Abstract
Forest soil organic carbon (SOC) is one of the largest reservoirs of terrestrial carbon (C) and is a major component of the global C cycle. Yet there is still uncertainty regarding how ecosystems, and the SOC they store, will respond to changes due to anthropogenic processes. Current and future reactive nitrogen (N) deposition to forest soils may alter biogeochemical processes and shift both the quantity and quality of stored SOC. We studied SOC storage and molecular-level composition after 22 years of N additions (100 kg N ha−1 y−1) in a temperate deciduous forest. SOC storage in surface soils increased by 0.93 kg m−2 due to a decline in microbial biomass (phospholipid fatty acids) and litter decomposition. N additions resulted in the selective preservation of a range of plant-derived compounds including steroids, lignin-derived, cutin-derived, and suberin-derived compounds that have anti-microbial properties or are non-preferred microbial substrates. This overall shift in SOC composition suggests limited sustainability and a decline in soil health. The reduction in microbial biomass and increase in specific SOC components demonstrate that long-term N fertilization negatively alters fundamental C cycling in forest soils. This study also demonstrates unequivocally that anthropogenic impacts on C and N cycling in forests at the molecular-level must be considered more holistically.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest soil organic carbon (SOC) is one of the largest terrestrial carbon (C) pools (~ 383 × 1015 g; Pan et al. 2011) that regulates global and regional C, nutrient and pollutant cycling, and controls C feedback to climate change (Schmidt et al. 2011; Lehmann and Kleber 2015). Soil C dynamics are strongly coupled to nitrogen (N) cycling, and the prolific use of fertilizers and fossil fuels has increased reactive N deposition globally (~ 150 × 1012 g y−1) into soils (Lamarque 2005; Galloway et al. 2008) well above pre-industrial levels. Although there has been a reduction in N emissions and deposition in much of Europe and the United States (Waldner et al. 2014; Li et al. 2016), with resulting decreases in wet deposition (Du et al. 2014), large increases in N deposition rates are observed in other regions, particularly in Asia (Vet et al. 2014). Furthermore, increasing deposition of reduced nitrogen forms indicate that atmospheric deposition of nitrogen will continue to result in ecosystem-level inputs that exceed critical loads to forest ecosystems (Pardo et al. 2015).
It is currently unclear how and to what extent long-term N inputs to soils from anthropogenic processes will alter forest SOC storage and C biogeochemical cycling (Aber et al. 1998; Gallo et al. 2005; Zak et al. 2017) and consequently alter ecosystem services. To date, studies have reported varying results, with N additions increasing, decreasing or causing no change in forest SOC storage (Janssens et al. 2010; Lu et al. 2011; Frey et al. 2014; Yue et al. 2016). SOC content and composition is controlled mainly by both plant inputs and microbial processing and degradation of SOC (Schmidt et al. 2011; Lehmann and Kleber 2015). Although N fertilization can stimulate plant growth and increase litterfall (Yue et al. 2016), it can also accelerate or slow down microbial processing of plant residues (Hobbie et al. 2012; Xia et al. 2017), thus altering SOC chemistry. Given the complexity of C cycling processes that influence soil biogeochemistry, and the importance of C chemistry in controlling nutrient availability, providing energy to soil micro- and macro-biota, and in regulating soil C sequestration, there is a critical need to use sensitive chemical characterization methods to understand how long-term N deposition may alter the molecular-level composition of SOC and biogeochemical controls on SOC accrual and turnover (Schmidt et al. 2011; Lehmann and Kleber 2015). Furthermore, responses of SOC to N additions are to date derived mostly from relatively short-term N-fertilization experiments (< 10 years of treatment) or limited types of SOC investigations (Feng et al. 2010; Liu et al. 2016; Pisani et al. 2015; Wu et al. 2015; Zak et al. 2017). Here, we hypothesize that there are indeed compound-specific SOC responses to long-term N fertilization that depend on the initial molecular size, source, and turnover of SOC. To test this hypothesis, we quantified SOC storage and composition with soil depth after 22 years of N fertilization (100 kg N ha−1 y−1 as of NH4NO3) in a temperate deciduous forest in Meadville, Pennsylvania, USA. A range of sensitive and targeted microbial- and plant-derived compounds were analyzed in O, A and B horizons. This included the isolation and quantification of lipids and other small molecules as well as compounds derived from cutin, suberin and lignin as well as microbial-specific phospholipid fatty acids (PLFAs) using gas chromatography-mass spectrometry (GC–MS). In addition, non-targeted solid-state 13C nuclear magnetic resonance (NMR) spectroscopy was used to measure potential shifts in the overall SOC composition.
