Abstract
Cryoconite holes host active microbial communities despite their extreme physical conditions. In the McMurdo Dry Valleys of Antarctica, these perennially cold, mini-ecosystems form ice lids that can persist for many years thereby isolating the cryoconite from nutrient and carbon inputs. Despite much recent work on cryoconite holes in Antarctica, little is known about nutrient dynamics and limitations in these ice-enclosed ecosystems. We used multiple biogeochemical approaches, including stable isotope signatures (δ15N and δ13C), nutrients concentrations (C, N, P), and enzyme activities, to evaluate what nutrients are likely limiting to biological activity in cryoconite hole sediments on Taylor, Canada, and Commonwealth glaciers in Taylor Valley, one of the McMurdo Dry Valleys. Nutrient concentrations (C, N, and P) varied in accordance with previous studies showing that the most inland of the three glaciers (Taylor Glacier) is the most oligotrophic. C-to-N ratios of Canada and Commonwealth cryoconite-hole sediments were close to the global mean for biologically-active sediments and soils, whereas Taylor Glacier cryoconite deviated from the global mean and were similar to the high C:N ratios seen in Taylor Valley soils. C and N stable isotope signatures on Commonwealth and Canada glaciers are congruent with values for efficient C and N fixation by nostocalean cyanobacteria, combined with higher levels of denitrification on Canada Glacier. In contrast, stable isotope signatures on the more oligotrophic Taylor Glacier are reflective of atmospheric deposition of N and C, or N inputs from nearby soils. Enzyme stoichiometric approaches further support extreme nutrient limitation on Taylor Glacier and indicate that P is the ultimate limiting nutrient across all three glaciers. Extremely high DIN-to-phosphate ratios also indicate P limitation across all three glaciers with Commonwealth Glacier being less severely P-limited than the other two glaciers. At a broader scale, this work provides a comprehensive framework for understanding how biogeochemical cycling of C, N and P vary across nutrient and climatic gradients in the cryobiosphere, and point towards the need for experimental work to test the relative controls of climate, microbes, and nutrients on biogeochemistry of cryoconite holes and other ecosystems of the cryosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The McMurdo Dry Valleys (MDV) represent the largest ice-free expanse of land in Antarctica and harbor some of the coldest and driest ecosystems on Earth (Levy, 2013). Mean annual temperatures average between − 18 and − 28 °C, with summer temperatures rarely reaching as high as 12 °C (Doran et al. 2008; Goordial et al. 2016). Precipitation amounts to less than 50 mm of water equivalents of snowfall annually, much of which sublimates before melting (Fountain et al. 2010). The major source of water in this polar desert is glacial melt that feeds the ephemeral streams, which only flow between 3 and 12 weeks each year (Conovitz et al. 2013). In these streams, microbial mats persist in a desiccated state for most of the year but respond quickly to the arrival of water (McKnight et al. 1999). In contrast, the soils in the MDV that are not immediately adjacent to streams or lakes or in areas that accumulate wind-blown snow are dependent on infrequent snowfall events for water and are among the driest and most oligotrophic soils on Earth (Barrett et al. 2007; Zeglin et al. 2009; Goordial et al. 2016; Solon et al. 2021).
Given the mostly dry and oligotrophic conditions of the MDV, any hotspots of biological activity can be important for regional biogeochemical cycles (Priscu et al. 1998) and this includes cryoconite holes (Foreman et al. 2007; Bagshaw et al. 2013). Cryoconite holes are estimated to occupy 4–6% of the ablation zone of glaciers in the MDV (Fountain et al. 2004), where they range in diameter from < 5 cm to over 1 m, in depth from 4 to 80 cm, and are typically covered with an ice lid up to 50 cm thick (Fountain et al. 2004; Tranter et al. 2004). The holes freeze completely in winter, and melt for 3–8 weeks each summer, but due to their ice lids they can remain isolated from the atmosphere for many years at a time (Fountain et al. 2004). The closed-system nature of Antarctic cryoconite holes can lead to extreme biogeochemical conditions such as low partial pressures of CO2 and supersaturation of O2 from photosynthesis (Tranter et al. 2004; Webster-Brown et al. 2015; Lutz et al. 2019).
Thus, the ice-lidded, isolated cryoconite holes of the MDV are an important model system for cryoconite ecosystems around the periphery of the Antarctic ice sheet that have not been as well-studied as the MDV. Their isolation and similarity of physical conditions also make them useful as natural replicated mesocosms for studying community ecology (Sommers et al. 2019b) and as model systems for understanding how cryospheric ecosystems might respond to global change drivers such as climate warming and nutrient deposition. There is much current interest in how cold ecosystems will respond to global warming and nutrient deposition, especially systems that are severely nutrient limited (Schmidt et al. 2016; Darcy et al. 2018b; Blume-Werry et al. 2020; Bueno de Mesquita et al. 2020). Previous studies have shown that there are strong nutrient and climatic gradients in the MDV. Precipitation decreases and summer air temperatures increase inland, and glacier equilibrium lines (where snow accumulation equals loss) increase inland as well (Fountain et al. 1999, 2010; Doran et al. 2002). A nutrient gradient is present for cryoconite holes in the MDV, in that cryoconite holes on Commonwealth Glacier near the coast are richer in nutrients compared to more inland glaciers (e.g. Taylor Glacier) (Porazinska et al. 2004; Bagshaw et al. 2013; Stanish et al. 2013), reflecting broad-scale nutrient gradients for the soils of the MDV (Virginia and Wall 1999; Barrett et al. 2007). This nutrient gradient is also reflected in biodiversity and production patterns, with cryoconite holes on Taylor Glacier having more depauperate micro-fauna and microbial communities than Commonwealth Glacier near the coast (Porazinska et al. 2004; Darcy et al. 2018a; Sommers et al. 2018, 2019a, b). Photosynthetic production, as estimated by chlorophyll a concentrations, decrease along the gradient from 0.44 (SE 0.05) µg g−1 on Commonwealth Glacier, to 0.24 (0.04) µg g−1 on Canada Glacier to 0.07 (0.008) µg g−1 on Taylor Glacier (Sommers et al. 2019a).
