Abstract
High-latitude boreal and arctic surface/inland waters contain sizeable reservoirs of dissolved organic matter (DOM) and trace elements (TE), which are subject to seasonal freezing. Specifically, shallow ponds and lakes in the permafrost zone often freeze solid, which can lead to transformations in the colloidal and dissolved fractions of DOM and TE. Here, we present results from experimental freeze-thaw cycles using iron (Fe)- and DOM-rich water from thaw ponds situated in Stordalen and Storflaket palsa mires in northern Sweden. After ten cycles of freezing, 85% of Fe and 25% of dissolved organic carbon (DOC) were removed from solution in circumneutral fen water (pH 6.9) but a much smaller removal of Fe and DOC (< 7%) was found in acidic bog water (pH 3.6). This removal pattern was consistent with initial supersaturation of fen water with respect to Fe hydroxide and a lack of supersaturation with any secondary mineral phase in the bog water. There was a nearly two- to threefold increase in the low-molecular-weight (LMW) fraction of organic carbon (OC) and several TEs caused by the repeated freeze-thaw cycles. Future increases in the freeze-thaw frequency of surface waters with climate warming may remove up to 25% of DOC in circumneutral organic-rich waters. Furthermore, an increase of LMW OC may result in enhanced carbon dioxide losses from aquatic ecosystems since this fraction is potentially more susceptible to biodegradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic-rich soils in high-latitude boreal and arctic regions contain large reservoirs of soluble organic carbon (Laudon et al. 2004; Pokrovsky et al. 2015). The “colloidal world” of organic-rich waters has received thorough attention in non-permafrost (Andersson et al. 2001, 2006; Gundersen and Steinnes 2003; Vasyukova et al. 2010) and permafrost regions (Guo et al. 2003; Stolpe et al. 2013; Pokrovsky et al. 2011, 2013, 2016a). The bulk of DOM present in organic-rich surface waters, which comprises colloidal humic and fulvic substances, is dramatically different from the original soil organic matter, likely because of a combination of photochemical (Shiller et al. 2006; Porcal et al. 2015; Doane 2017) and microbial degradation (Bosch et al. 2010; Fritzsche et al. 2012; Oleinikova et al. 2017a). Both processes led to the removal of organic carbon (Corg) and trace elements (TE) via coagulation of amorphous iron (Fe) hydroxide, loss of carbon via CO2 emissions, and production of low-molecular-weight (LMW) fractions of Corg and TE (Wetzel 1995; Moran and Zepp 1997; Bertilsson et al. 1999; Brinkmann et al. 2003; Oleinikova et al. 2017a, b). Another important factor potentially transforming DOM is the freezing of shallow boreal and Arctic ponds and lakes (Arp et al. 2011; Manasypov et al. 2015). Even more intense organic colloid transformation may take place during the transfer of DOM from the active layer to the underlying permafrost with successive freeze-thaw cycles due to full freezing of the soil column (Shur et al. 2005).
The cryo-transformation of organic and organo-mineral colloids due to freezing and thawing of aqueous solutions may coagulate colloids, decrease the concentration of organic carbon (Spencer et al. 2007), change the molecular structure of DOM (Belzile et al. 2002; Chen et al. 2016; Xue et al. 2015, 2016), and precipitate insoluble Fe and aluminum (Al) hydroxides as well as silica- and aluminium-rich minerals (Wada and Nagasto 1983; Keung et al. 1984; Dietzel 2005; Kokelj and Burn 2005; Ireson et al. 2013). Previous studies on freezing of natural water to optimize DOM (Giesy and Briese 1978; Spencer et al. 2007) and nutrient (Ron Vaz et al. 1994; Fellman et al. 2008) preservation have demonstrated partial removal of DOC, phosphorus (P), and trace elements (TE) from the dissolved fraction (< 0.22 or 0.45 µm). Cryogenic concentrations of major ions during freezing at the soil surface (Levy et al. 2012) and within the active layer (Lundin and Johnsson 1994; Kokelj and Burn 2005; Jessen et al. 2014) have also been reported. However, to our knowledge, there has been no experimental evaluation of the transformation of organic and organo-mineral colloids during freeze and thaw cycles.
The overall aim of our study is to evaluate the possible transformation of dissolved and colloidal carbon and TE due to seasonal freezing and thawing of organic-rich surface waters in the high latitude boreal and Arctic inland waters. We quantified colloidal transformation by measuring concentrations of DOC and TE in different size fractions during subsequent freeze and thaw cycles in surface waters from two contrasting thaw ponds located in the discontinuous permafrost zone in northern Sweden. We hypothesized that (1) dissolved + colloidal (< 0.45 µm) organic carbon and TEs would be removed from solution via coagulation, (2) the precipitation of secondary mineral phases would depend on the initial saturation state of the fluid with respect to these minerals, and (3) the breakdown of large organic and organo-mineral colloids would lead to production of small-size organic molecules.
Materials and methods
Study sites
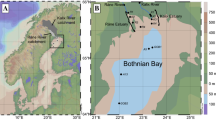
The two thaw ponds are situated in two nearby peat palsa complexes (Storflaket and Stordalen) located about 3 km apart within the Stordalen valley (68°20′N, 18°58′E) in northern Sweden. The palsa mires are characterized by a peat plateau with a homogeneous peat thickness of about 0.5 m overlaying silty lacustrine sediments of glacial origin (Klaminder et al. 2008). The mean annual air temperature in the region between 2000 and 2009 was 0.6 ± 0.4 °C (mean ± standard deviation). Annual precipitation was 340 ± 56 mm with average snow coverage lasting from October to May. All climatological data were recorded at the Abisko Scientific Research Station (www.polar.se/abisko). The maximum thickness of the active layer (i.e., the seasonally thawing zone in late September) was about 0.5 m in the hummocks and between 1 and 3 m in the hollows in both of the palsa mires (Åkerman and Johansson 2008, Johansson et al. 2013). Detailed descriptions of the two palsa mires are published elsewhere (Malmer et al. 2005; Alewell et al. 2011; Johansson et al. 2013 Thompson et al. 2015). The Stordalen thaw pond is acidic (pH 3.6) with low concentrations of calcium (Ca), dissolved inorganic carbon (DIC), magnesium (Mg), and strontium (Sr), as shown in Table 1. The Storflaket thaw pond is circumneutral (pH 6.9) and demonstrates a higher concentration of total dissolved solids (TDS). The two shallow thaw ponds (< 0.5 m deep; Fig. S1 of Online Resource 1) were rich in DOC (55 and 112 mg/l in Storflaket and Stordalen, respectively) and fully oxygenated at the time of sampling (Table 1).