Materials and methods
Site description and soil sampling
The nitrogen (N) fertilization experiment at the Allegheny College Bousson Environmental Research Reserve in northwestern Pennsylvania (41°36′N, 80°2′W) was started in 1994. This 80-year-old forest is an N-rich site dominated by black cherry (Prunus serotina; 60% of the aboveground biomass) and sugar maple (Acer saccharum; 28% of the aboveground biomass (Bowden et al. 2000; Chan et al. 2005)). Litterfall is 2.1 Mg C ha−1 y−1 and total soil N (to 60 cm) is about 10,000 kg N ha−1. The soils are Alfisols (a silty-loam texture) and are moderately well drained with a bulk density of 0.52 ± 0.01 g cm−3, pH of 4.0 and a cation exchange capacity of 3.73 cmolc kg−1 in the upper 15 cm of the mineral soil. Alfisols cover 13.5% of ice-free land area and contain 8% of soil C globally (Eswaran et al. 1993; Pries et al. 2017). Invasive, non-native earthworms are not observed at this site, either at the surface or in soil pits.
There are three control and three N-fertilized 15 × 15 m plots. Ammonium nitrate (NH4NO3) is added in six aqueous additions using a backpack sprayer during the growing season, from May/June through August/September each year with an annual rate of 100 kg N ha−1 y−1 (Bowden et al. 2000). The application rate was selected to (1) accelerate atmospheric N deposition and examine potential changes in forest responses to long-term N deposition, and (2) be comparable to addition rates in a similar N-addition study at the Harvard Forest Long-Term Ecological Research site (Bowden et al. 1990). When the study was initiated (1994) total inorganic wet N deposition was 6.1 kg N ha−1 y−1 in Kane, PA and 5.3 kg N ha−1 y−1 in Chautauqua, NY (NADP 2018), with about 35% of N being deposited as NH +4 and 65% as NO −3 . Interpolating NADP maps of total wet plus dry deposition indicated that total N deposition at Bousson in 2000 was between 16 and 18 kg N ha−1 y−1. More recently (2017), total wet inorganic deposition in Kane is 4.8 kg N ha−1 y−1 (55% NH +4 , 45% NO −3 ) and total wet plus dry deposition (2016) is between 12 and 14 kg N ha−1 y−1 (NADP 2018). Over the course of the study, sulfate and H+ deposition have also declined dramatically (NADP 2018).
After surface litter removal, soils from O horizon (depth of approximately 1–3 cm), A horizon (depth of approximately 3–8 cm), and top 10 cm of the B horizon were collected from two 20 × 20-cm-area soil blocks within each plot on May 9, 2016. Therefore, six soil blocks (two blocks in each of the three plots) were collected from both control and N-fertilized plots and they were analyzed individually. All samples were sieved (2 mm), lyophilized, ground with a ball mill before chemical analyses. The dried mass of each horizon of each soil block was weighed and recorded. Soil pH (determined in deionized water) after 21 years of N amendment indicated a slight decline in both the O and A horizons (unpublished data). With N addition, soil pH decreased from 4.19 ± 0.31 (control plots) to 3.69 ± 0.13 (N amended plots) in the O horizon and from 3.97 ± 0.31 (control plots) to 3.54 ± 0.08 (N amended plots) in the A horizon.
Total carbon (C) and nitrogen (N) and soil fractionation
The total C and N contents in soils were determined with a Thermo Flash 2000 elemental analyzer. A subset of mineral soil samples (A and B horizons) were fractioned by density using sodium polytungstate following Sollins et al. (2006) and divided into three pools including light (< 1.85 g cm−3), aggregate (1.85–2.4 g cm−3), and heavy (> 2.4 g cm−3) fractions (Hatton et al. 2012).
Biomass production and decomposition
Litterfall was collected monthly from two 1.0 m2 litter collectors on each plot and dried at 105 °C for 48 h. Three 15 × 15-cm-area soil samples from each plot for root mass analysis were collected in autumn of 2010 in the manner described previously (Bowden et al. 2014), with the exception that the B-horizon soils were collected using a gas-powered 9.62-cm diameter diamond-bit stainless steel soil corer (Earthquake, 9800B). Roots (0–1 mm dia.) were removed from each soil sample by hand, and dried at 105 °C for 48 h. Aboveground tree growth was estimated by measuring tree diameters at 1.35 m, and applying species-specific biomass equations (Tritton and Hornbeck 1982) for each of the trees on the plots.
