/H2O (383/382) and Suca (301). 542 is B1 (428) plus 114 Da. This can be Asn, Orn or two Gly. Ion trap CA yields the same ions and in addition 795. The mass difference 542–795 corresponds to Dab + OHHis. 795—96 yields 699. This loss is typical for OHHis. Additional ions are 777 (795—H2O) and 620.5 (668.5—96/2). 795 + 114 corresponds to 909 (again Asn, Orn or 2 Gly). Ions of higher mass (except 1298) are of too low abundance. The sequence Ala–Thr–Ser–cOHOrn would account for the mass difference 909–1,298 (71 + 101 + 87 + 130). Through the combination of mass analysis and in silico analysis, the following ten amino acids long peptide backbone for the pyoverdine of P. entomophila could be determined (Suca-Chr)–Ala–Asn–Dab–OHHis–Gly–Gly–Ala–Thr–Ser–cOHOrn where Chr represents the chromophore and Suca succinamide. The abbreviation cOHOrn stands for cyclo-hydroxy-ornithine (3-amino-1-hydroxy-piperidone-2).
Five pyoverdine-negative Tn5 mutants (1C11, 1E10, 1G9, 1H12 and 3C6) were isolated; which showed a complete loss of pyoverdine fluorescence under UV light and were unable to grow on CAA supplemented with the strong iron chelator EDDHA. These mutants were still able to decolorize chrome azurol S, suggesting the production of a second siderophore. HPLC analysis of the culture supernatant showed that for all mutants no pyoverdine was detected anymore while pseudomonine was found at a level comparable to the wild type. The mutants were characterized at the molecular level and were shown to have Tn5 insertions in genes predicted to be involved in pyoverdine synthesis (see Fig. 2) namely in PSEEN3234 (1G9 and 1E10 at genome position 3491208 and 3490460, respectively), in pvsA encoding the chromophore NRPS PSEEN3234 (Mossialos et al. 2002; 1C11 at genome position 1904651) and in pvdA coding for a putative l-ornithine N5-oxygenase (PSEEN2323; Ge and Seah 2006; 3C6 at genome position 3450500).
Genomic, genetic and structural analysis of pyoverdine of P. putida KT2440
A search of the genome sequence of P. putida KT2440 revealed 26 genes with predicted roles in pyoverdine biosynthesis, regulation and transport which are distributed across three different loci on the genome as observed for P. entomophila. The results are summarized in Fig. 2.
In silico pyoverdine backbone structural prediction of KT2440 by Ravel and Cornelis (2003) predicted following structure: OHAsp–Lys–OHAsp–Dab–Gly–Ser–hOrn. Comparison of the IEF profile with the reference strains showed that the IEF pattern of KT2440 was identical to the one of P. putida G4R. In addition both pyoverdines have the same mass (1,073). Based on these results the pyoverdine of P. putida KT2440 has following peptide chain: Asp–Orn–(OHAsp-Dab)–Gly–Ser–cOHOrn which fits closely, but not entirely, the peptide chain predicted from the NRPS genes.
Three Tn5 insertions caused a pyoverdine-negative phenotype, including two in the NRPS gene PP_4220 (3E2 and 5A12 at genome position 4779691 and 4783050, respectively) confirming the role of the gene in pyoverdine biosynthesis. Interestingly, one Tn5 insertion was obtained in gene PP_4218 (5C10 at genome position 4768248), a lipase/esterase family protein lying downstream of the NRPS genes. This enzyme is predicted to act on carboxylic esters. To our knowledge this is the first time that a mutant in this gene has been described. Next to the NRPS genes lies a syrP gene in P. entomophila and P. putida genomes. It has been recently shown that the syrP gene is involved in the hydroxylation reaction of the aspartate residue in NRPS encoded syringomycin E and related non ribosomal peptides (Singh et al. 2008). A syrP homologue is found in P. putida KT2440 (Fig. 2, PP_4222) and in the pyoverdine biosynthetic genes of P. syringae and P. fluorescens Pf0-1 of which both pyoverdines contain a HO-Asp residue. A homologue of the syrP gene is also found in P. entomophila (Fig. 2, PSEEN3233) which does not contain a HO-Aps residue, but a HO-His residue, possibly this gene has in P. entomophila a different substrate.