The Stordalen pond is representative for the large zone of frozen peat bogs, where active thermokarst processes cause peat thaw and small pond formation. The low pH, high DOC, and low TDS are very typical for hundreds of thousands of small thaw ponds and lakes in Western Siberia (Pokrovsky et al. 2011, 2013, 2016a; Shirokova et al. 2013; Manasypov et al. 2014, 2015 and Polishchuk et al. 2017) and elsewhere. The typical feature of all these ponds is that they are located within ombrotrophic bogs and receive no groundwater because of their geomorphologic position in the landscape and the frozen nature of the surrounding organic soil. The Storflaket pond is more similar to minerotrophic bogs in that it has a circumneutral pH and higher concentrations of major ions such as Ca and DIC. Such hydrochemical features are also typical of thaw slump-affected ponds and lakes in northern Canada (Kokelj et al. 2009) as well as small rivers and streams draining boreal and subarctic peatland regions like those in Western Siberia (Pokrovsky et al. 2015, 2016b). Together, these two studied ponds are generally representative of common, yet distinctly different types of surface waters in boreal and subarctic wetlands.
Sampling, size fractionation, and analyses
On 21 October 2016, after the first ca. 2 cm of ice formation (Fig. S1 of Online Resource 1), the thaw ponds were sampled and brought to the laboratory for filtration and colloidal separation within 1 h after sampling. All the analytical approaches used in this study followed methods developed for boreal DOM-rich waters (Pokrovsky et al. 2011, 2016a, b; Shirokova et al. 2013). The analytes are listed and defined by type in Table 1. Approximately 1 L of unfiltered surface water was collected in sterile pre-cleaned light-protected polypropylene bottles for 0.45- and 0.22-µm filtration followed by a centrifugal ultrafiltration (UF) through 50- and 3-kDa single-use Amicon Ultracell 15-ml cartridges [see the description of the preparation and cleaning procedure and discussion of possible artifacts of ultrafiltration in Oleinikova et al. (2017a)]. The centrifugal ultrafiltration was run at 4 °C using an Eppendorf 5920 R refrigerated centrifuge (4000 rpm, 20 min). Vacuum filtration (0.45 µm) was performed using a Mityvac MV8255 PVC hand pump. The filtration and ultrafiltration allowed us to separate two group of colloids: the high molecular weight (HMW50 kDa–0.45 µm) and low molecular weight (LMW3–50 kDa) as well as the low-molecular-weight fraction (LMW< 3 kDa).
The pH was measured at the in situ temperature of the ponds (4 °C). Chloride and sulfate concentrations were measured by ion chromatography (Dionex 2000i) with uncertainties of 2% and detection limits of 0.05 mg l−1. The concentrations of DOC and DIC were determined using a Shimadzu TOC-VSCN Analyzer with an uncertainty of 3% and a detection limit of 0.1 mg/l as derived for the specific instrument in the Géosciences Environnement Toulouse (GET) laboratory (Prokushkin et al. 2011). The UV absorbance of the filtered samples was measured at 254 nm using a quartz 10-mm cuvette on a Cary-50 spectrophotometer. The specific UV absorbency (SUVA254, l mg−1 m−1) was used as a proxy for the aromatic C, molecular weight, and source of DOM (Weishaar et al. 2003; Ilina et al. 2014 and references therein). All major and trace elements were measured with an ICP-MS Agilent 7500 ce using both argon (Ar) and helium (He) modes to diminish the interferences. Indium and rhenium were used as internal standards of the interference types at concentrations of ~ 3 µg/l. The typical uncertainty for elemental concentration measurement ranged from 5 to 10% at 1–1000 μg/l to 10–20% at 0.001–0.1 μg/l. The MilliQ field blanks were collected and processed to monitor any potential sample contamination introduced by our sampling and handling procedures. The DOC blanks of filtrates and ultrafiltrates never exceeded 0.1 mg/l. For all major and most trace elements, the concentrations in the blanks were below analytical detection limits (≤ 0.1–1 ng/l for Cd, Ba, Y, Zr, Nb, REE, Hf, Pb, Th, U; 1 ng/l for Ga, Ge, Rb, Sr, Sb; ~ 10 ng/l for Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As). The international geostandard SLRS-5 (Riverine Water Reference Material for Trace Metals) was used to check the validity and reproducibility of analysis. All certified major (Ca, Mg, K, Na, Si) and trace element (Al, As, B, Ba, Co, Cr, Cu, Fe, Ga, Li, Mn, Mo, Ni, Pb, all REEs, Sb, Sr, Th, Ti, U, V, Zn) concentrations of the SLRS-5 standard and the measured concentrations agreed with an uncertainty of 10–20%. The agreement with SLRS-5 for Cd, Cs, and Hf was between 30 and 50%, although the uncertainty in the analyses of these elements was between 5 and 10%.
The tests of Fe(II) presence at the beginning and end of freezing cycles were performed using the conventional ferrozine method (Viollier et al. 2000), employing a standard addition technique, to account for the presence of high DOM concentrations. The uncertainty of this technique was ± 10% and the quantification limit 50 µg l−1 Fe(II).
Experimental freezing and thawing cycles
Sterile-filtered (< 0.22 µm) waters were subjected to full freezing at − 18 °C in the freezer (12–24 h) and thawing at 4 °C (typically 24 h) in the refrigerator. After each cycle, a subsample of water was collected in a sterile laminar hood box under continuous stirring with a magnet stir bar and processed for filtration (< 0.45 µm) or ultrafiltration (3 and 50 kDa) as described below. After complete melting of ice in the refrigerator, we measured pH at 4 °C in the laboratory. Each sample was subjected to ten freeze-thaw cycles run in duplicate. The ten cycles chosen in this study corresponded to the typical range of annual soil freeze-thaw cycles across the high-latitude boreal and Arctic zone, which is between 5 and 15 (Henry 2008). Limited volumes of initial samples precluded a larger number of cycles and did not allow detailed colloidal characterization after each freeze-thaw cycle. As such, only at the 0th, 3rd, and 10th cycles was the 0.45-µm-filtered solution separated into colloidal fractions (3 and 50 kDa) as described above.