Despite our knowledge of the nutrient, diversity and productivity gradients in MDV cryoconite sediments, it is not known which nutrients are the most limiting to life in these ice-enclosed ecosystems, but it has been hypothesized that phosphorus (P) is the most likely limiting nutrient based on high dissolved N-to-P ratios (Foreman et al. 2007), and rapid uptake of P following nutrient pulses (Telling et al. 2014). However, cryoconite sediments on Arctic glaciers (Svalbard) appear to be N-limited based on low N-to-P ratios and high C-to-N in the sediments (Stibal et al. 2008; Lutz et al. 2017), thus more work is needed to definitively show that different nutrients are limiting on Antarctic versus Arctic glaciers. It is also unknown if cryoconite sediments show patterns of enzyme activities, stable isotope fractionation, and nutrient stoichiometry in line with other similar biological systems such as soils and aquatic sediments. Given all of these unknowns, the goal of the present study was to use measures of enzyme activity, stable isotopes, and nutrient levels in cryoconite-hole sediments to help understand if carbon (C), nitrogen (N), phosphorus (P) or some combination are limiting microbial communities in cryoconite holes on Commonwealth, Canada, and Taylor Glaciers in the MDV. Enzyme stoichiometries have proven to be a reliable predictor of nutrient limitations in other extreme cold environments (Schmidt et al. 2008, 2016; Jiang et al. 2019; Bueno de Mesquita et al. 2020; Hu et al. 2021), including MDV soils (Zeglin et al. 2009). Likewise, stable isotopes of carbon and nitrogen can be used to help understand sources of nutrient inputs (Barrett et al. 2006; Burkins et al. 2000, 2001), an important aspect in the present study, and to deduce histories of nutrient cycling. For example, the nitrogen cycle has distinct isotopic fractionations associated with a number of microbially mediated steps (e.g. Bowman et al. 1996; Bauersachs et al. 2009; Stüeken et al. 2016), but this approach has only recently been applied to Antarctic and Arctic cryoconite holes (Lutz et al. 2019, Novotná-Jaroměřská et al. 2021). Therefore, the present study is the first systematic analysis of cryoconite hole sediments among glaciers, incorporating independent biogeochemical approaches (i.e., enzyme and nutrient stoichiometries and stable isotopes) to gain an understanding of which nutrients may be limiting microbial activity in cryoconite holes.
Materials and methods
Field sites and sampling
We sampled 30 frozen cryoconite holes from each of Taylor (77.7406°S, 162.1157°E), Canada (77.6196°S, 162.0011°E), and Commonwealth (77.5699°S, 162.2955°E) glaciers between November 7–17, 2016 (Fig. 1) as described elsewhere (Darcy et al. 2018a; Sommers et al. 2019a). The glaciers sampled represent a gradient in biological and sediment deposition extending from the inland Taylor Glacier (an outlet glacier of the Antarctic ice sheet) to Commonwealth Glacier 30 km eastward adjacent to the Ross Sea (Fortner and Lyons 2018). We sampled holes while frozen early in the polar spring to avoid temporal heterogeneity in the degree to which nutrients dissolve into the water column as cryoconite hole sediment begins to melt (“ionic pulse”; Telling et al. 2014). These frozen samples should represent nutrient levels and enzyme activities from the end of the previous Austral summer, and our work in other cold systems has shown that freezing does not alter soil enzyme activity even for samples stored frozen for many years (Weintraub et al. 2007; King et al. 2008; Bueno de Mesquita et al. 2020). To collect a sample from each cryoconite hole, we used a 10-cm diameter SIPRE corer to drill through the ice lid and frozen water column, extracting a bottom “puck” of frozen sediment. The sediment puck was stored in a sterile polyethylene bag at − 20 °C for one to three weeks before being melted, homogenized, and subset for analysis. To melt and homogenize samples, each cleaned (rinsed off with DI water) sediment puck was placed in a beaker (sterilized with 70% ethanol and UV light) at 4 °C for 12–24 h to melt. When sediments were incompletely melted after that time, they were placed at room temperature for up to 4 h for final melting. Sediments were mixed and allowed to settle before excess meltwater was poured off. Two separate 125-ml conical flasks per sample each received 5 g of the homogenized sample for extraction of phosphate and dissolved inorganic nitrogen. All sediment subsamples and the remainder of the sediment were stored in separate fresh polyethylene bags at − 70 °C for up to three years before proceeding with all chemical analyses and removing 1–2 g for stable isotope analysis.
Nutrient concentrations and stable isotopes
Cryoconite hole sediment (from now on referred to as cryoconite) total organic C (TOC), total organic N (TON), and C and N isotopes were measured via elemental analyzer (EA) mass spectrometry at the Colorado Boulder Earth Systems Stable Isotope Lab (CUBESIL). To prepare samples for analysis, we first freeze-dried each for 24 h, then crushed each to a powder using a motorized agate mortar and pestle. Approximately 100 mg of each sample was decarbonated prior to analysis for C content and isotopes, though un-decarbonated samples were run for N. To remove carbonates, sample powder was placed into a 10 mL centrifuge tube, to which 5 mL of 10% HCl was added, vortexed, and allowed to react for one hour. Samples were then centrifuged at 5000 rpm for 5 min, decanted to remove the acid, rinsed with 5 mL of milliQ (18 MΩ) water, and then vortexed, centrifuged, and decanted. Samples were rinsed with water two more times by centrifuging and decanting.
We weighed out 20–100 mg of subsamples for both isotope N and C analyses into 5 × 8 mm tin capsules. Samples and standards were combusted at 1020 °C in a Thermo Flash EA in the presence of 200 mL/min oxygen, with He carrier gas (90 mL/min). Combusted sample gas was converted to CO2 and N2, separated in a gas chromatograph, and analyzed on a Thermo Delta V plus. To correct for linearity and drift, a series of isotopic standards (acetanilide #1 and #2, l-glutamic acid, and pugel) were prepared and run alongside sample powders. Raw output from Thermo’s IsoDat software was processed and corrected using the Isoverse pipeline (https://www.isoverse.org/). Stable isotope data for each element (XE) is presented in delta notation:
where R is the ratio of heavy to light isotope in a sample or standard, either 15N/14N or 13C/12C, and is reported in units of permil (‰). Standards for N and C were N2 in air and the Vienna Pee Dee Belemnite (V-PDB) for C, respectively.
Dissolved inorganic nitrogen (DIN), nitrate and ammonium concentrations were measured from 5 g of subsamples of cryoconite extracted in 25 mL 0.5 M K2SO4 by shaking for 1 h at 250 rpm. Samples were centrifuged for 3 min at 4000 rpm, then filtered through a Whatman GF/F glass fiber filter and stored at 4 °C for up to six months before analysis by the CSU Soil, Water, and Plant Testing Lab. Ions were determined colorimetrically using cadmium reduction (EPA Method 353.2) with an O.I. Analytical Flow Solution 3000 (O.I. Corporation, College Station, TX, USA). Phosphate was measured from 5 g of subsamples of cryoconite extracted in 25 mL 0.5 M NaHCO3 using the same procedure as the K2SO4 extraction. Phosphate was measured in the same lab colorimetrically using EPA method 365.2 on a Spectronic 20D+ (Thermo Fisher Scientific, Waltham, MA, USA).