Leaf litter samples for decomposition estimates were collected from 9 m2 catchment screens placed in the middle of each nitrogen addition plot and on the outside edge of each control plot and were homogenized by treatment. Leaf pack composition for mixed litter bags was determined through an autumnal collection in 2014 of litter caught in two 1.0 m2 screens per plot, dried for 48 h at 105°C, and sorted to species. Litter bags (20 × 30 cm for leaves, 0.18 mm2 mesh no-see-μm netting) contained the air-dried equivalent of 2.0 g of 105 °C-dried leaf litter. Four bags for each of black cherry (Prunus serotina), sugar maple (Acer saccharum), or mixed leaf litter (53.8% sugar maple, 29.5% black cherry, 16.7% beech (Fagus grandifolia)) were deployed per plot in each treatment, in mid-October 2015, the time of maximum autumnal litterfall. Litter bags were placed directly atop the O horizon and were buried under the leaves. Bags were collected in October of the following year; litter was oven-dried for 48 h at 105°C and weighted.
Targeted organic matter compound analysis by gas chromatography–mass spectrometry (GC–MS)
Specific organic matter compounds with various microbial- and plant-derived sources were isolated and quantified using GC–MS. PLFAs were extracted by a modified Bligh-Dyer method and analyzed by GC–MS (Frostegård and Bååth 1996). Briefly, 0.5–2.5 g soil samples (depending on the C content) were extracted using 16 ml of methanol, 8 ml of chloroform, and 6 ml of 0.15 M sodium citrate buffer (acidified to pH 4). The lipid extract in CHCl3 phase was collected after centrifugation and separation. The lipid extract was then fractionated into neutral, glycol, and polar lipids with 10 ml of CHCl3, 20 ml of acetone, and 10 ml of CH3OH, respectively by silica column chromatography. The PLFAs in the polar fraction were concentrated, dried by N2 gas, and derivatized into methyl esters. The methyl esters were extracted with 5 ml of hexane and chloroform mixture (4:1, v/v) three times, dried under N2 gas in a 2 ml glass vial. Extracts were derivatized prior to analysis by GC–MS (Feng and Simpson 2009).
Acyclic lipids, plant-derived steroids, sugars, as well as the cutin-, suberin-, and lignin-derived compounds were sequentially extracted by dichloromethane/methanol extraction, base hydrolysis and CuO oxidation, respectively and analyzed by GC–MS using established methods (Otto and Simpson 2007; Otto et al. 2005). First, 0.1–1.0 g of soil sample (depending on the C content) was sonicated with 30 ml dichloromethane, dichloromethane and methanol mixture (1:1, v:v), and methanol (each for 15 min). The combined solvent extracts were then filtered through Whatman GF/A and GF/F glass filters, concentrated by rotary evaporation, and dried by N2 gas in a 2 ml glass vial. Second, the solvent-extraction residues were air dried and base hydrolyzed with 20 ml of 1 M methanolic KOH at 100 °C for 3 h. The extracts were sonicated twice with 15 ml dichloromethane:methanol (1:1, v:v), centrifuged and acidified to pH 1. The combined supernatants were rotary evaporated, and the hydrolysable lipids were liquid–liquid extracted three times with 30 ml diethyl ether. The extracts were dried with Na2SO4, concentrated by rotary evaporation, and dried by N2 gas in a 2 ml glass vial. Finally, the base hydrolyzed residues were air dried and oxidized with 1 g CuO, 100 mg ammonium iron (II) sulfate hexahydrate [Fe(NH4)2(SO4)2·6H2O] and 15 ml of 2 M NaOH in Teflon lined bombs at 170 °C for 2.5 h. The extracts were acidified to pH 1 and kept for 1 h at room temperature in the dark to prevent the polymerization of cinnamic acids. Then, the supernatants were solid-phase extracted using HLB cartridges and dried with anhydrous Na2SO4. The extracts were concentrated by rotary evaporation and dried by N2 gas in a 2 ml glass vial and then derivatized prior to GC–MS analysis (Otto and Simpson 2006, 2007).