Thermodynamic modeling of metal speciation in the course of freeze-thaw cycles in the colloids
Trace element (metal cations) complexation with organic matter in filtrates and ultrafiltrates was calculated using the Visual MINTEQ (vMinteq) computer code (Gustafsson 2011, version 3.0, for Windows) in conjunction with the NICA-Donnan humic ion-binding model (Benedetti et al. 1995; Kinniburgh et al. 1999; Milne et al. 2003) and the vMinteq (CRITICAL) database. The vMinteq implements a database for the binding of metal cations to discrete carboxylic sites of the humic and fulvic acids (Allison and Perdue 1994). The calculation is performed assuming a constant content of 10 µeq carboxylate per mg DOC (Oliver et al. 1983). We considered a ratio of DOM to DOC of 2 and that 100% DOM is fulvic acid. This approach works efficiently for boreal organic-rich waters (Vasyukova et al. 2010; Ilina et al. 2016; Pokrovsky et al. 2016a). The input parameters of the model were the in situ temperature, pH, DIC, DOC, anions, cations, and filtered (0.45 µm) or ultrafiltered (3 and 50 kDa) TE concentrations at the 0th, 3rd, and 10th freezing-thawing cycles. The model was run at 4 °C, which corresponded to the temperature of the solution before filtration and ultrafiltration and after complete melting of ice in the refrigerator. The model calculation yielded a theoretical degree of complexation for divalent metals [barium (Ba), Ca, cadmium (Cd), cobalt (Co), copper (Cu), Mg, manganese (Mn), nickel (Ni), lead (Pb), Sr, zinc (Zn), Al3+, FeIII, lanthanum (La3+), thorium (ThIV), and uranyl (UVIO22+)] with DOM and the degree of solution supersaturation with respect to possible authigenic minerals such as amorphous Al hydroxide, allophane, imogolite, ferrihydrite, and lepidocrocite, which most likely form under the soil and groundwater conditions in the high-latitude boreal and Arctic Sweden (Gustafsson et al. 1995; Lundström et al. 2000).
Results
Impact of freezing on the total dissolved concentration, colloidal composition, and low-molecular-weight fraction
We identified three groups of elements according to the change in element concentrations in filtrates of progressively decreasing pore size from 0.45 µm to 3 kDa: (1) strongly affected by decreasing pore size (> 5× concentration change): Fe, DOC, P, all trivalent and tetravalent hydrolysates, Pb2+, and UVIO22+; (2) moderately affected by decreasing pore size (between 2 and 3× concentration change): alkaline earth elements (Ca, Mg, Sr, and Ba), V, Cr, Mo, and As and divalent metals (Cu, Ni, Co, Zn, Mn, and Cd); (3) not affected (within 20–30% variation) by the size separation procedure: Li, B, Si, Na, K, Rb, and Sb. The evolution of the DOC, Fe, Al, and TE compositions of surface waters during filtration and ultrafiltration and their comparison with other boreal and subarctic settings are described in Supplement 2 (Online Resource 2).
The freezing yielded generally transparent ice on the walls of containers with concentrated brown ice in the center (Fig. S3-1 of Online Resource 3). After the first thawing, the water from Storflaket produced a brown/yellow precipitate at the bottom of the container. In contrast, no precipitate was visible in the Stordalen palsa mire water even after the tenth cycle of freezing and thawing. The pH and proportion of Fe(II) of both samples remained very stable during the freeze-thaw cycles (within ± 0.05 pH units and ± 5%, respectively). The variations of pH are illustrated in Fig. S3-2 of Online Resource 3. After ten freeze-thaw cycles, the concentrations of DOC (< 0.45 µm) decreased by ca. 25 ± 5% and 6 ± 1% in the Storflaket and Stordalen waters, respectively. The decreases in concentrations of Fe, Al, P, Ti, V, Ge, Y, Nb, Cd, REEs, Pb, Th, and U were between 40 and 90% of their initial concentrations in the Storflaket water but only 5–15% in the Stordalen water (Table 2). Chromium (Cr), Mn, Co, Ni, Cu, and Ba decreased by 15–30% in the Storflaket and < 10% in the Stordalen water. Note the sizeable removal of Si (75 ± 10%) in both waters. Examples of element concentration changes during freezing/thawing cycles of Stordalen and Storflaket waters are shown in Online Resource 3 (Fig. S3-3 and S3-4, respectively). Depending on their affinity to Fe or DOC within size fractions after each freezing and thawing, the elements are divided into two groups. In both Stordalen and Storflaket waters, Al, Ga, As, Ti, V, Cr, Y, Nb, Zr, Mo, REEs, Cd, Hf, Pb, Th, and U followed the behavior of Fe during freeze-thaw cycles, whereas alkaline-earth elements and divalent transition metals decreased their concentration concomitant to that of DOC. The separation into two groups was based on the degree of correlation (R2 of the linear relationship) between the elements and DOC and Fe (see details in Online Resource 2).
We observed a strong contrast in the organic carbon and trace metal concentrations in the course of freezing/thawing of two ponds. The Corg/Fe and Corg/Al remained fairly constant or only slightly (within 10%) decreased in Stordalen water (Fig. 1a) but increased by a factor of 6 ± 1 in the course of the freezing experiment of Storflaket water (Fig. 1b, c). The OC demonstrated net and clear enrichment in the low-molecular-weight fraction (LMW< 3 kDa) (Fig. 2a, b). The proportion of OC in the LMW< 3 kDa fraction increased from 23% at the beginning to 57% at the tenth cycle and from 19 to 47% for Stordalen and Storflaket mires, respectively (Fig. 3a, b). Further, in the LMW< 3 kDa fraction of DOM, the SUVA254 nm, which reflects the content of aromatic DOM, decreased from 4.0 to 2.4 in Stordalen and from 4.3 to 1.8 in Storflaket in the course of ten freeze-thaw cycles. At the same time, the decrease of SUVA254 nm in the 0.45-µm fraction was absent in Stordalen and constituted only 15% of the initial amount in Storflaket (Table 2).
Percentages of elements in low- and high-molecular-weight fractions during freeze-thaw experiments. Element proportions in the dissolved low-molecular-weight fraction (LMW< 3 kDa) in Stordalen (a) and Storflaket (b) waters and the fractions of high-molecular-weight colloids (HMW50 kDa–0.45 µm) in Stordalen (c) and Storflaket (d) waters. (E) Cr, Mo, and V in LMW< 3 kDa fractions of Storflaket waters. (f) Si proportions in LMW< 3 kDa and high-molecular-weight colloid (HMW50 kDa–0.45 µm) fractions in Stordalen waters
The distribution of major and trace elements between colloids of different sizes was heavily impacted by freezing and thawing cycles in neutral Storflaket water compared with acidic Stordalen water. Thus, the percentage of Fe and Al found in the LMW< 3 kDa fraction increased by a factor of 3 over ten cycles of freezing/thawing in the Storflaket waters, whereas this change in the Stordalen water was within ± 10% of the total dissolved fraction (see Fig. 2c–f for concentrations and Fig. 3a, b for relative fractions).