Rates of enzyme activity
We measured activities (nmol h−1 g−1 dry sediment) of seven enzymes associated with C, N, and P acquisition, including beta-glucosidase (BG), alpha-glucosidase (AG), beta-xylase (BXYL), cellobiosidase (CBH), N-acetylglucosamine (NAG), leucine aminopeptidase (LAP); and phosphatase (PHOS). Sediment slurries consisting of 1 g of each sample were placed in 125 mL 0.5 M bicarbonate buffer (pH = 7), homogenized at 3000 rpm for 1 min using an Ultra-Turrax homogenizer (IKA Works Inc., USA), and incubated in the dark at 4 °C using controls, fluorescent substrates, and volumes as described in Weintraub et al. (2007) and modified by King et al. (2008, 2010) for cold sediments and soils. Fluorescence was measured using a Synergy HT Multi-Detection Microplate Reader (Biotek, USA) at 4, 22, 23 and 46 h of incubation and the highest values on a per hour basis were noted early in the incubation (after 4 h) so those are the values reported. Because enzyme activity in soils and sediments is driven primarily by the concentration of enzyme producing cells and the relative demand for each nutrient (in this case C, N, or P), raw enzyme activity estimates need to be normalized by some estimate of biomass in order to ascertain relative demand for various elements. Most studies normalize enzyme rates by organic C concentration (Sinsabaugh et al. 2009; Hill et al. 2012; Schmidt et al. 2016) or organic matter concentration (Zeglin et al. 2009). Those approaches, however, are problematic for cryoconite sediments since some of the organic C in cryoconite holes is likely relic recalcitrant ancient carbon that is not being actively cycled (cf. McCrimmon et al. 2018) and therefore should not scale with biomass or trophic status of the sediments. To compare rates of enzyme activities across the three glaciers, we normalized enzyme rates (nmol h−1 g dry sediment−1) by DNA concentration (µg DNA g dry sediment−1) in each sample to factor out differences in microbial biomass across the glaciers, to yield enzyme activity estimates with units of nmol h−1 µg DNA−1. For stoichiometric analyses to compare the relative rates of different enzyme activities, normalization by biomass is not needed as biomass would cancel out in the numerator and denominator (cf. Zeglin et al. 2009; Hill et al. 2012). In these analyses the relative activity of extracellular enzymes directed at acquiring various nutrients reflect the relative allocation of microbial resources to the acquisition of C (BG, AG, BXYL, and CBH), N (NAG and LAP), and P (PHOS), including in cold soils in Antarctica and high elevation plant-free soils (Schmidt et al. 2016; Bueno de Mesquita et al. 2020). For comparison to past studies of soils in the Dry Valleys (Zeglin et al. 2009), we used the following enzyme ratios: BG/LAP, and LAP/PHOS.
Data analyses
Statistical analyses were conducted in the R language, version 3.6.2 (Ihaka and Gettleman 1996), in R Studio, version 1.2.1335 (R Studio Team 2018). To reduce bias and provide more reproducible results, molar ratios were log transformed before any analyses (Isles 2020). Also, all C-enzyme concentrations were added together (same for N-enzymes) before statistical tests were run. Outliers were detected with boxplots and removed. Finally, Analysis of Variance (ANOVA) was used to test for significant difference between glaciers and if significance was determined, then Tukey HSD was used for a post hoc test (‘agricolae’ package).
Results
Concentrations of TOC and TON showed the up-valley pattern of the decreasing concentrations in cryoconite sediments from Commonwealth to Canada to Taylor Glacier (Fig. 2). C-to-N ratios for cryoconite sediments were close to values from soils and sediments worldwide of 14.3 (Cleveland and Liptzin 2007, solid line in Fig. 2), but higher than the Redfield ratio for aquatic bacteria, zooplankton, and phytoplankton of 6.6 (dashed line in Fig. 2; Redfield 1958; Chrzanowski and Kyle 1996). Specifically, C-to-N ratios were 10.7 (SE = 1), 12.2 (1.2), and 19.7 (3.5) for Commonwealth, Canada and Taylor glaciers, respectively (disregarding a high C outlier on Commonwealth) and pairwise comparisons of log means indicated each glacier’s C:N was significantly different from the others (Tukey HSD, Taylor v. Canada/Commonwealth P < 0.0001, Canada v. Commonwealth P = 0.002). For comparison, the mean C-to-N ratio of Taylor Valley soils was 24.6 (SE = 3.0) (Fig. 2).
Organic C and N content of sediments from cryoconite holes on Commonwealth (black triangles), Canada (blue circles) and Taylor (red squares) glaciers compared to Taylor Valley soils (brown circles, Burkins et al. 2001). The dashed line represents the “Redfield ratio” of 6.6 (C:N) which is the C:N of many aquatic bacteria (Chrzanowski and Kyle 1996) and zooplankton and phytoplankton in the oceans (Redfield 1958; Falkowski 2000). The solid line represents the global mean for soil organic matter C:N of 14.3, including tundra soils (Cleveland and Liptzin 2007). Mean (and SE) C:N values were 10.7 (1.0), 12.3 (1.2) and 19.7 (3.5) for Commonwealth, Canada and Taylor glaciers, respectively; and 24.6 (3.0) for the Taylor Dry Valley soils. Taylor Valley soil data are from Burkins et al. (2001)
Stable isotopic results for both N and C fell into distinct populations for each glacier (Fig. 3). Commonwealth Glacier had the least depleted δ15N values (− 3 to 1‰), followed by Canada (− 4 to 7‰ with one outlier at 15‰), and Taylor (− 17 to − 3‰). Commonwealth δ15N values were fairly consistent across TON concentrations > 200 ppm, whereas Canada δ15N values were positively correlated with TON (r2 = 0.45, slope = 0.036), and Taylor values were all negative and had extremely low TON (Fig. 3). Patterns of δ13C and TOC values displayed similar relationships, with Commonwealth having high δ13C and high TOC, Canada positively correlated δ13C and TOC values, and Taylor the lowest δ13C and TOC values (Fig. 3). To better ascertain the possible sources of the isotope signatures, Fig. 4 shows the relationship between δ13C and δ15 N for cryoconite holes, Taylor Valley soils, and the range of values from the literature for cyanobacteria that fix N and have mechanisms for concentrating inorganic C (green box in Fig. 4).