All compounds were analyzed by GC–MS using an Agilent 7890B gas chromatograph equipped with a 5977B mass spectrometer with electron impact (70 eV) ionization. Samples (1 μl) were injected at an inlet temperature of 280°C onto a HP-5MS fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness). The oven temperature program was: 65°C for 2 min, followed by an increase of 6°C min−1 to 300°C, followed by a 20 min isothermal hold. The carrier gas (helium) flow rate was 1 ml min−1. Data acquisition was performed using Agilent Mass Hunter GC–MS Acquisition (version B.07.03.2129) and data were processed using Agilent Enhanced ChemStation software (version E.02.02.1431). External standards were used for quantification: tetracosane (analytical standard grade, Sigma-Aldrich), 1-docosanol (approximately 98%, Sigma-Aldrich), methyl tricosanoate (analytical standard grade, Fluka Analytics) and ergosterol (> 75%, Sigma-Aldrich) for solvent extracts; methyl tricosanoate for base hydrolysis products; and syringic acid (minimum 98%, Sigma-Aldrich) and syringaldehyde (98%, Sigma-Aldrich) for copper oxidation products. Compounds were identified using Wiley Registry (9th edition) plus NIST (2008) mass spectral databases, mass spectral library of soil compounds, and published mass spectra. Mean and standard errors of six replicates were calculated for each soil horizon of either the control or N-fertilized plots.
Solid-state 13C nuclear magnetic resonance (NMR) analysis
Solid-state 13C nuclear magnetic resonance (NMR) analysis was performed to assess changes in C chemistry. The mineral soil samples from A and B horizons were repeatedly extracted with hydrofluoric acid (0.3 M), rinsed with deionized water, and lyophilized to concentrate the organic matter for better detection by 13C NMR (Schmidt et al. 1997). The soils from O horizon and HF-washed soils from A and B horizons were characterized by solid-state 13C cross polarization magic angle spinning (CPMAS) NMR. Solid-state 13C NMR spectra were collected using a 500 MHz Bruker BioSpin Avance III spectrometer equipped with a 4 mm H–X MAS probe, using a ramp-cross-polarization pulse program with a spinning rate of 11 kHz, a contact time of 1 ms, and a recycle delay of 1 s (Conte et al. 2004). Four regions of the 13C NMR spectra were defined and calculated for their relative abundance (Baldock et al. 1992): (1) alkyl C (0–50 ppm; from cutin, suberin, lignin, lipids, and amino acid side chains), O-alkyl C (50–110 ppm; from carbohydrates, peptides, and methoxyl C in lignin), aromatic + phenolic C (110–165 ppm; from lignin and amino acids found in peptides), and carboxyl + carbonyl C (165–210 ppm; from fatty acids and peptides).
Statistical analyses
One and two-way analyses of variance (ANOVA) with Tukey’s posthoc comparison were used to examine the main and interactive effects of N fertilization and soil horizon on the soil chemical properties (i.e., C and N contents and concentrations and/or ratios of SOC components) and effects of N on litter decomposition. Root litter mass change after 1 year was assessed with a paired t test. Because the N fertilization often interacted with the soil horizon to affect the soil chemical properties, analyses of simple main effects for N fertilization in each soil horizon were also conducted. The main and simple main effects of N fertilization were considered as significant when P < 0.05. Two-tailed Pearson correlations were conducted to explore the relationships among soil chemical properties. Detailed data and statistical results are available in Tables S4–S6.
Results
Total carbon (C) and nitrogen (N) and soil density fractions
With long-term N-fertilization, total soil C content increased significantly in both the O and A horizons respectively (Table 1). Total N content did not significantly differ in all soil horizons (Table 1). As such, the N-additions only impacted total soil C content but not the N content in this forest. In the O horizon, total soil C content increased from 22.2 to 30.2% with N-addition relative to the control plots. In the A horizon, soil C content increased from 6.77% (control) to 10.06% with N-fertilization. Soil C content did not change significantly in the B horizon (Table 1). Soil C storage in each horizon was calculated from the product of soil C content (with units converted from % to kg-C kg-soil−1) and the soil mass of a horizon (in kg-soil m−2). Overall soil C storage in the O, A, and B horizons increased 17%, from 5.32 ± 0.71 kg m−2 in the control plots to 6.25 ± 0.34 kg m−2 in the N-fertilized plots (P = 0.27; Fig. 1). Soil C storage (in kg-C m−2) increases were horizon-specific as most of the accrual occurred in the O horizon (an increase of 90% compared to the control; P = 0.001), followed by the A (+11%; P = 0.64) and B (+6%; P = 0.66) horizons (Fig. 1; Table 2).
Both A and B horizons were separated into density fractions to determine any changes in C distribution amongst different pools with long-term N-addition. The light fraction increased significantly in the A horizon and contained more than twice as much C compared to the control (Fig. 2). This shift in C distribution indicates that more plant-derived C accumulated in the light fraction with long-term N-enrichment. The distribution in soil C did not change significantly amongst the other density fractions in either the A or B horizon (Fig. 2) demonstrating that long-term N-fertilization impacted the processing of plant-derived inputs.