The concentration of HMW50 kDa–0.45 µm colloidal forms of organic matter remained constant (Stordalen) or decreased (Storflaket) in the course of freeze-thaw cycles (Fig. 2a, b). The relative proportion of Fe, Al, and Ti in HMW50 kDa – 0.45 µm colloids systematically decreased in the course of freeze-thaw cycles in the Storflaket waters (Fig. 2c–h). At the end of the tenth cycle, this decrease relative to the initial proportion was a factor of 15, 13, and 40 for Fe, Al, and Ti, respectively (Fig. 3 D). At the same time, the HMW50 kDa–0.45 µm fraction of these metals only slightly decreased in concentrations during freeze-thaw cycles in the Stordalen waters (Fig. 3 C). Significant increases in the LMW< 3 kDa fractions of the oxyanions Mo, Cr, and V were observed in circumneutral Storflaket waters (Fig. 3e). Finally, there was remarkable formation of colloidal Si in Stordalen waters as the proportion of Si in the HMW50 kDa–0.45 µm fraction increased from 1.3% at the beginning to 43% after ten cycles and the proportion of Si in the LMW< 3 kDa fraction decreased from 95 to 25% (Fig. 3f).
Thermodynamic modeling: metal-DOM complexation and mineral saturation indices
The vMinteq model yielded metal cation complexation with DOM in both LMW< 3 kDa and dissolved (< 0.45 µm) fractions during the freezing cycles (Fig. S4 of Online Resource 4). The fraction of metal complexed to DOM is defined as the ratio of metal-DOM complexes to total dissolved metal (organic + inorganic forms). With this approach, we calculated the degrees of TE associations with DOM and possible changes in metal speciation during the experiment. In the Stordalen thaw pond (pH 3.6), organic complexes of Na and K accounted for 45% of total dissolved metals, whereas all other elements were fully bound to DOM (98.5 to 100% of organic complexes). Element speciation in Storflaket thaw pond (pH 6.9) showed that organic complexes of Na and K constitute < 10% of dissolved metals. Between 20 and 40% of alkaline earth elements (Ca, Mg, Sr, Ba) and 60–80% of Co, Zn, Cd, and Ni were complexed to DOM. Finally, 95–100% of UO22+, Pb, Cu, Fe(III), Al, La, Ce, and Th was complexed to DOM.
We used the calculated saturation indices of aqueous solutions with respect to possible solid phases to evaluate the potential of the fluids to precipitate insoluble minerals of Fe, Al, and Si during freezing (Table S4 of Online Resource 4). The Stordalen water was strongly undersaturated (SI < − 7) with respect to all possible secondary minerals including amorphous Al(OH)3, ferrihydrite, lepidocrocite, imogolite, and kaolinite regardless of the organic matter size class (< 3 kDa and < 0.45 µm) and the number of freezing cycles. The Storflaket water exhibited a different saturation state as the initial solution (prior to freezing) was oversaturated with respect to Al(OH)3, ferrihydrite, lepidocrocite, imogolite, and kaolinite (SI = 0.43, 1.3, 4.4, 1.0, and 3.6, respectively). The initial LMW< 3 kDa fraction of this sample was undersaturated (SI < − 1) with respect to all possible minerals except kaolinite (SI = 0.86). After freezing, the 0.45-µm fraction of Storflaket water equilibrated with ferrihydrite and kaolinite and was undersaturated with respect to Al(OH)3 and imogolite (SI = − 0.78 and − 1.92, respectively). Further removal of Fe and Al in the course of the experiment led to undersaturation with respect to ferrihydrite and kaolinite (SI = − 0.50 and − 0.52, respectively) after the tenth cycle of freezing/thawing.
Discussion
Chemical nature of colloids
The colloidal phase (3 kDa–0.45 μm) of the acidic thaw pond water at Stordalen and circumneutral thaw pond at Storflaket exhibited consistent organic carbon and TE concentrations (ca. ± 10%) with the values reported for surface waters of the discontinuous permafrost zone (Table S2-1). The elemental composition of colloids, the decrease of organic C, Fe, Al, and TE concentrations during ultrafiltration (Figs. S2-1, S2-2, S2-3 of Online Resource 2), and Fe-normalized TE distribution coefficients between colloids and the LMW fraction (Fig. S2-4) show the similarities between our results for two thaw ponds at Abisko to previously investigated bog waters and adjacent lakes and rivers in other permafrost and non-permafrost regions. The trivalent and tetravalent hydrolysates and Pb2+ are incorporated into Fe(Al) hydroxides of high molecular weight, which are stabilized by OM. In contrast, LMW and colloidal organic complexes are responsible for speciation of alkaline earth and divalent transition metals. As such, we believe that the results of the freezing experiments of thaw pond waters in this study are representative of similar landscape features, which are widespread in high-latitude boreal and Arctic regions.
Production of the low-molecular-weight fraction
We show a sizeable production of low-molecular-weight OC (< 3 kDa) for both acidic and neutral organic-rich waters. This phenomenon has not been reported by earlier studies of frozen and thawed DOM-rich pond water (i.e., Giesy and Briese 1978). In Storflaket, the Corg increase in the LMW< 3 kDa fraction was due to generation of non-aromatic OM in the course of freeze-thaw cycles. It can be further hypothesized that the decrease in SUVA and the increase in LMW OC occurred because of removal of aromatic carbon and the appearance of aliphatic organic material during the freeze-thaw process. The molecular nature of the initial and newly produced OC fraction in permafrost-affected waters is unknown and requires special molecular-level DOM study. Analogous to peat waters in northern Scotland (Batchelli et al. 2009; Krachler et al. 2010, 2012) and organic-rich rivers of the temperate zone (Remucal et al. 2012), the OC fraction may include small humic molecules and oligomeric lignin phenols, aliphatic compounds, as well as small-size fulvic molecules from degradation of plant residues. In the course of freeze-thaw experiments, the generation of the LMW Corg fraction may occur via disintegration of HMW organic colloids. The breakup of large DOM molecules was accompanied by the removal of aromatic carbon from the LMW fraction as SUVA254 nm in LMW< 3 kDa decreased from the beginning to the tenth freeze-thaw cycle by a factor of 1.6 and 2.3 in Stordalen and Storflaket, respectively. Therefore, we conclude that the aromatic carbon was preferentially removed from the LMW fraction and aliphatic compounds were generated in the course of freeze-thaw cycles.
The appearance of LMW organic ligands within the hydrological continuum “depressions → rivers → ponds → lakes” has been reported in the discontinuous permafrost zone (Roehm et al. 2009; Pokrovsky et al. 2011, 2016a; Shirokova et al. 2013). The first process responsible for LMW OC generation is the photodegradation of soil-derived allochthonous DOM in lakes (Lindell et al. 1995; Roiha et al. 2012) and bogs (Oleinikova et al. 2017b) of the high-latitude boreal zone. The second process of LMW ligand generation in the surface waters of permafrost zones is the biodegradation of large organic colloids and HMW aromatic carbon of allochthonous origin by heterotrophic bacterioplankton (Sleighter et al. 2014; Shirokova et al. 2017; Oleinikova et al. 2017a). The present study adds the possibility of a third and previously unknown mechanism of LMW organic carbon and metal complex generation in surface waters—full freezing of shallow organic-rich water bodies. We hypothesize that this process may occur in all bog waters, both neutral and acidic, of the high-latitude boreal and Arctic regions and requires several thaw and freeze phenomena over the course of the year.