A Relationship between total nitrogen and δ15N isotope from cryoconite hole sediments. Each glacier falls into a distinct population, with Taylor the most depleted in δ15N and the lowest total N. B Relationship between total carbon and δ13C from decarbonated cryoconite hole sediments. As with N, the glaciers define distinct populations. Taylor, again, has the lowest concentration of C and the most depleted δ13C values
Relationship between δ13C and δ15N signatures in cryoconite hole sediments and soils of Taylor Valley. Green box shows the range of lab and field values for δ13C and δ15N from cyanobacteria that concentrate inorganic C and fix N2 (Vuorio et al. 2006; Zerkle et al. 2008; Bauersachs et al. 2009; Stricker et al. 2021). Symbols for cryoconite and soils are the same as in Fig. 2, except open circles are from a soil site that had significantly higher biological activity (as determined by chlorophyll a content) than the rest of the soils studied in Taylor Valley (Burkins et al. 2000). Linear regression line to glacier cryoconite values (r2 = 0.69)
We measured activity of key extracellular enzymes to gain an understanding of the relative demand for C, N and P. Enzyme activity per µg DNA was significantly higher on Taylor Glacier compared to both Commonwealth and Canada glaciers (Fig. 5) for C– an N– processing enzymes (Tukey HSD, P < 0.0001) and Phosphatase (Tukey P < 0.03), suggesting that microbes on Taylor Glacier are more nutrient limited than microbes on the two glaciers closer to the coast. These differences were greater for N- and C-acquiring enzymes (Fig. 5a, b) providing evidence of N and C limitation in some holes on Taylor Glacier. Activity levels of P-acquiring enzymes showed less difference among the three glaciers but were almost an order of magnitude higher than activity of N- and C-processing enzymes on all three glaciers, supporting the hypothesis that P is the ultimate limiting nutrient on all three glaciers.
Enzyme activity (per µg DNA) of N–, C– and P-acquiring enzymes in cryoconite-hole sediments from Commonwealth, Canada and Taylor glaciers. A Activity of N-acquiring enzymes was significantly higher (P < 0.0001) on Taylor Glacier than on Commonwealth and Canada glaciers (note log scale). B Combined activity of all four C-acquiring enzymes showed the same pattern and degree of significance as N-acquiring enzymes (P < 0.0001). C Phosphatase activity varied less across the glaciers but was still significantly higher on Taylor Glacier compared to Commonwealth (P = 0.03). Overall phosphatase activity was an order of magnitude higher than the other enzymes on all three glaciers, reinforcing that phosphorus is the most limiting nutrient
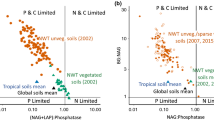
In order to compare our enzyme data to data from MDV soils, we used the same enzyme ratios (LAP-to-PHOS and BG-to-LAP) as those used by Zeglin et al. (2009) for Dry Valley soils. The LAP:PHOS data show strong P limitation in most of the holes sampled (Fig. 6, note the log scale), with Taylor Glacier having a more balanced mix of P and N limitation, similar to Dry Valley soils (Fig. 6). The BG:PHOS data also show strong P limitation (relative to C-limitation) across most of the cryoconite holes sampled and all of the soils sampled by Zeglin et al. (2009).
Enzyme stoichiometric plots to estimate the relative degree of N–, C– and P-limitation in cryoconite hole sediments from Commonwealth (black), Canada (blue), and Taylor (red) glaciers. A The ratio of N-processing (LAP) to P-processing enzyme (PHOS) activities indicates that most cryoconite holes were P-limited. For comparison, the brown circles represent enzyme stoichiometries for MDV soils (Zeglin et al. 2009), which show more balanced P and N limitation. B The ratio of C-processing (BG) to P-processing enzyme (PHOS) activities indicates that most cryoconite holes were P-limited. Dry Valley soils (brown circles) were likewise more P-limited than C-limited
Another stoichiometric approach to investigate relative N and P availability in cryoconites and soils is the ratio of DIN to phosphate-P (Barrett et al. (2007). Phosphate-P was over an order of magnitude higher on Commonwealth Glacier compared to the other two glaciers (P < 0.0001) (Fig. 7) and DIN levels were significantly higher on Taylor than Canada Glacier (P < 0.005) (Fig. 7b). DIN-to-phosphate ratios showed an almost two orders of magnitude lower N:P on Commonwealth Glacier (55, SE 8.3), compared to Canada (2078, SE 335) and Taylor (2348, SE 289) Glaciers. Pairwise tests of log means indicated significantly higher P:N ratios on Canada and Taylor vs. Commonwealth (Tukey HSD, P < 0.0001).
Phosphate and Dissolved Inorganic N (DIN) concentrations in cryoconite sediments from all three glaciers. A The mean phosphate concentration was an order of magnitude higher on Commonwealth Glacier than on the other two glaciers (P < 0.0001). B DIN levels were significantly higher on Commonwealth Glacier than Canada and Taylor glaciers (P < 0.0001). C DIN to P ratios indicate extreme P limitation on Canada and Taylor glaciers compared to Commonwealth Glacier (P < 0.0001)
Discussion
The gradient in nutrient status across the three glaciers seen in previous studies (Porazinska et al. 2004; Bagshaw et al. 2013; Stanish et al. 2013) is readily apparent in our data (Fig. 2) which show the highest C and N concentrations on Commonwealth and the lowest on Taylor Glacier. In addition, the levels of organic C and N in Taylor Glacier cryoconite sediments overlap to some extent with the previously measured levels of C and N in Taylor Valley soils (brown circles in Fig. 2), although most soils in the Taylor Valley have much lower levels of nutrients (especially N) compared to cryoconite sediments (note log scale in Fig. 2). The combined C-to-N ratio for cryoconite sediments across all three glaciers of 13.5 (SE = 1.4, n = 30) was close to the global average for soil organic matter of 14.3 (Fisk and Schmidt 1995; Cleveland and Liptzin 2007) but higher than the Redfield Ratio for aquatic bacteria and marine phytoplankton and zooplankton of 6.6 (Redfield 1958; Chrzanowski and Kyle 1996; Falkowski 2000; Chrzanowski and Grover 2008). The overall agreement of cryoconite sediment C:N with the global mean for soil organic matter is an indication that cryoconite microbes process organic matter (OM) in cryoconite holes in a manner similar to soils. It should be pointed out, however, that the C:N of Taylor Glacier sediments (19.7, SE 3.5) approaches the high C:N of Taylor Valley soils (24.6, SE 3.0). These higher C:N values could indicate incomplete processing of OM in Taylor Glacier sediments and Taylor Valley soils, perhaps due to nutrient and/or climatic limitations; or they may be the result of the influence of ancient recalcitrant organic C in Taylor Valley soils and sediments. Work in the Arctic has shown that cryoconite microbes do not use ancient OM in cryoconite holes (McCrimmon et al. 2018), but no similar work has been done on ancient Antarctic cryoconite OM.