Genomic, genetic and structural analysis of pseudomonine
A CAS-positive fraction was purified from the pyoverdine-negative mutant 1E10 of P. entomophila using the same methodology as for the medium scale pyoverdine purification. The EI mass spectrum is directly comparable to the data published in Mercado-Blanco et al. (2001) confirming that the siderophore purified is pseudomonine. The molecular ion is observed at m/z 330, and fragment ions are observed at m/z 82, 95, 110, 121, 127, 138, and 204 (data not shown).
A genome search identified fifteen ORFs which are involved in the synthesis and transport of pseudomonine in P. entomophila L48 (Fig. 3). The same gene cluster (except for one ORF) consisting out of highly similar genes with identical organization was present in the pseudomonine producer P. fluorescens WCS374 (Fig. 3).
Of these 15 ORFs only pmsCEAB has been studied in P. fluorescens WCS374 (Mercado-Blanco et al. 2001). It has been shown in that study that the pmsCEAB genes are co-transcribed and that the expression is iron-regulated. In addition it was demonstrated that salicylic acid (SA) biosynthesis was encoded by pmsCEAB. PmsC is a putative isochorismate synthetase which catalyzes the conversion of chorismate to isochorismate and PmsB is a putative isochorismate-pyruvate lyase which catalyzes the cleavage of isochorismate to give SA and pyruvate. The constructed ∆pmsCEAB mutant of P. entomophila showed a complete loss of pseudomonine production as verified by HPLC (data not shown) confirming that the genes are involved in pseudomonine production. Mercado-Blanco et al. (2001) showed that deletions affecting pmsC dimished SA production, whereas deletion of pmsB resulted in a complete loss.
The third ORF pmsE is annotated as a putative 2,3-dihydroxybenzoate-AMP ligase and shows strong similarities with 2,3-dihydroxybenzoate-AMP ligases from different species. This enzyme activates the carboxylate group of 2,3-dihydroxybenzoic acid (2,3-DHB) via an ATP-dependent PPi-exchange reaction. Since SA (2-hydroxybenzoic acid) and not 2,3-dihydroxybenzoic acid is a moiety of pseudomonine it is assumed that the actual substrate of PmsE is not 2,3-DHB but the structurally very similar SA. This would also explain why a putative isochorismate hydrolase, which catalyzes the hydrolysis of isochorismate to 2,3-dihydroxybenzoic acid, the substrate of 2,3-dihydroxybenzoate-AMP ligase, is not present in the operon. In addition, BlastX analysis with pmsB of P. entomophila showed that pmsB was detected in Pseudomonas sp. producing the SA containing siderophore pyochelin [PchB of P. fluorescens CHA0 (57% identity), P. fluorescens 5 (38% identity), P. aeruginosa spp. (58% identity)] and Paracoccus denitrificans, which also produces a SA containing siderophore, parabactin, but not in the genomes of for example Acinetobacter baumannii sp., which produces the 2,3-DHB containing siderophore acinetobactin.
The region also contained a putative pyridoxal-dependent histidine decarboxylase (HDC) gene pmsA. HDC catalyzes decarboxylation of histidine to histamine, the second moiety of pseudomonine. Histamine is also a precursor of the siderophore anguibactin produced by Vibrio anguillarum, and it was found that the gene, which shows high similarities to PmsA of P. entomophila, was essential for its biosynthesis (Tolmasky et al. 1995).
Thus the pmsCEAB genes give probably raise to 2 precursors of pseudomonine, an activated SA, and histamine. Upstream of pmsCEAB two NRPS genes, basB and basAD, were annotated (PSEEN2500 and PSEEN2503, respectively). The conserved amino acid sequences for the cyclization (Cy) and adenylation domain (A) were identified in BasAD. The NRPS predictor predicted the amino acid threonine as the substrate for the A domain which fits together with the Thr in the molecular structure of pseudomonine. In BasB only one condensation (C) domain (C2) and a peptidyl carrier domain were identified. Both NRPS are probably responsible for the adenylation and cyclization of the amino acid threonine (probably by basAD) and the incorporation of the activated SA and the histamine giving raise to pseudomonine. A chain-terminating TE domain was not found in the NRPS genes. But probably termination occurs by the transfer of the siderophore chain to histamine to make an amide linkage, catalyzed by a C-terminal condensation domain rather than TE domains. This has been described for anguibactin (cosubstrate amine is histamine) and vibriobactin (cosubstrate amine is norspermidine; Crosa and Walsh 2002).