Biomass production and decomposition
Litterfall and root biomass were monitored to determine changes in plant-derived inputs with N-enrichment (Fig. 3). Interestingly, no significant differences were observed in litterfall flux, aboveground production, and fine root mass in the O and A horizon (Fig. 3). Fine root mass was observed to increase in the B horizon (Fig. 3) indicating the potential for increased root-derived inputs deeper within the soil profile. The mass remaining after 1 year of litter decomposition for black cherry leaf litter, sugar maple leaf litter, and mixed leaf litter was 69.7% ± 1.8%, 84.6% ± 0.9%, and 72.0% ± 1.1%, respectively. With N addition, the corresponding values increased to 74.0% ± 1.3%, 89.3% ± 0.7%, and 79.7% ± 1.0%, respectively. Clearly, litter decomposition was reduced significantly with N-fertilization (Fig. 4). This reduction in decomposition did not vary with litter type (Fig. 4).
Litterfall, aboveground production, and standing fine root (0–1 mm diameter) mass in control and N-fertilized plots (mean ± standard error; n = 3 plots per treatment; litterfall flux in each plot is the average of data from two 1 m2 collectors; fine root mass in each plot is the average of data from three 15 × 15-cm-area soil blocks). Significant difference between treatment and control at P < 0.05 is indicated with an asterisk
Percent mass remaining of leaf litter samples after 1 year of in situ litter decomposition at control and N-fertilized plots (mean ± standard error; n = 12). Mixed leaf litter included 53.8% sugar maple (Acer saccharum Marshall), 29.5% black cherry (Prunus serotina Ehrh.), and 16.7% American beech (Fagus grandifolia Ehrh.) leaf litter. Significant differences between treatment and control at P < 0.05 are indicated with asterisks
Targeted organic matter compound analysis by gas chromatography–mass spectrometry (GC–MS)
Total PLFA concentrations (Fig. 5) significantly decreased in both O and A horizons indicating a reduction in microbial biomass with N-fertilization. The concentration of PLFAs also declined in the B horizon but not significantly (Fig. 5). With N-enrichment, the distribution of gram-negative, gram-positive and fungal PLFAs was also altered and all three microbial classes declined markedly (Fig. 5). When bacteria are faced with substrate constraints or other stresses such as drought, they produce more of the cyclopropane PLFAs as compared to the monounsaturated versions (Bossio and Scow 1998; Feng and Simpson 2009). A ratio of these PLFAs (cy17:0/16:1ω7c and cy19:0/18:1ω7c) can be compared to ascertain the degree of stress exhibited by microbes. These ratios increased significantly with N-fertilization (Fig. 5) suggesting that microbial stress was enhanced with long-term N-addition.
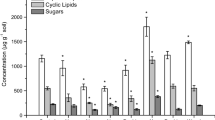
Several targeted organic matter compounds shifted with long-term N-enrichment (Fig. 6; Table 2). Plant-derived steroids, lignin-derived, cutin-derived compounds, acyclic lipids, suberin-derived compounds and simple sugars all exhibited changes in their concentration and storage but to varying extents and depending on soil depth. Plant-derived steroids, which include (ω-sitosterol, stigmasterol, sitosterone, stigmasta-3,5-dien-7-one, and campesterol, increased markedly (+132%) and more so than other plant-derived compounds (Fig. 6; Table 2). Significant increases were also observed for lignin-, cutin-, and suberin-derived compounds in the O horizon. The storage of specific organic matter compounds was also altered in the A and B horizons but not significantly.
Changes in the storage of plant-derived soil organic carbon components (in g m−2 soil) in control and N-fertilized plots (mean ± standard error; n = 6). Percentages in figures are the percent change compared to the control. Significant differences between treatment and control at P < 0.05 are indicated with asterisks. Plant-derived biopolymer compounds are underlined. Detailed quantification of specific organic matter compounds is provided in the Supplementary Materials
Solid-state 13C nuclear magnetic resonance (NMR) analysis
Solid-state 13C NMR spectroscopy provides an overview of soil organic matter composition but lacks the sensitivity of targeted compound analysis (Simpson and Simpson 2012). The distribution of various organic matter classes is listed in Table 1. In each soil horizon, only small or no difference was observed in each of the compound class groups. The most dominant group was the O-alkyl region (50–110 ppm) which contains resonances mostly from carbohydrates and peptides but may have contributions from lignin (methoxyl group; Preston et al. 1997). Alkyl-C did not vary with N-fertilization, likely reflecting small changes in plant-derived waxes and other lipids. Similarly, the aromatic and phenolic C and carboxyl and carbonyl C regions did not exhibit major differences with N-enrichment. The alkyl/O-alkyl C ratio is used to assess the relative degradation stage of organic matter because as oxidative degradation increases, the resonances from more easily degraded substrates will result in a decrease in the O-alkyl signal intensity. These ratios (Table 1) for the A and B horizon were similar and did not provide evidence for any differences in the relative degradation state of organic matter in these horizons. The O horizon reflected a small difference in the alkyl/O-alkyl ratio but this is likely due to the difference in O-alkyl C in this horizon. It is important to note that the error associated with this type of NMR analysis for relative comparisons of different organic matter classes is ~ 5% (Dria et al. 2002) and indicates that solid-state 13C NMR lacks the ability to detect small changes in organic matter composition.