We gained further insight into nutrient dynamics and N and C cycling in cryoconite sediments using stable isotope proxies. Figure 3a shows the relationship between total N concentration and δ15N values for cryoconite hole sediments. Commonwealth δ15N values ranged from − 3 to 1‰ (mean, − 0.37‰) most likely indicating a strong influence of cyanobacterial N-fixation. For comparison, Mo-Fe based nitrogenases in a variety of cyanobacteria yield biomass with δ15N values of about 0 to − 2‰ (Zerkle et al. 2008; Bauersachs et al. 2009) and Vuorio et al. (2006) report a range of − 2.1 to 1.6‰ (mean − 0.5‰) for the N-fixing, nostocalean cyanobacterium Gloeotrichia in an oligotrophic Finnish lake (Fig. 4). Benthic cyanobacterial mats in the moats of Lake Fryxell, Lake Hoare and Lake Bonney, all in the Taylor Valley, have mean δ15N values of − 1.25, − 3.9 and − 3.9‰, respectively (Lawson et al. 2004) suggesting a similar reliance on N-fixation in Lake Fryxell, the lake closest to Commonwealth Glacier. In addition, cryoconite holes on Commonwealth Glacier are dominated by N-fixing, nostocalean cyanobacteria in the genus Nostoc (Sommers et al. 2019a), that are closely related to Gloeotrichia (Domínguez-Escobar et al. 2011), whereas Nostoc are relatively rarer in Canada Glacier cryoconite holes and very rare in Taylor Glacier cryoconite holes (Sommers et al. 2019a). The dominance of Nostoc and less nutrient limitation on Commonwealth Glacier likely allow more photosynthesis to occur, resulting in higher oxygenation of cryoconite sediments which would inhibit denitrification. Canada Glacier δ15N values indicate a mix of influence of N-fixation and denitrification across the glacier (Fig. 4). That is, Canada Glacier exhibited a broader range of δ15N values that increased significantly with TON concentration (Fig. 3a). This pattern is consistent with more denitrification (which causes enrichment of 15N) occurring in Canada Glacier cryoconite holes with higher TON, and more N-fixation occurring in holes with lower TON.
Of the three glaciers, Taylor showed the least influence of either N-fixation or denitrification on δ15N values (Fig. 3). In fact, the most depleted δ15N values seen in Taylor Glacier cryoconite holes are similar to atmospheric NO3− observed in Antarctica (Savarino et al. 2007) and soil δ15N values across the MDV. For comparison, δ15N values of nitrate in MDV soils range from − 9.5 to − 26.2‰ (Michalski et al. 2005), whereas soil organic matter δ15N values range from − 16.6 to 4.7 (Burkins et al. 2000), as shown in Fig. 4. The depleted 15 N values seen in Taylor Glacier cryoconite and most Taylor Valley soils likely reflect a strong influence of atmospheric nitrate deposition. The most depleted δ15N values (− 16.6 to − 38.2‰) in atmospheric NO3− are observed in the Antarctic summer, which is the period of cryoconite formation. Thus, the range of δ15N in Taylor Glacier cryoconite closely resembles the range seen in Taylor Valley soils that probably reflect long-term deposition of atmospheric nitrate with little biological N-fixation or denitrification. This low δ15N signal from biological activity corresponds with low relative abundance of N-fixing Nostoc spp. on Taylor Glacier (Sommers et al. 2019a), and may indicate that severe P-limitation (discussed below) is preventing the development of N-fixing cyanobacteria there. This would also explain the very high values for enzymes responsible for recycling N in Taylor cryoconite holes (Fig. 5a).
As with isotopic results for N, those for δ13C fall into distinct populations for each glacier with values for Commonwealth and Canada glaciers overlapping with one another and Taylor having significantly more depleted δ13C values (Fig. 3b). The depleted δ13C values for Taylor Glacier are very similar to the δ13C values of soils of the Taylor Valley as shown in Fig. 4. This could mean that what little C is present in Taylor cryoconite is predominantly un-processed soil C (or ancient C), or that the biologic processes in Taylor Glacier cryoconite are similar to Taylor Valley soils. This similarity of δ13C values for Taylor valleys soils and Taylor Glacier cryoconite holes is mirrored in our nutrient results shown in Fig. 2, in that values for TOC and C:N of Taylor Glacier cryoconite holes are the closest to values for Taylor Valley soils of the three glaciers.
The enriched δ13C values recorded in cryoconite sediments on Commonwealth and Canada glaciers indicate that photosynthetic organisms in these holes (which can be sealed off from atmospheric inputs of CO2 for many years) might be CO2 limited. Enriched cryoconite δ13C values, close to atmospheric values (− 5‰), can indicate that all initial carbon (as atmospheric CO2) was processed through photosynthesis after the holes were isolated from the atmosphere by formation of an ice lid. More intense competition for CO2 in cryoconite holes on Commonwealth and Canada glaciers is also supported by past work that showed much higher concentrations of chlorophyll a in cryoconite sediments on Commonwealth (0.44 µg g−1) and Canada (0.24 µg g−1) glaciers, compared to Taylor Glacier (0.07 µg g−1) (Sommers et al. 2019a). In addition, many cyanobacteria have CO2-concentrating mechanisms that lead to enriched δ13C values in aquatic and soil systems (Raven et al. 2008; Morales-Williams et al. 2017; Stricker et al. 2021). For example, a nostocalean cyanobacterium (Gloeotrichia) yielded δ13C values from − 14.4 to − 5.9‰ in a cold, oligotrophic Finnish lake (Vuorio et al. 2006) that overlap with the δ13C values of Commonwealth and Canada Glaciers, but not Taylor Glacier (Fig. 4). Thus, the δ13C values and higher production observed on Commonwealth and Canada Glaciers is very likely due to nostocalean cyanobacterial activity.
To further explore nutrient limitations in cryoconite sediments of the Taylor Valley, we measured activity of key extracellular enzymes that have been used as an indicator of nutrient limitations in soils and sediments throughout the world (Weintraub et al. 2007; Sinsabaugh et al. 2009, 2012; Hill et al. 2012), including sites in the Dry Valleys and similar extreme environments (Zeglin et al. 2009; Schmidt et al. 2016; Jiang et al. 2019; Bueno de Mesquita et al. 2020). A summary plot of enzyme stoichiometry (N-processing enzymes/phosphatase) for every cryoconite hole sampled in this study (Fig. 6) shows that most cryoconite holes are P limited. Despite this overall pattern, it is interesting that two holes on Commonwealth Glacier and five holes on Taylor Glacier are more N than P limited, but we do not have enough replication or metadata to explain why at the present time. In comparison, Taylor Valley soils (Zeglin et al. 2009; brown circles in Fig. 6a) show more balanced P and N limitation as has been noted in other peri-glacial sediments, for example at high-elevation, plant-free sites in the Alaska Range (Schmidt et al. 2016).