Mechanism of Fe, Al, and TE removal during freezing via coagulation of high-molecular-weight colloids
Thermodynamic modeling of the solution saturation state with respect to common secondary minerals of the boreal zone demonstrates the possibility of precipitation of Fe, Al hydroxide, and semi-amorphous aluminosilicates from the Storflaket circumneutral water. The decrease of dissolved (< 0.45 µm) Fe, Al, and Si during freezing-thawing cycles strongly suggests the on-going precipitation of secondary phases. In the acidic Stordalen water, we hypothesize that the increase in HMW colloidal Si (Fig. 3f) is due to formation of amorphous aluminosilicates. The aggregation of Fe hydroxide HMW colloids into particles and removal of dissolved Fe in neutral waters of Storflaket (pH 6.9) are consistent with the fact that the surface charge of Fe(III) hydroxide at these conditions is low (the pH of the zero charge point is close to 7.0, Haq et al. 1991), which can inhibit repulsive forces and favor aggregation (Carlson and Schwertmann 1981). In contrast, because of repulsion between positively charged Fe oxyhydroxide particles and colloids at pH 3.6 (Stumm 1992), the freezing of acidic Stordalen bog waters did not coagulate dissolved Fe.
The main cause of organic and organo-mineral colloid coagulation in thaw lakes and ponds is annual freezing of the whole water column, which leads to the concentration of Fe, organic carbon, and related divalent metals and trivalent and tetravalent hydrolysates in the particulate phase (Manasypov et al. 2015). A mechanism of TE removal during such coagulation is the incorporation of TEs into Fe oxy(hydr)oxides via coprecipitation during full freezing. It is known that two colloidal pools are responsible for TE speciation in boreal aquatic environments, namely, (1) small organic-rich colloids and LMW< 1 kDa DOM and (2) high-molecular-weight Fe-rich colloids (Baalousha et al. 2006; Stolpe et al. 2013; Krachler et al. 2012). The LMW colloids dominate metal speciation in acidic thaw pond water and are highly stable during freeze-thaw. The HMW colloids occur mostly in neutral mire/fen waters of the permafrost zone and are subjected to strong removal during water freezing. If small streams are fed by mire waters that are subjected to full freezing, the observed impact of freezing on the DOC and element concentration may partially explain the high variability of the chemical composition of small streams that drain peat mires. Therefore, we hypothesize that highly variable interannual patterns of DOC, Fe, Al, and TE concentrations in small streams draining mires and peatlands having similar lithologies and environmental settings (Pokrovsky et al. 2015, 2016b; Huser et al. 2012; Tiwari et al. 2016) may be partially due to different regimes of freezing and the numbers of freeze-thaw cycles that affect adjacent bog waters.
Possible transformation of surface water colloidal chemistry and organic carbon under changing climate and increasing freeze-thaw frequency
Across high-latitude boreal and Arctic zones, the frequency of annual soil freeze-thaw cycles is projected to increase significantly in both warm and dry winters by 2050 (Henry, 2008). Soil freezing has long been recognized to exert a strong impact on DOC and nutrients (i.e., Wang and Bettany 1993; Ron Vaz et al. 1994; Haei et al. 2010; Fitzhugh et al. 2001). Results of the present study suggest that the increase in the frequency of freeze/thaw cycles in surface waters will (1) impoverish the neutral (fen-like) waters of DOC, Fe, Al, and a number of low-soluble trace elements and (2) produce low-molecular-weight organic ligands and complexes with metals. LMW ligand production is expected at the beginning and end of winter because of repetitive freezing. In addition to shallow bog waters of the high-latitude boreal zone, a larger territory where the soil temperature fluctuates around zero is the discontinuous permafrost zone of western Siberian peatlands (e.g., Romanovsky et al. 2010). Therefore, interstitial soil solutions of Siberian peatlands may become enriched in LMW organic carbon during annual freezing/thawing cycles.
The production of LMW organic ligands has important consequences for Arctic ecosystems. The LMW fraction of DOM is expected to be more bioavailable and susceptible to microbial transformation than the HMW colloids, consistent with the results of microbial processing of natural colloids from different terrestrial and aquatic sources (cf., Roehm et al. 2009; Berggren et al. 2010; Yang et al. 2016). As a result, the climate warming in high-latitude boreal and Arctic regions may increase the delivery of the potentially bioavailable LMW fraction of carbon and micronutrients such as V, Mo, and Cr in the coastal zone because of simultaneous enhancement of the photo-, bio-, and cryo-transformation of organic and organo-ferric colloids in the inland waters. At the same time, freezing and thawing of acidic peat waters may increase the concentration of HMW colloidal Si and decrease the concentration of bioavailable LMW< 3 kDa Si.
Low-molecular-weight organic ligands increase as water moves from hollows to depressions and from thaw ponds to thermokarst lakes (Pokrovsky et al. 2016a). Indeed, large (terminal) lakes accumulate effluents of small temporary water channels, suprapermafrost waters, hollows, and depressions. These shallow (< 0.5 m) water bodies are subjected to intermittent freezing and thawing at the beginning and end of winter. We argue that the full freezing of water in hollows and depressions of the permafrost zone may generate the LMW fraction of DOC and affect delivery of metal micronutrients to the rivers and larger lakes. In particular, small Fe- and DOC-rich rivers with neutral pH may represent key locations for the preferential removal of Fe, Al, and some TEs by freezing.
Conclusions
This study demonstrated a systematic change in DOC and metal concentrations and colloidal composition in the course of freeze-thaw cycles of pond water from discontinuous permafrost peat mires. The high-molecular-weight colloids (50 kDa–0.45 µm) of organic carbon, Fe, Al, and other metals were preferentially removed from solution during freeze-thaw cycles. The impact of freezing on Fe hydroxide coagulation and TE coprecipitation was much smaller in acidic bog waters than in the circumneutral fen waters. This is consistent with the supersaturation of the latter with respect to Fe and Al oxy(hydr)oxides. The freezing of acidic bog water yielded an increase in HMW colloidal Si at the expense of the LMW< 3 kDa fraction. We show an increase in (1) the concentration of the low-molecular-weight (< 3 kDa) fraction of organic matter in water of thaw ponds and (2) the relative proportion of the LMW< 3 kDa fraction of Fe, Al, and micronutrients (Mo, V, Cr) in fen waters after several cycles of freezing and thawing. As climate changes in high latitudes, we expect an increase in LMW forms of organic matter and metal micronutrients due to simultaneous enhancement of photo-, bio-, and cryo-transformation of colloids in surface waters. In particular, the increase in the frequency of freeze-thaw cycles may increase the delivery of bioavailable carbon and micronutrients from high-latitude boreal inland waters to the Arctic Ocean.