Between those two NRPS genes a putative l-lysine 6-monooxygenase (NADPH-dependent; basC, PSEEN2502) was annotated. The role of BasC is not clear. It is similar to BasC homologues of siderophore biosynthesis gene clusters of A. baumannii sp. (68–69% identity), Vibrio sp. (58–59% identity) and A. salmonicida subsp. salmonicida (60% identity). BasC is predicted to be involved in the oxidation of histamine, yielding N-hydroxyhistamine, one of the constituents of acinetobactin. Similarly AngU a putative monooxygenase, is also predicted to be involved in N-hydroxyhistamine production of anguibactin (Crosa and Walsh 2002). But in contrast to acinetobactin and anguibactin histamine and not N-hydroxyhistamine is a constituent of pseudomonine (Fig. 4). Therefore BasC must have a different function in P. entomophila.
Upstream of the biosynthesis genes putative transport genes bauBCDE were annotated (PSEEN2497 and PSEEN2498).
Utilization of exogenous pyoverdines by P. entomophila and P. putida KT2440
To estimate the diversity of exogenous pyoverdines that may be utilized by P. entomophila and P. putida, pyoverdines were purified from 24 different Pseudomonas strains and isolates, which had been shown to each produce a unique siderotype profile (Fig. 1; Table 2). These strains represented 11 environmental isolates collected from the Woluwe River (Brussels, Belgium), 7 type strains, and 6 strains from different origins. The pyoverdine produced by P. fluorescens 5, 6 Woluwe and 2 type strains (P. brenneri LMG 23068T and P. kilonensis LMG 21624T) could be identified based on an identical mass and IEF profile as the relevant reference strain (Table 2).
The pyoverdines were supplied to a pyoverdine-deficient mutant strain (1E10) of P. entomophila that had been overlaid as a dilute suspension onto CAA medium containing EDDHA. Growth was clearly restored by provision of some of the purified pyoverdines, including the cognate pyoverdine (type 41; Tables 2, 3) and the three P. aeruginosa types (type I, II and III; Table 3) of P. aeruginosa isolates from the Woluwe (Pirnay et al. 2005). The pyoverdine of eight strains (P. putida KT2440, P. libanensis LMG 21606T, W2Feb31B, P. fluorescens Pf0-1, P. syringae subsp. syringae LMG 1247T, P. fluorescens ATCC 17400, P. brassicacearum LMG 21623T and P. fluorescens LMG 14562) led only to a very weak but reproducible growth stimulation. This growth stimulation was considered as background and recorded as negative. Thus, it is apparent that P. entomophila is able to utilize a very large range (16 out of 24 pyoverdines) of exogenous pyoverdines produced by other pseudomonads.
Similarly, a growth stimulation assay was carried out with a pyoverdine-negative mutant 5A12 of P. putida KT2440. In contrast to P. entomophila, growth of a pyoverdine-negative P. putida KT2440 mutant was only well stimulated by its cognate pyoverdine and the pyoverdine of P. syringae subsp. syringae LMG 1247T. Surprisingly these two pyoverdines do not show a large overlap in structure. Pyoverdine of isolate W2Aug36 and P. agarici LMG 2112T were only able to stimulate growth very weakly. For all the other pyoverdines no growth stimulation was visible. It was observed that the pyoverdine-negative mutant of P. putida KT2440 does not grow well in iron limiting conditions and that the strain is sensitive for iron stress while giving a very weak or negative CAS reaction (S. Matthijs, unpublished results). To overcome this iron stress sensitivity the assay with the pyoverdine-negative mutant of P. putida was repeated on a lower concentration of EDDHA (0.25 mg ml−1) and for the overlay a 5 times higher number of cells was overlaid on the CAA + EDDHA plate, but with no significant effect on the outcome.
Despites the relatedness between both species, we can conclude that there is no overlap between the pyoverdines which are able to stimulate their respective growth. These two strains were also not able to utilize each other cognate pyoverdine. This observation is supported by the genome searches which did not identify a receptor with significant similarity to the fpvA receptor (PSEEN2325) of P. entomophila in P. putida KT2440 and vice versa.