Another way to visualize the relative demand for N, P and C in cryoconite holes is to separately compare the enzyme activity levels for N, P and C processing enzymes on each glacier. Figure 5 shows that phosphatase activity is an order of magnitude higher than the combined values of all N-processing enzymes and the combined value of all C-processing enzymes across all three glaciers. This suggests strong P-limitation across all three glaciers. In addition, these analyses show that activity of all enzymes (per µg DNA) is significantly higher on Taylor Glacier compared to the less oligotrophic glaciers (Fig. 5), indicating that nutrients in general are more limiting on Taylor Glacier. This finding is supported by previous work showing lower nutrient levels on Taylor Glacier (Porazinska et al. 2004; Stanish et al. 2013) and our findings of much lower C and N concentrations and higher C:N on Taylor Glacier (Fig. 2).
Further evidence of P limitation in cryoconite holes can be gleaned by comparing the DIN and phosphate-P concentrations across the three glaciers (Fig. 7). The extremely low levels of phosphate-P in Canada and Taylor cryoconite compared to Commonwealth cryoconite indicate higher biological demand for P and hence probable P-limitation on Canada and Taylor glaciers. However, the high inorganic N-to-P ratio on Commonwealth Glacier (55, SE 8.3) still indicates P limitation, although it is much less severe than the other two glaciers where the inorganic N-to-P ratios were much higher (Fig. 7).
In summary, we used multiple, independent biogeochemical approaches to gain an understanding of which nutrients are likely limiting microbial activity in cryoconite holes of the Taylor Valley, Antarctica. As with other recent studies of microbial activity in the cryobiosphere (Barrett et al. 2007; Zeglin et al. 2009; Schmidt et al. 2016; Darcy et al. 2016, 2018b; Vimercati et al. 2019; Bueno de Mesquita et al. 2020), the present study reveals that nutrient dynamics in Antarctic cryoconite holes are somewhat variable on each glacier, but show strong, predictable patterns of nutrient limitation across the broader landscape. Overall, based on enzyme stoichiometry (Figs. 5, 6) and ratios of P to DIN (Fig. 7), P is likely the ultimate limiting nutrient across the three main glaciers of Taylor Valley as previously hypothesized by Foreman et al. (2007) and Telling et al. (2014). However, the degree of P limitation varies among the three glaciers with Commonwealth Glacier being the least P limited. Carbon-to-nitrogen ratios of cryoconite sediments (Fig. 2) on Commonwealth and Canada glaciers are close to global means, but the more oligotrophic sediments on Taylor Glacier have significantly higher C:N indicating some N-limitation. N-limitation on Taylor Glacier is also strongly indicated by the very high activity (per unit biomass) of N-acquiring enzymes (Fig. 5a) compared to the other two glaciers, and lack of apparent N-fixation on Taylor glacier as indicated by the δ15N data (Fig. 4). In contrast, the δ15N of Canada, and especially Commonwealth glaciers, indicate higher rates of N-fixation are likely occurring on those glaciers.
From these results we can now hypothesize that extreme P limitation (and perhaps some other environmental factors) on Taylor Glacier is limiting establishment of N-fixing bacteria (likely Nostoc, which are common on the other two glaciers), which in turn is keeping biomass and diversity levels very low on Taylor Glacier. The lower biomass on Taylor Glacier is also consistent with more negative δ13C values there compared with the other two glaciers. Less negative δ13C likely result from photosynthesis by C-concentrating cyanobacteria in an environment without atmospheric equilibration. More negative values on Taylor Glacier would therefore be consistent with lower overall rates of photosynthesis. Laboratory (and perhaps field) nutrient addition experiments (cf. Darcy et al. 2018b; Mindl et al. 2007; Teufel et al. 2016) to test these hypotheses are planned in the near future. Similar fertilization experiments have recently been used to show that colonization of glacial sediments by N-fixing nostocalean cyanobacteria at plant-free sites in the Alaska Range and High Andes (> 5000 m.a.s.l.) are not climate limited, but rather are limited by P availability (Darcy et al. 2018b, Knelman et al. 2021).
Data availability
Data will be made available upon request.
Code availability
Not applicable.
Material availability
Data will be made available upon request.
References
Bagshaw EA, Tranter M, Fountain AG, Welch K, Basagic HJ et al (2013) Do cryoconite holes have the potential to be significant sources of C, N, and P to downstream depauperate ecosystems of Taylor Valley, Antarctica? Arct Antarct Alp Res 45:440–454
Barrett JE, Virginia RA, Hopkins DW, Aislabie J, Bargagli R, Bockheim JG, Campbell IB, Lyons WB, Moorhead DL, Nkem JN, Sletten RS, Steltzer H, Wall DH, Wallenstein MD (2006) Terrestrial ecosystem processes of Victoria Land, Antarctica. Soil Biol Biochem 38:3019–3034
Barrett JE, Virginia RA, Lyons WB, McKnight DM, Priscu JC, Doran PT, Fountain AG, Wall DH, Moorhead DL (2007) Biogeochemical stoichiometry of Antarctic Dry Valley ecosystems. J Geophys Res 112:G01010
Bauersachs T, Schouten S, Compaoré J, Wollenzien U, Stal LJ, Sinninghe Damsté JS (2009) Nitrogen isotopic fractionation associated with growth on dinitrogen gas and nitrate by cyanobacteria. Limnol Oceanogr 54:1403–1411
Blume-Werry G, Krab EJ, Olofsson J et al (2020) Invasive earthworms unlock arctic plant nitrogen limitation. Nat Commun 11:1766. https://doi.org/10.1038/s41467-020-15568-3
Bowman WD et al (1996) Symbiotic N2 fixation in alpine tundra: ecosystem input and variation in fixation rates among communities. Oecologia 108:345–350
Bueno de Mesquita CP, Brigham LM et al (2020) Evidence for phosphorus limitation in high-elevation unvegetated soils, Niwot Ridge, Colorado. Biogeochemistry 147:1–13
Burkins MB, Virginia RA, Chamberlain RA, Wall DH (2000) The origin of soil organic matter in Taylor Valley, Antarctica. Ecology 81:2377–2391
Burkins MB, Virginia RA, Wall DH (2001) Organic carbon cycling in Taylor Valley, Antarctica: quantifying soil reservoirs and soil respiration. Glob Change Biol 7:113–125