References
Åkerman HJ, Johansson M (2008) Thawing permafrost and thicker active layers in sub-Arctic Sweden. Permafr Periglac Process 19:279–292. https://doi.org/10.1002/ppp.626
Alewell C, Giesler R, Klaminder J, Leifeld J, Rollog M (2011) Stable carbon isotopes as indicators for environmental change in palsa peats. Biogeosciences 8:1769–1778
Allison JD, Perdue EM (1994) Modeling metal–humic interaction with Minteqa2. In: Senesi N, Miano TM (eds) Humic substances in the global environment and implications on human health. Elsevier Science B.V, Amsterdam, pp 927–942
Andersson PS, Dahlqvist R, Ingri J, Gustafsson O (2001) The isotopic composition of Nd in a boreal river: a reflection of selective weathering and colloidal transport. Geochim Cosmochim Acta 65(4):521–527
Andersson K, Dahlqvist R, Turner D, Stolpe B, Larsson T, Ingri J, Andersson P (2006) Colloidal rare earth elements in a boreal river: changing sources and distributions during the spring flood. Geochim Cosmochim Acta 70(13):3261–3274
Arp CD, Jones BM, Urban FE, Grosse G (2011) Hydrogeomorphic processes of thermokarst lakes with grounded-ice and floating-ice regimes on the Arctic coastal plain, Alaska. Hydrol Process 25:2422–2438
Baalousha M, Kammer FVD, Motelica-Heino M, Baborowski M, Hofmeister C, Le Coustumer P (2006) Size-based speciation of natural colloidal particles by flow field flow fractionation, inductively coupled plasma-mass spectroscopy, and transmission electron microscopy/X-ray energy dispersive spectroscopy: colloids-trace element interaction. Environ Sci Technol 40(7):2156–2162
Batchelli S, Muller FLL, Baalousha M, Lead JR (2009) Size fractionation and optical properties of colloids in an organic-rich estuary (Thurso, UK). Mar Chem 113:227–237
Belzile C, Gibson JAE, Vincent WF (2002) Colored dissolved organic matter and dissolved organic carbon exclusion from lake ice: implications for irradiance transmission and carbon cycling. Limnol Oceanogr 47(5):1283–1293
Benedetti M, Milne C, Kinniburgh D, van Riemsdijk W, Koopal L (1995) Metal ion binding to humic substances: application of the non-ideal competitive adsorption model. Environ Sci Technol 29:446–457
Berggren M, Ström L, Laudon H, Karlsson J, Jonsson A, Giesler R, Bergström AK, Jansson M (2010) Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecol Lett 13:870–880. https://doi.org/10.1111/j.1461-0248.2010.01483
Bertilsson S, Stepanauskas R, Cuados-Hansson R, Granéli W, Wikner J, Tranvik L (1999) Photochemically induced changes in bioavailable carbon and nitrogen pools in a boreal watershed. Aquat Microb Ecol 19:47–56
Bosch J, Fritzsche A, Totsche KU, Meckenstock RU (2010) Nanosized ferrihydrite colloids facilitate microbial iron reduction under flow conditions. Geomicrobiol J 27(2):123–129
Brinkmann T, Horsch P, Sartorius D, Frimmel FH (2003) Photoformation of low molecular-weight organic acids from brown water dissolved organic matter. Environ Sci Technol 37:4190–4198
Carlson L, Schwertmann U (1981) Natural ferrihydrites in surface deposits from Finland and their association with silica. Geochim Cosmochim Acta 45:421–429
Chen J, Xue S, Lin Y, Wang C, Wang Q, Han Q (2016) Effect of freezing-thawing on dissolved organic matter in water. Desalin Water Treat 57:17230–17240
Dietzel M (2005) Impact of cyclic freezing on precipitation of silica in Me-SiO2–H2O systems and geochemical implications for cryosoils and sediments. Chem Geol 216(1–2):79–88
Doane TA (2017) A survey of photogeochemistry. Geochem Trans 18:1. https://doi.org/10.1186/s12932-017-0039-y
Fellman JB, D’Amore DV, Hood E (2008) An evaluation of freezing as a preservation technique for analyzing dissolved organic C, N and P in surface water samples. Sci Total Environ 392:305–312
Fitzhugh RD, Driscoll CT, Groffman PM, Tierney GL, Hardy JP (2001) Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56(2):215–238
Fritzsche A, Bosch J, Rennert T, Heister K, Braunschweig J, Meckenstock RU, Totsche KU (2012) Fast microbial reduction of ferrihydrite colloids from a soil effluent. Geochim Cosmochim Acta 77:444–456
Giesy JP, Briese LA (1978) Particulate formation due to freezing humic waters. Water Resour Res 14(3):542–544
Gundersen P, Steinnes E (2003) Influence of pH and TOC concentration on Cu, Zn, Cd, and Al speciation in rivers. Water Res 37:307–318
Guo L, Lehner JK, White DM, Garland DS (2003) Heterogeneity of natural organic matter from the Chena River, Alaska. Water Res 37:1015–1022
Gustafsson J (2011) Visual MINTEQ ver. 3.1. http://vminteq.lwr.kth.se/. Assessed 1 Dec 2015
Gustafsson JP, Bhattacharya P, Bain DC, Fraser AR, McHardy WJ (1995) Podzolisation mechanisms and the synthesis of imogolite in Northern Scandinavia. Geoderma 66:167–184
Haei M, Öquist MG, Buffam I, Agren A, Blomkvist P, Bishop K, Löfvenius MO, Laudon H (2010) Cold winter soils enhance dissolved organic carbon concentrations in soil and stream water. Geophys Res Lett 37:L08501. https://doi.org/10.1029/2010GL042821
Haq I, Parveen S, Mehmood K (1991) Surface properties of iron hydroxide. Phys Chem 10:1–10
Henry HAL (2008) Climate change and soil freezing dynamics: historical trends and projected changes. Clim Change 87:421–434
Huser BJ, Fölster J, Köhler SJ (2012) Lead, zinc, and chromium concentrations in acidic headwater streams in Sweden explained by chemical, climatic, and land-use variations. Biogeosciences 9:4323–4335
Ilina SM, Drozdova OY, Lapitskiy SA, Alekhin YV, Demin VV, Zavgorodnyaya YuA, Shirokova LS, Viers J, Pokrovsky OS (2014) Size fractionation and optical properties of dissolved organic matter in the continuum soil solution-bog-river and terminal lake of a boreal watershed (North Karelia, Russia). Org Geochem 66:14–24
Ilina SM, Lapitsky SA, Alekhin YV, Viers J, Benedetti M, Pokrovsky OS (2016) Speciation, size fractionation and transport of trace element in the continuum soil water–mire-lake-river–large oligotrophic lake of a high-latitude boreal and Arctic watershed. Aquat Geochem 22(1):65–95