Discussion
Pseudomonas entomophila L48 is an entomopathogenic bacterium which kills upon ingestion Drosophila melanogaster as well as insects from different orders (Vodovar et al. 2006). A genomic search revealed the putative pyoverdine biosynthesis, transport and regulation gene clusters, consisting of in total 28 genes which are distributed across three different loci. Inactivation of l-ornithine N5-oxygenase and two NRPS genes, all predicted to be involved in pyoverdine biosynthesis, by Tn5 mutagenesis resulted in complete loss of pyoverdine production confirming their predicted role. In silico and structural analysis of the pyoverdine showed that P. entomophila produces a structurally new pyoverdine with a peptide backbone of ten amino acids. Comparison of the IEF profile of this pyoverdine with the siderotypes of a diverse collection of 450 Pseudomonas strains (S. Matthijs, unpublished results) showed that this siderotype is unique, and has only been observed in P. entomophila L48.
In addition to pyoverdine, the secondary siderophore pseudomonine, which has been previously identified in P. fluorescens AH2 isolated from spoiled Nile perch from Lake Victoria (Africa) (Anthoni et al. 1995) and the plant growth-promoting rhizobacterium P. fluorescens WCS374 (Mercado-Blanco et al. 2001), was identified in the supernatant of a pyoverdine-negative P. entomophila mutant. Construction of a pmsCEAB mutant in strain L48 resulted in a loss of pseudomonine production confirming the role of the genes in pseudomonine biosynthesis. Since at the time that pseudomonine was identified only the pmsCEAB genes were sequenced and analysed, the genome was searched for other pseudomonine biosynthesis genes surrounding pmsCEAB and 15 ORFs were identified in total. A putative role could be assigned to all the ORFs, except for basC. Pseudomonine is assembled from a salicylic acid moiety, which is synthesised by the gene products of pmsCB and activated by pmsE. The second constituent, histamine, is synthesized by the gene product of pmsA. After the condensation of threonine to SA, histamine is condensed to and the pseudomonine is released. A putative extracytoplasmic sigma factor (ECF σ) and transport genes could also be identified.
The pyoverdine of P. putida KT2440 was also identified. It is identical to the pyoverdine produced by another P. putida strain G4R. In contrast to the pyoverdine of P. entomophila, it is not unique and has been found in several Pseudomonas species in our collection (S. Matthijs, unpublished results). The pyoverdine clusters have also been identified and Tn5 mutants confirmed the role of two genes in pyoverdine biosynthesis. If an alternative iron uptake system to pyoverdine exists at all, its siderophore should have a rather low affinity for iron since only a very weak CAS decolouration activity could be observed.
Heterologous pyoverdine growth stimulation assays demonstrate that P. entomophila, in contrast to P. putida is able to utilize a high variety of structurally distinct pyoverdines produced by other Pseudomonas species. Based on this study it is clear that P. entomophila has the tools to compete efficiently for ferric iron. It would be interesting to verify the importance of iron stress during the infection of Drosophila melanogaster.
References
Anthoni U, Christophersen C, Nielsen PH, Gram L, Petersen BO (1995) Pseudomonine, an isoxazolidone with siderophoric activity from Pseudomonas fluorescens AH2 isolated from Lake Victorian Nile perch. J Nat Prod 58:1786–1789. doi:10.1021/np50125a026
Barelmann I, Taraz K, Budzikiewicz H, Geoffroy VA, Meyer JM (2002) The structures of the pyoverdins from two Pseudomonas fluorescens strains accepted mutually by their respective producers. Z Naturforsch 57c:9–16
Beiderbeck H, Taraz K, Meyer JM (1999) Revised structures of the pyoverdins from Pseudomonas putida CFBP 2461 and from Pseudomonas fluorescens CFBP 2392. Biometals 12:331–338. doi:10.1023/A:1009227520314
Briskot G, Taraz K, Budzikiewicz H (1986) Pyoverdine-type siderophores from Pseudomonas aeruginosa. Z Naturforsch C 41:497–506