Discussion
Impacts on soil organic carbon (SOC) storage and microbial biomass
We observed significant increases in SOC storage in the entire soil profile (O, A and B horizons) after 22 years of N fertilization. Increases in soil C sequestration have been linked to increased litterfall inputs at the soil surface, because N fertilization may increase plant productivity (Aber et al. 1998; Frey et al. 2014). However, litterfall (2010–2016) in the Control plots was 492 ± 42 g m−2 y−1, compared to 478 ± 35 g m−2 y−1 in the N-fertilized plots (Fig. 3) indicating that aboveground inputs were not enhanced by N fertilization. Additionally, there was no increase in growth rates of aboveground biomass (2005–2009) between the control plots (7.9 ± 1.0 Mg ha−1 y−1) and the fertilized plots (7.3 ± 3.7 Mg ha−1 y−1; Fig. 3). Interestingly, our study at this same forest that experimentally doubled the annual aboveground litterfall inputs for 20 years did not observe any net gain in soil C storage, because additional plant inputs were decomposed by soil microbiota (Wang et al. 2017). This companion study reported a small decrease in SOC with doubling of aboveground inputs and this was attributed to enhanced decomposition, likely due to soil priming, of these additional inputs (Wang et al. 2017).
We also did not find significant differences in standing root biomass between control and N-fertilized plots at O and A horizons, although we do not know how root productivity or turnover compared between the treatments (Fig. 3). Therefore, aboveground or belowground plant inputs alone are unlikely to have contributed to the large gains in SOC storage in the O and A horizons. Because SOC in the O horizon is mainly from partially decomposed plant residues, the +90% SOC accrual is most likely caused by a reduction in SOC processing. To examine this further, soil density fractions were isolated because the light fraction is typically an indicator of plant-derived compounds that are not as degraded as SOC found in the mineral fractions (Sollins et al. 2009). In the A horizon, there was a higher mass of light fraction (density < 1.85 g m−3; mainly derived from plant residues) in N-fertilized plots (43.52 ± 3.34 mg C/g soil) than the control plots (19.58 ± 11.52 mg C/g soil; Fig. 2), suggesting that the plant-derived inputs were less decomposed. This is consistent with observations reported in other studies where N amendments decreased SOC degradation (Frey et al. 2014; Li et al. 2017).
The combined observations of increased SOC storage, no significant changes in litterfall, and an increase in the light fraction with N fertilization (Figs. 1, 2 and 3) collectively suggest a decline in microbial degradation of soil organic matter. Several studies have demonstrated that N addition to soil can reduce microbial biomass and alter microbial diversity (Feng et al. 2010; Frey et al. 2014; Morrison et al. 2016; Pisani et al. 2015; Ramirez et al. 2012). We therefore measured microbial PLFAs, which are excellent indicators of microbial biomass and microbial community structure (Bossio and Scow 1998; Feng and Simpson 2009; Frostegård and Bååth 1996; Zelles et al. 1995). With N fertilization, PLFA concentrations declined markedly in the O and A horizons (Fig. 5). The decline in PLFA concentrations indicates a reduction in microbial biomass with N fertilization that likely resulted in increased SOC storage. When facing substrate constraints, drought or changes in temperature, bacteria increase production of cyclopropane PLFAs and decrease production of monounsaturated PLFAs (Bossio and Scow 1998; Feng and Simpson 2009). We observed that ratios of cyclopropane PLFAs to their monounsaturated precursors (cy17:0/16:1ω7c and cy19:0/18:1ω7c) and the ratio of total saturated to total monounsaturated PLFAs increased for all soil horizons (all P < 0.01; Fig. 4 and Table S1) indicating that in addition to a decline in total microbial biomass, microbes were under stress with long-term N fertilization. Short-chain (< C20) acyclic lipids (including n-alkanes, n-alkanols, and n-alkanoic acids) which are predominantly microbially-derived (Otto et al. 2005), also decreased in O and A horizons (Table S1). Consequently, our data indicate that both biomass and subsequent microbially-derived inputs declined with long-term N fertilization. Furthermore, the observed decrease in PLFA concentrations in O and A horizons in our study is greater than decreases reported from most short-term fertilization studies (Liu et al. 2016), supporting the hypothesis that bacterial and fungal biomass continue to decline with long-term N fertilization (Treseder 2008). A shift in microbial community composition was also observed with N-fertilization (Fig. 5 and Table S1) in conjunction with decreases in bacteria (both gram-negative and gram-positive), fungi, and actinomycetes. Other studies have also reported altered microbial community composition with N fertilization (Allison et al. 2008; Bhatnagar et al. 2018). Although the localized