Ireson AM, van der Kamp G, Ferguson G, Nachshon U, Wheater HS (2013) Hydrogeological processes in seasonally frozen northern latitudes: understanding, gaps and challenges. Hydrogeol J 21:53–66
Jessen S, Holmslykke HD, Rasmussen K, Richardt N, Holm PE (2014) Hydrology and pore water chemistry in a permafrost wetland, Ilulissat, Greenland. Water Resour Res 50:4760–4774
Johansson M, Callaghan TV, Bosiö J, Åkerman HJ, Jackowicx-Korzynski M, Christensen TR (2013) Rapid responses of permafrost and vegetation to experimentally increased snow cover in sub-Arctic Sweden. Environ Res Lett. https://doi.org/10.1088/1748-9326/8/3/035025
Keung W, Leung S, Carmichael G (1984) Solute redistribution during normal freezing. Water Air Soil Pollut 21:141–150
Kinniburgh DG, van Riemsdijk WH, Koopal LK, Borkovec M, Benedetti MF, Avena MJ (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf A 151:147–166
Klaminder J, Yoo K, Rydberg J, Giesler R (2008) An explorative study of mercury export from a thawing palsa mire. J Geophys Res Biogeosci 113:1–9
Kokelj SV, Burn CR (2005) Geochemistry of the active layer and near-surface permafrost, Mackenzie delta region, Northwest Territories, Canada. Can J Earth Sci 42:37–48
Kokelj SV, Zajdlik B, Thompson MS (2009) The impacts of thawing permafrost on the chemistry of lakes across the subarctic Boreal-Tundra transition, mackenzie Delta Region, Canada. Permafr Periglac Process 20:185–199
Krachler R, Krachler RF, von der Kammer F, Süphandag A, Jirsa F, Ayromlou S, Hofmann T, Keppler BK (2010) Relevance of peat-draining rivers for the riverine input of dissolved iron to the ocean. Sci Tot Environ 408:2402–2408
Krachler R, von der Kammer F, Jirsa F, Süphandag A, Krachler RF, Plessl C, Vogt M, Keppler BK, Hofmann T (2012) Nanoscale lignin particles as sources of dissolved iron to the ocean. Global Biogeochem Cycles 26:GB3024
Laudon H, Stephan K, Buffam I (2004) Seasonal TOC export from seven boreal catchments in northern Sweden. Aquat Sci 66:223–230
Levy JS, Fountain AG, Welch KA, Berry Lyons W (2012) Hypersaline “wet patches” in Taylor Valley, Antarctica. Geophys Res Lett 39:L05402. https://doi.org/10.1029/2012GL050898
Lindell MJ, Granéli HW, Tranvik LJ (1995) Enhanced bacterial growth in response to photochemical transformation of dissolved organic matter. Limnol Oceanogr 40:195–199
Lundin LC, Johnsson H (1994) Ion dynamics of a freezing soil monitored in situ by time domain reflectometry. Water Resour Res 30:3471–3478
Lundström US, Van Breemen N, Bain DC, Van Hees PAW, Giesler R, Gustafsson JP, Ilvesniemi H, Karltun E, Melkerud PA, Olsson M, Riise G, Wahlberg O, Bergelin A, Bishop K, Finlay R, Jongmans AG, Magnussont T, Mannerkoski H, Nordgren A, Nyberg L, Starr M, Strand LT (2000) Advances in understanding the podzolization process resulting from a multidisciplinary study of three coniferous forest soils in the Nordic Countries. Geoderma 84:335–354
Malmer N, Johansson T, Olsrud M, Christensen TR (2005) Vegetation, climate changes and net carbon sequestration in a North-Scandinavian subarctic mire over 30 years. Glob Change Biol 11:1895–1909
Manasypov RM, Pokrovsky OS, Kirpotin SN, Shirokova LS (2014) Thermokarst lake waters across permafrost zones of Western Siberia. Cryosphere 8:1177–1193. https://doi.org/10.5194/tc-8-1177-2014
Manasypov RM, Vorobyev SN, Loiko SV, Kritzkov IV, Shirokova LS, Shevchenko VP, Kirpotin SN, Kulizhsky SP, Kolesnichenko LG, Zemtzov VA, Sinkinov VV, Pokrovsky OS (2015) Seasonal dynamics of organic carbon and metals in thermokarst lakes from the discontinuous permafrost zone of western Siberia. Biogeosciences 12:3009–3028
Milne CJ, Kinniburgh DG, van Riemsdijk WH, Tipping E (2003) Generic NICA-donnan model parameters for metal-ion binding by humic substances. Environ Sci Technol 37:958–971
Moran MA, Zepp RG (1997) Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol Oceanog 42:1307–1316
Oleinikova O, Shirokova LS, Drozdova OY, Gerard E, Lapitskiy SA, Bychkov AY, Pokrovsky OS (2017a) Transformation of organo-ferric colloids by heterotrophic bacterium Pseudomonas saponiphila. Geochim Cosmochim Acta 205:313–330
Oleinikova O, Drozdova OY, Lapitskiy SA, Bychkov AY, Pokrovsky OS (2017b) Dissolved organic matter degradation by sunlight coagulates organo-mineral colloids and produces low-molecular weight fraction of metals in boreal humic waters. Geochem Cosmochim Acta 211:97–114
Oliver B, Thurman E, Malcolm R (1983) The contribution of humic substances to the acidity of colored natural waters. Geochim Cosmochim Acta 47:2031–2035
Pokrovsky OS, Shirokova LS, Kirpotin SN, Audry S, Viers J, Dupré B (2011) Effect of permafrost thawing on organic carbon and trace element colloidal speciation in the thermokarst lakes of western Siberia. Biogeosciences 8:565–583
Pokrovsky OS, Shirokova LS, Kirpotin SN, Kulizhsky SP, Vorobiev SN (2013) Impact of Western Siberia heat wave 2012 on greenhouse gases and trace metal concentration in thaw lakes of discontinuous permafrost zone. Biogeosciences 10:5349–5365
Pokrovsky OS, Manasypov RM, Shirokova LS, Loiko S, Krickov I, Kopysov S, Kolesnichenko LG, Zemtsov VA, Kulizhsky SP, Vorobyev SN, Kirpotin SN (2015) Permafrost coverage, watershed area and season control of dissolved carbon and major elements in western Siberia rivers. Biogeosciences 12:6301–6320
Pokrovsky OS, Manasypov RM, Loiko SV, Shirokova LS (2016a) Organic and organo-mineral colloids of discontinuous permafrost zone. Geochim Cosmochim Acta 188:1–20
Pokrovsky OS, Manasypov RM, Loiko S, Krickov IA, Kopysov SG, Kolesnichenko LG, Vorobyev SN, Kirpotin SN (2016b) Trace elements transport in western Siberia rivers across a permafrost gradient. Biogeosciences 13:1877–1900
Polishchuk YM, Bogdanov AN, Polischuk VY, Manasypov RM, Shirokova LS, Kirpotin SN, Pokrovsky OS (2017) Size-distribution, surface coverage, water, carbon and metal storage of thermokarst lakes (> 0.5 ha) in permafrost zone of the Western Siberia Lowland. Water 9:228. https://doi.org/10.3390/w9030228