Budzikiewicz H (1993) Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol Rev 104:209–228. doi:10.1111/j.1574-6968.1993.tb05868.x
Budzikiewicz H (1997) Siderophores of fluorescent pseudomonads. Z Naturforsch [C] 52:713–720
Cornelis P, Matthijs S (2002) Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ Microbiol 4:787–798. doi:10.1046/j.1462-2920.2002.00369.x
Cox CD, Graham R (1979) Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol 137:357–364
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi:10.1128/MMBR.66.2.223-249.2002
de Chial M, Ghysels B, Beatson SA, Geoffroy V, Meyer J-M, Pattery T, Baysse C, Chablain P, Parsons YN, Winstanley C, Cordwell SJ, Cornelis P (2003) Identification of type II and type III pyoverdine receptors from Pseudomonas aeruginosa. Microbiology 149:821–831. doi:10.1099/mic.0.26136-0
Demange P, Bateman A, Mertz C, Dell A, Piémont Y, Abdullah M (1990) Structures of pyoverdins Pt, siderophores of Pseudomonas tolaasii NCPPB 2192, and pyoverdins Pf, siderophores of Pseudomonas fluorescens CCM 2798. Identification of an unusual natural amino acid. Biochemistry 29:11041–11051. doi:10.1021/bi00502a005
Dennis JJ, Zylstra GJ (1998) Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl Environ Microbiol 64:2710–2715
Ge L, Seah SY (2006) Heterologous expression, purification, and characterization of an L-ornithine N 5-hydroxylase involved in pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa. J Bacteriol 188:7205–7210. doi:10.1128/JB.00949-06
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi:10.1016/S0022-2836(83)80284-8
Höfte M, Buysens S, Koedam N, Cornelis P (1993) Zinc affects siderophore-mediated high affinity iron uptake systems in the rhizosphere Pseudomonas aeruginosa 7NSK2. Biometals 6:85–91. doi:10.1007/BF00140108
Jülich M, Taraz K, Budzikiewicz H, Geoffroy V, Meyer JM, Gardan L (2001) The structure of the pyoverdin isolated from various Pseudomonas syringae pathovars. Z Naturforsch 56c:687–694
Jurkevitch E, Hadar Y, Chen Y (1992) Differential siderophore utilization and iron uptake by soil and rhizosphere bacteria. Appl Environ Microbiol 58:119–124
Koedam N, Wittouck E, Gaballa A, Gillis A, Höfte M, Cornelis P (1994) Detection and differentiation of microbial siderophores by isoelectric focusing and chrome azurol S overlay. Biometals 7:287–291. doi:10.1007/BF00144123
Matthijs S, Abbaspour Tehrani K, Laus G, Jackson RW, Cooper RM, Cornelis P (2007) Thioquinolobactin, a Pseudomonas siderophore with antifungal and anti-Pythium activity. Environ Microbiol 9:425–434. doi:10.1111/j.1462-2920.2006.01154.x
Matthijs S, Budzikiewicz H, Schäfer M, Whatelet B, Cornelis P (2008) Ornicorrugatin, a new siderophore from Pseudomonas fluorescens AF76. Z Naturforsch 63:8–12
Mercado-Blanco J, van der Drift KMGM, Olsson PE, Thomas-Oates JE, van Loon LC, Bakker PAHM (2001) Analysis of the pmsCEAB gene cluster involved in the biosynthesis of salicylic acid and the siderophore pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J Bacteriol 183:1909–1920. doi:10.1128/JB.183.6.1909-1920.2001
Meyer J-M (1992) Exogenous siderophore-mediated iron uptake in Pseudomonas aeruginosa: possible involvement of porin OprF in iron translocation. J Gen Microbiol 138:951–958
Meyer J-M, Abdallah MA (1978) The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol 107:319–328
Meyer J-M, Gruffaz C, Raharinosy V, Bezverbnaya I, Schäfer M, Budzikiewicz H (2008) Siderotyping of fluorescent Pseudomonas: molecular mass determination by mass spectrometry as a powerful pyoverdine siderotyping method. Biometals 21:259–271. doi:10.1007/s10534-007-9115-6
Moon CD, Zhang XX, Matthijs S, Schäfer M, Budzikiewicz H, Rainey PB (2008) Genomic, genetic and structural analysis of pyoverdine-mediated iron acquisition in the plant growth-promoting bacterium Pseudomonas fluorescens SBW25. BMC Microbiol 8:7. doi:10.1186/1471-2180-8-7
Mossialos D, Ochsner U, Baysse C, Chablain P, Pirnay JP, Koedam N, Budzikiewicz H, Fernández DU, Schäfer M, Ravel J, Cornelis P (2002) Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol Microbiol 45:1673–1685