physiological mechanisms are not well understood (Treseder 2008), our results clearly demonstrate marked alterations to the microbial community composition and reduced active microbial biomass in O and A horizons with long-term N fertilization (Frey et al. 2014). These upper layers of soil are where microbial communities thrive and are most active in degradation of plant-derived inputs. Although plant litter inputs did not increase in surface soils with N fertilization, the 1-year leaf litter decomposition, both in single and mixed assemblages, was significantly reduced by N fertilization (P < 0.001; Fig. 4). Therefore, multiple observations collectively support that the reduction in microbial biomass suppressed the microbial processing of SOC which resulted in higher SOC storage with N fertilization over time.
Selective preservation of specific soil organic carbon (SOC) components
The suppression of SOC microbial processing also resulted in a large shift in SOC composition and the concentration of specific SOC components (Fig. 6). Plant-derived steroids (β-sitosterol, stigmasterol, sitosterone, stigmasta-3,5-dien-7-one, and campesterol) increased more than all other studied plant-derived compounds (Fig. 6). Storage of plant-derived steroids increased by 4.57 g m−2, with increases of +281%, +103%, and +50% in O, A, and B horizons, respectively (Fig. 6). Summed across the three horizons, the three measured steroids increased by 132% (P = 0.02). The more complex plant biopolymers (e.g., lignin and acid insoluble fraction of plant residues) have been observed to be retained preferentially compared to small extractable molecules during litter decomposition with N addition (Xia et al. 2017). However, we found that plant-derived steroids, although small molecules, have the greatest percentage increase in storage and contribute significantly to the SOC accrual. This suggests that the component-specific SOC response to long-term N fertilization is not necessarily a result of the molecular size of the SOC components or the hypothesized chemical recalcitrance of plant-derived compounds. More importantly, these plant-derived steroids represent the largest group of plant secondary compounds and may have antibacterial and antifungal properties (Smania et al. 2003; Adamczyk et al. 2015). Their concentrations correlated significantly with the SOC content and with indicators of microbial composition (e.g., fungal/bacterial PLFA ratio) and microbial stress indicators (e.g., cy17:0/16:1ω7c; two-tailed Pearson’s, all P < 0.01; Figs. S2 and S3). Taken collectively, our results suggest a new hypothesis wherein accumulation of plant-derived steroids in N-fertilized plots may have suppressed microbial biomass, thus decreasing organic matter decomposition, and subsequently increasing soil C sequestration.
Lignin is the second largest form of plant-derived C after cellulose and a major contributor of aromatic and phenolic components in soils (Kögel-Knabner 2002; Bianchi 2011). Lignin-derived phenols (including ether-bound vanillyl, syringyl, and cinnamyl phenolics) also increased significantly (+94% in total; P = 0.01; Fig. 6) with N fertilization. As with the plant-derived steroids, accrual of lignin-derived phenols occurred in all soil horizons and was most significant in the O horizon (Fig. 6). The lignin oxidation indicators, i.e., lignin-derived acid to aldehyde ratios of vanillyls and syringyl (Hedges et al. 1988), did not significantly increase with N fertilization in any horizon (Table S1). This observation, together with the overall reduced microbial biomass, demonstrates that lignin oxidation was suppressed with N fertilization. In addition, the slightly increased aromatic and phenolic C in the NMR spectra (Table 1), mainly originating from lignin and amino acids of peptides (Baldock et al. 1992; Preston et al. 1997), further confirms the increased preservation of lignin in all soil horizons. The concentration of lignin-derived phenols in previously studied N-fertilized soils has shown a range of responses, including increases (Frey et al. 2014), decreases (Pisani et al. 2015), and significant alterations (Feng et al. 2010; Theuerl et al. 2010; Wu et al. 2015), possibly due to site-specific alteration in microbial community. A recent meta-analysis suggested that short-term increases of lignin-derived phenols to N fertilization seem to diminish with longer-term N fertilization (Liu et al. 2016), and no significant increase in lignin phenols was found in longer-term (14.5 and 16 years) N-fertilized forests (Theuerl et al. 2010; Thomas et al. 2012). Our results from long-term N fertilization strongly support SOC accrual with significant contributions from lignin. As with the concentrations of plant-derived steroids, the concentrations of lignin-derived phenols correlated significantly with the SOC content and microbial indicators (two-tailed Pearson’s, all P < 0.1; Fig. S2) further suggesting that lignin preservation is directly connected to our other observations.