Porcal P, Dillon PJ, Molot LA (2015) Temperature dependence of photodegradation of dissolved organic matter to dissolved inorganic carbon and particulate organic carbon. PLoS ONE 10(6):e0128884. https://doi.org/10.1371/journal.pone.0128884
Prokushkin AS, Pokrovsky OS, Shirokova LS, Korets MA, Viers J, Prokushkin SG, Amon R, Guggenberger G, McDowell WH (2011) Sources and export fluxes of dissolved carbon in rivers draining larch-dominated basins of the Central Siberian Plateau. Environ Res Lett 6:045212. https://doi.org/10.1088/1748-9326/6/4/045212
Remucal CK, Cory RM, Sander M, McNeill K (2012) Low molecular weight components in an aquatic humic substance as characterized by membrane dialysis and orbitrap mass spectrometry. Environ Sci Technol 46(17):9350–9359
Roehm CL, Giesler R, Karlsson J (2009) Bioavailability of terrestrial organic carbon to lake bacteria: the case of a degrading high-latitude boreal and Arctic permafrost mire complex. J Geophys Res 114. Art No G03006
Roiha T, Tiirola M, Cazzanelli M, Rautio M (2012) Carbon quantity defines productivity while its quality defines community composition of bacterioplankton in high-latitude boreal and Arctic ponds. Aquat Sci 74:513–525
Romanovsky VE, Drozdov DS, Oberman NG, Malkova GV, Kholodov AL et al (2010) Thermal state of permafrost in Russia. Permafr Periglac Process 21:136–155
Ron Vaz MDR, Edwards AC, Shand CA, Cresser MS (1994) Changes in the chemistry of soil solution and acetic-acid extractable-P following different types of freeze-thaw episodes. Eur J Soil Sci 45(3):353–359
Shiller AM, Duan S, van Erp P, Bianchi TS (2006) Photo-oxidation of dissolved organic matter in river water and its effect on trace element speciation. Limnol Oceanogr 51(4):1716–1728
Shirokova LS, Pokrovsky OS, Kirpotin SN, Desmukh C, Pokrovsky BG, Audry S, Viers J (2013) Biogeochemistry of organic carbon, CO2, CH4, and trace elements in thermokarst water bodies in discontinuous permafrost zones of Western Siberia. Biogeochemistry 113:573–593
Shirokova LS, Bredoire R, Rols JL, Pokrovsky OS (2017) Moss and peat leachate degradability by heterotrophic bacteria: the fate of organic carbon and trace metals. Geomicrobiol J. https://doi.org/10.1080/01490451.2015.1111470
Shur Y, Hinkel KM, Nelson FE (2005) The transient layer: implications for geocryology and climate-change science. Permafr Periglac Process 16:5–17
Sleighter RL, Cory RM, Kaplan LA, Abdulla HAN, Hatcher PG (2014) A coupled geochemical and biogeochemical approach to characterize the bioreactivity of dissolved organic matter from a headwater stream. J Geophys Res Biogeosci 119:1520–1537
Spencer RGM, Bolton L, Baker A (2007) Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations. Water Res 41(13):2941–2950
Stolpe B, Guo L, Shiller AM, Aiken GR (2013) Abundance, size distributions and trace-element binding of organic and iron-rich nanocolloids in Alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim Cosmochim Acta 105:221–239
Stumm W (1992) Chemistry of the solid-water interface. Wiley, New York, p 428
Thompson MS, Giesler R, Kalsson J, Klaminder J (2015) Size and characteristics of the DOC pool in near-surface high-latitude boreal and Arctic mire permafrost as a potential source for nearby freshwaters. Arctic Antarct Alpine Res 47(1):49–58
Tiwari T, Lidman F, Laudon H, Lidberg W, Ågren AM (2016) GIS-based prediction of stream chemistry using landscape composition, wet areas, and hydrological flow pathways. J Geophys Res Biogeosci. https://doi.org/10.1002/2016JG003399
Vasyukova E, Pokrovsky OS, Viers J, Oliva P, Dupré B, Martin F, Candaudap F (2010) Trace elements in organic- and iron-rich surficial fluids of boreal zone: assessing colloidal forms via dialysis and ultrafiltration. Geochim Cosmochim Acta 74:449–468
Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Van Cappellen P (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15(6):785–790
Wada S, Nagasto A (1983) Formation of silica microplates by freezing dilute silicic acid solution. Soil Sci Plant Nutr 29:93–95
Wang FL, Bettany JR (1993) Influence of freeze-thaw and flooding on the loss of soluble organic-carbon and carbon-dioxide from soil. J Environ Qual 22(4):709–714
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Wetzel RG (1995) Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter simple substrates for rapid bacterial metabolism. Limnol Oceanogr 40:1369–1380
Xue S, Wen Y, Hui X, Zhang L, Zhang Z, Wang J, Zhang Y (2015) The migration and transformation of dissolved organic matter during the freezing processes of water. J Environ Sci 27:168–178
Xue S, Wang C, Zhang Z, Song Y, Liu Q (2016) Photodegradation of dissolved organic matter in ice under solar irradiation. Chemosphere 144:816–826
Yang Z, Wullschleger SD, Liang L, Graham DE, Gu B (2016) Effects of warming on the degradation and production of low-molecular-weight labile organic carbon in an Arctic tundra soil. Soil Biol Biochem 95:202–211
Acknowledgements
We are grateful to AE S.D. Sebestyen for his editorial corrections and three anonymous reviewers for their insightful and constructive comments and suggestions. We thank C. Benker for the English editing and acknowledge the support from the Russian Scientific Fund grant no. 15-17-10009 and RFFI (RFBR) grant nos. 17-05-00348_a and 17-05-00342_a and the Swedish Research Council (VR; 2013-5001). We thank E. Lundin and O. Oleinikova for help with sampling and Fe(II) analysis, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stephen D. Sebestyen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pokrovsky, O.S., Karlsson, J. & Giesler, R. Freeze-thaw cycles of Arctic thaw ponds remove colloidal metals and generate low-molecular-weight organic matter. Biogeochemistry 137, 321–336 (2018). https://doi.org/10.1007/s10533-018-0421-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0421-6