Pirnay JP, Matthijs S, Colak H, Chablain P, Bilocq F, Van Eldere J, De Vos D, Zizi M, Triest L, Cornelis P (2005) Global Pseudomonas aeruginosa biodiversity as reflected in a Belgian river. Environ Microbiol 7:969–980. doi:10.1111/j.1462-2920.2005.00776.x
Poole K, Young L, Neshat S (1990) Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol 172:6991–6996
Raaijmakers JM, Van Der Sluis I, Koster M, Bakker PAHM, Weisbeek PJ, Schippers B (1995) Utilization of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Can J Microbiol 41:126–135
Rausch C, Weber T, Kohlbacher O, Wohlleben W, Huson DH (2005) Specificity prediction of adenylation domains in nonribosomal peptide synthetases (NRPS) using transductive support vector machines (TSVMs). Nucleic Acids Res 33:5799–5808. doi:10.1093/nar/gki885
Ravel J, Cornelis P (2003) Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbial 11:195–200
Risse D, Beiderbeck H, Taraz K, Budzikiewicz H, Gustine D (1998) Corrugatin, a lipopeptide siderophore from Pseudomonas corrugata. Z Naturforsch 53c:295–304
Salah-el-Din ALM, Kyslic P, Stephan D, Abdallah MA (1997) Bacterial iron transport: structure elucidation by FAB-MS and by 2 D NMR (1H, 13C, 15N) of pyoverdin G4R, a peptidic siderophore produced by a nitrogen-fixing strain of Pseudomonas putida. Tetrahedron Lett 53:12539–12552
Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Défago G, Haas D, Keel C (2000) Autoinduction of 2, 4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225. doi:10.1128/JB.182.5.1215-1225.2000
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi:10.1016/0003-2697(87)90612-9
Singh GM, Fortin PD, Koglin A, Walsh CT (2008) beta-Hydroxylation of the aspartyl residue in the phytotoxin syringomycin E: characterization of two candidate hydroxylases AspH and SyrP in Pseudomonas syringae. Biochemistry 47:11310–11320. doi:10.1021/bi801322z
Stachelhaus T, Mootz HD, Marahiel MA (1999) The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol 6:493–505. doi:10.1016/S1074-5521(99)80082-9
Teintze M, Hossain MB, Barnes CL, Leong J, van der Helm D (1981) Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry 20:6446–6457. doi:10.1021/bi00525a025
Tolmasky ME, Actis LA, Crosa JH (1995) A histidine decarboxylase gene encoded by the Vibrio anguillarum plasmid pJM1 is essential for virulence: histamine is a precursor in the biosynthesis of anguibactin. Mol Microbiol 15:87–95. doi:10.1111/j.1365-2958.1995.tb02223.x
Vieira J, Messing J (1991) New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189–194
Vodovar N, Vallenet D, Cruveiller S, Rouy Z, Barbe V, Acosta C, Cattolico L, Jubin C, Lajus A, Segurens B, Vacherie B, Wincker P, Weissenbach J, Lemaitre B, Médigue C, Boccard F (2006) Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nat Biotechnol 24:673–679. doi:10.1038/nbt1212
Voisard C, Bull C, Keel C, Laville J, Maurhofer M, Schnider U, Défago G, Haas D (1994) Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches. In: O’Gara F, Dowling D, Boesten B (eds) Molecular ecology of rhizosphere microorganisms. VCH, Weinheim, pp 67–89
Wong-Lun-Sang S, Bernardini JJ, Hennard C, Kyslic P, Dell A, Abdallah MA (1996) Bacterial siderophores: structure elucidation, 2 D 1H and 13C NMR assignments of pyoverdins produced by Pseudomonas fluorescens CHA0. Tetrahedron Lett 37:3329–3332. doi:10.1016/0040-4039(96)00569-2
Yunta F, García-Marco S, Lucena JJ, Gómez-Gallego M, Alcázar R, Sierra MA (2003) Chelating agents related to ethylenediamine bis(2-hydroxyphenyl)acetic acid (EDDHA): synthesis, characterization, and equilibrium studies of the free ligands and their Mg2+, Ca2+, Cu2+, and Fe3+ chelates. Inorg Chem 42:5412–5421. doi:10.1021/ic034333j
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthijs, S., Laus, G., Meyer, JM. et al. Siderophore-mediated iron acquisition in the entomopathogenic bacterium Pseudomonas entomophila L48 and its close relative Pseudomonas putida KT2440. Biometals 22, 951–964 (2009). https://doi.org/10.1007/s10534-009-9247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9247-y