Chrzanowski TH, Grover JP (2008) Element content of Pseudomonas fluorescens varies with growth rate and temperature: A replicated chemostat study addressing ecological stoichiometry. Limnol Oceanogr 53:1242–1251
Chrzanowski TH, Kyle M (1996) Ratios of carbon, nitrogen and phosphorus in Pseudomonas fluorescens as a model for bacterial element ratios and nutrient regeneration. Aquat Microb Ecol 10:115–122
Cleveland CC, Liptzin D (2007) C:N: P stoichiometry in soil: is there a redfield ratio for the microbial biomass? Biogeochemistry 85:235–252
Conovitz PA, McKnight DM, Macdonald LH, Fountain AG, House HR (2013) Hydrologic processes influencing streamflow variation in Fryxell Basin, Antarctica. In: Priscu JC (ed) Antarctic research series. American Geophysical Union, Washington DC, pp 93–108
Darcy JL, Schmidt SK (2016) Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biol Biochem 95:156–163
Darcy JL, Gendron E, Sommers P, Porazinska DL, Schmidt SK (2018a) Island biogeography of cryoconite hole bacteria in Antarctica’s Taylor Valley and around the world. Front Ecol Evol. https://doi.org/10.3389/fevo.2018.00180
Darcy JL, Schmidt SK, Knelman JE, Cleveland CC, Castle SC, Nemergut DR (2018b) Phosphorus, not nitrogen, limits plants and microbial primary producers following glacial retreat. Sci Adv 4:eaaq094
Domínguez-Escobar J, Beltrán Y, Bergman B et al (2011) Phylogenetic and molecular clock inferences of cyanobacterial strains within Rivulariaceae from distant environments. FEMS Microbiol Lett 316:90–99
Doran PT, McKay CP, Clow GD, Dana GL, Fountain AG, Nylen TH, Lyons WB (2002) Valley floor climate observations from the McMurdo Dry Valleys, Antarctica, 1986–2000. J Geophys Res. https://doi.org/10.1029/2001JD002045
Doran PT, McKay CP, Fountain AG, Nylen T, McKnight D, Jaros C, Barrett JE (2008) Hydrologic response to extreme warm and cold summers in the McMurdo Dry Valleys, East Antarctica. Antarct Sci 20:499–509
Falkowski PG (2000) Rationalizing elemental ratios in unicellular algae. J Phycol 36:3–6
Fisk MC, Schmidt SK (1995) Nitrogen mineralization and microbial biomass N dynamics in three alpine tundra communities. Soil Sci Soc Am J 59:1036–1043
Foreman CM, Sattler B, Mikucki JA, Porazinska DL, Priscu JC (2007) Metabolic activity and diversity of cryoconites in the Taylor Valley, Antarctica. J Geophys Res Biogeosci 112:G04532
Fortner SK, Lyons WB (2018) Dissolved trace and minor elements in cryoconite holes and supraglacial streams, Canada Glacier, Antarctica. Front Earth Sci. https://doi.org/10.3389/feart.2018.00031
Fountain AG, Lewis KJ, Doran PT (1999) Spatial climatic variation and its control on glacier equilibrium line altitude in Taylor Valley, Antarctica. Glob Planet Change 22:1–10
Fountain AG, Tranter M, Nylen TH, Lewis KJ, Mueller DR (2004) Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. J Glaciol 50:35–45
Fountain AG, Nylen TH, Monaghan A, Basagic HJ, Bromwich D (2010) Snow in the McMurdo dry valleys, Antarctica. Int J Climatol 30:633–642
Goordial J, Davila A et al (2016) Nearing the cold-arid limits of microbial life in permafrost of an upper dry valley, Antarctica. ISME J 10:1613–1624
Hill BH, Elonen CM, Seifert LR, May AA, Tarquino E (2012) Microbial enzyme stoichiometry and nutrient limitation in US streams and rivers. Ecol Ind 18:540–551
Hu W, Schmidt SK, Sommers P, Darcy JL, Porazinska DL (2021) Multi-trophic patterns of primary succession following retreat of a high-elevation glacier. Ecosphere 12:e03400
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Isles PD (2020) The misuse of ratios in ecological stoichiometry. Ecology 101:e03153
Jiang Y, Lei Y, Qin W, Korpelainen H, Li C (2019) Revealing microbial processes and nutrient limitation in soil through ecoenzymatic stoichiometry and glomalin-related soil proteins in a retreating glacier forefield. Geoderma 338:313–324
King AJ, Meyer AF, Schmidt SK (2008) High levels of microbial biomass and activity in unvegetated tropical and temperate alpine soils. Soil Biol Biochem 40:2605–2610
King AJ, Freeman KR, Lozupone CA, Knight R, Schmidt SK (2010) Biogeography and habitat modelling of high-alpine bacteria. Nat Commun 1:53
Knelman JE et al (2021) Cyanobacteria in early soil development of deglaciated forefields: Dominance of non-heterocytous cyanobacteria and phosphorus limitation of N-fixing Nostocales. Soil Biol Biochem 154:108127
Lawson J, Doran PT, Kenig F, Des Marais DJ, Priscu JC (2003) Stable carbon and nitrogen isotopic composition of benthic and pelagic organic matter in lakes of the McMurdo Dry Valleys, Antarctica. Aquat Geochem 10:269–301
Levy J (2013) How big are the McMurdo Dry Valleys? Estimating ice-free area using Landsat image data. Antarct Sci 25:119–120
Lutz S, Anesio AM, Edwards E, Benning LG (2017) Linking microbial diversity and functionality of Arctic glacial surface habitats. Environ Microbiol 19:551–565
Lutz S, Ziolkowski LA, Benning LG (2019) The biodiversity and geochemistry of cryoconite holes in Queen Maud Land, East Antarctica. Microorganisms. https://doi.org/10.3390/microorganisms7060160
McCrimmon DO, Bizimis M, Holland A, Ziolkowski LA (2018) Supraglacial microbes use young carbon and not aged cryoconite carbon. Org Geochem 118:63–72
McKnight DM, Niyogi DK, Alger AS, Bomblies A, Conovitz PA, Tate CM (1999) Dry valley streams in Antarctica: ecosystems waiting for water. Bioscience 49:985–995
Michalski G, Bockheim JG, Kendall C, Thiemens M (2005) Isotopic composition of Antarctic Dry Valley nitrate: Implications for NOy sources and cycling in Antarctica. Geophys Res Lett 32:L13817
Mindl B, Anesio AM, Meirer K et al (2007) Factors influencing bacterial dynamics along a transect from supraglacial runoff to proglacial lakes of a high Arctic glacier. FEMS Microbiol Ecol 59:307–317
Morales-Williams AM, Wanamaker AD, Downing JA (2017) Cyanobacterial carbon concentrating mechanisms facilitate sustained CO2 depletion in eutrophic lakes. Biogeosciences 14:2865–2875
Novotná Jaroměřská TN, Trubač J, Zawierucha K, Vondrovicová L, Devetter M, Žárský JD (2021) Stable isotopic composition of top consumers in Arctic cryoconite holes: revealing divergent roles in a supraglacial trophic network. Biogeosciences 18:1543–1557
Porazinska DL, Fountain AG, Nylen TH, Tranter M, Virginia RA, Wall DH (2004) Biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley glaciers, Antarctica. Arct Antarct Alp Res 36:84–91
Priscu JC, Fritsen CH, Adams EE, Giovannoni SJ, Paerl HW, McKay CP, Doran PT, Gordon DA, Lanoil BD, Pinckney JL (1998) Perennial Antarctic lake ice: an oasis for life in a polar desert. Science 280:2095–2098
R Studio Team (2018) R studio: integrated development for R. R Studio Inc, Boston
Raven JA, Cockell CS, De La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos Trans Roy Soc Lond B 363:2641–2650
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Savarino J, Kaiser J, Morin S, Sigman DM, Thiemens MH (2007) Nitrogen and oxygen isotopic constraints on the origin of atmospheric nitrate in coastal Antarctica. Atmos Chem Phys 7:1925–1945
Schmidt SK, Reed SC, Nemergut DR (2008) The earliest stages of ecosystem succession in high-elevation (5000 m.a.s.l.), recently de-glaciated soils. Proc Roy Soc B 275:2793–2802
Schmidt SK, Porazinska D, Concienne B-L, Darcy JL, King AJ, Nemergut DR (2016) Biogeochemical stoichiometry reveals P and N limitation across the post-glacial landscape of Denali National Park, Alaska. Ecosystems 19:1164–1177
Sinsabaugh RL, Follstad-Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Ann Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Hill BH, Folstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic matter acquisition in soil and sediment. Nature 462:795–798
Solon A, Mastrangelo C, Vimercati L, Sommers P, Darcy JL, Gendron E, Porazinska DL, Schmidt SK (2021) Gullies and moraines are islands of biodiversity in an arid, mountain landscape, Asgard Range, Antarctica. Front Microbiol 12:654135
Sommers P, Darcy JL, Gendron EMS, Stanish LF, Bagshaw EA, Porazinska DL, Schmidt SK (2018) Diversity patterns of microbial eukaryotes mirror those of bacteria in Antarctic cryoconite holes. FEMS Microbiol Ecol 94:167
Sommers P, Darcy JL, Porazinska DL, Gendron EMS, Fountain AG, Zamora F et al (2019a) Comparison of microbial communities in the sediments and water columns of frozen cryoconite holes in the McMurdo Dry Valleys, Antarctica. Front Microbiol 10:65
Sommers P, Porazinska DL, Darcy JL, Zamora F, Fountain AG, Schmidt SK (2019b) Experimental cryoconite holes as mesocosms for studying community ecology. Polar Biol 42:1973–1984
Stanish LF, Bagshaw EA, McKnight DM, Fountain AG, Tranter M (2013) Environmental factors influencing diatom communities in Antarctic cryoconite holes. Environ Res Lett. https://doi.org/10.1088/1748-9326/8/4/045006
Stibal M, Tranter M, Telling J, Benning LG (2008) Speciation, phase association and potential bioavailability of phosphorus on a Svalbard glacier. Biogeochemistry 90:1–13
Stricker E, Crain G, Rudgers J et al (2021) What could explain δ13C signatures in biocrust cyanobacteria of drylands? Microb Ecol 81:134–145
Stüeken EE, Kipp MA, Koehler MC, Buick R (2016) The evolution of Earth’s biogeochemical nitrogen cycle. Earth-Sci Rev 160:220–239
Telling J, Anesio AM, Tranter M, Fountain AG, Nylen T, Hawkings J, Singh VB, Kaur P, Musilova M, Wadham JL (2014) Spring thaw ionic pulses boost nutrient availability and microbial growth in entombed Antarctic Dry Valley cryoconite holes. Front Microbiol 5:694
Teufel AG, Li W, Kiss AJ, Morgan-Kiss RM (2016) Impact of nitrogen and phosphorus on phytoplankton production and bacterial community structure in two stratified Antarctic lakes: a bioassay approach. Polar Biol 40:1007–1022
Tranter M, Fountain AG, Fristsen CH, Lyons WB, Priscu JC, Statham PJ, Welch KA (2004) Extreme hydrochemical condition in natural microcosms entombed within Antarctic ice. Hydrol Process 18:379–387
Vimercati L, Solon AJ, Krinsky A, Arán P, Porazinska DL, Darcy JL, Dorador C, Schmidt SK (2019) Nieves penitentes are a new habitat for snow algae in one of the most extreme high elevation environments on Earth. Arct Antarct Alp Res 51:190–200
Virginia RA, Wall DH (1999) Soil animals in an extreme environment: how soils structure communities in the McMurdo Dry Valleys, Antarctica. Bioscience 49:973–983
Vuorio K, Meili M, Sarvala J (2006) Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshw Biol 51:807–822
Webster-Brown JG, Hawes I, Jungblut AD, Wood SA, Christenson HK (2015) The effects of entombment on water chemistry and bacterial assemblages in closed cryoconite holes on Antarctic glaciers. FEMS Microbiol Ecol 91(12):144
Weintraub MN, Scott-Denton LE, Schmidt SK, Monson RK (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–338
Zeglin LH, Sinsabaugh RL, Barrett JE, Gooseff MN, Takacs-Vesbach CD (2009) Landscape distribution of microbial activity in the McMurdo Dry Valleys: linked biotic processes, hydrology, and geochemistry in a cold desert ecosystem. Ecosystems 12:562–573
Zerkle AL, Junium CK, Canfield DE, House CH (2008) Production of 15N-depleted biomass during cyanobacterial N2-fixation at high Fe concentrations. J Geophys Res 113:G03014
Acknowledgements
We thank the late Diana Nemergut whose visionary leadership launched this project. This work would not have been possible without support from US Antarctic Program support staff and contractors. We thank T. Nylen and B. Hodges from UNAVCO for invaluable GPS support, and Kaelin Cawley for helpful comments. Chemistry of extracted nutrients was performed by the Colorado State University Soil, Water, and Plant Testing Lab. This work was funded by NSF Polar Programs Award 1443578. BWJ was funded by NSF EAR postdoctoral fellowship 1725784.
Funding
NSF Polar Programs Award 1443578 to SKS, PS, and AGF. NSF EAR postdoctoral fellowship 1725784 to BWJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or competing interests.
Additional information
Responsible Editor: Jacques C. Finlay
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schmidt, S.K., Johnson, B.W., Solon, A.J. et al. Microbial biogeochemistry and phosphorus limitation in cryoconite holes on glaciers across the Taylor Valley, McMurdo Dry Valleys, Antarctica. Biogeochemistry 158, 313–326 (2022). https://doi.org/10.1007/s10533-022-00900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00900-4