The cutin- and suberin-derived compounds are valuable indicators for understanding the relative contributions of aboveground and belowground plant inputs to soil (Angst et al. 2016; Crow et al. 2009; Otto and Simpson 2006). The storage and accumulation of cutin-derived compounds increased (+59%) more than suberin-derived compounds (+15%; Fig. 6). This resulted in an increase in the cutin to suberin ratio in N-fertilized soils (PO-horizon = 0.06; Table S1). Hence, N fertilization increased contributions from aboveground plant C more than contributions from belowground plant C. Thus, the compositional shift in SOC retains more of a signature characteristic of aboveground plant inputs over belowground inputs in N-rich forests (Wu et al. 2015; Peng et al. 2017). Similarly, the long-chain acyclic lipids (≥C20; including n-alkanes, n-alkanols, and n-alkanoic acids; mainly derived from aboveground plant inputs in this forest (Wang et al. 2017) also showed higher accrual compared to the suberin-derived compounds (Fig. 6). This observation further supports the shift of SOC composition to higher contributions from aboveground inputs. In addition, the alkyl C in the NMR spectra, mainly originating from plant cutin, suberin, waxes, and microbe-derived lipids in the soil, was slightly higher in N-fertilized plots than the controls for O horizon but was similar between control and N-fertilized plots for A and B horizons (Table 1). This observation also supports the finding of increased preservation of cutin, suberin, and long-chain lipids in the O horizon. The increased plant cutin, suberin, and long-chain lipids in the A horizon were not captured by the alkyl C in NMR spectra likely because 13C NMR spectra do not detect subtle changes to SOC whereas targeted biomarker analyses are more sensitive for measuring SOC composition (Simpson and Simpson 2012).
Polymeric carbohydrates, such as cellulose, are the most abundant form of plant inputs (Kögel-Knabner 2002; Bianchi 2011). 13C NMR analysis showed that the O-alkyl C (mainly derived from cellulose) decreased with N fertilization for all horizons (Table 1). The decreased cellulose-derived O-alkyl C may be explained by the N-stimulated cellulase enzyme activity and selectively accelerated cellulose degradation (Hobbie et al. 2012; Thomas et al. 2012). Similarly, the simple carbohydrates (including mannose, glucose, sucrose, and trehalose) did not increase significantly with N fertilization (Fig. 6). This is likely due to accelerated sugar degradation with N fertilization and is consistent with the observed O-alkyl C degradation. However, different from the O-alkyl C, the sugars were slightly lower in the A horizon and slightly higher in B horizon. In the A horizon, these changes coincide with the highest degree of stress in bacteria indicated by the PLFA stress ratios (Fig. 5) as well as a significant decrease in PLFA and microbial short-chain lipids storage (Table 2). Thus, the lack of favorable microbial substrates, such as cellulose and related carbohydrates, in the A horizon may also be responsible for the large microbial suppression in this horizon (Billings and Ziegler 2008; Watzinger 2015; Müller et al. 2017). The small increase in sugar storage in the B horizon with N fertilization may also be due to leaching of these compounds from the O and A soil horizons. Overall, carbohydrates, either in polymeric or simple form, displayed the least accrual with N fertilization in all studied plant-derived SOC components.
Implications for biogeochemical processes and ecosystem services
Here, we demonstrate that the 22 years of N fertilization reduced the microbial processing and altered the biogeochemistry of several specific SOC components. The component-specific SOC accrual did not directly link to the molecular size but appeared to associate with SOC composition or antimicrobial properties and sources. We found higher contributions of relatively stable or antimicrobial plant-derived compounds (e.g. steroids, lignin, cutin, long-chain lipids, and suberin) over microbial-derived compounds (e.g., PLFAs and short-chain lipids) and relatively labile plant-derived compounds (e.g., carbohydrates). A recent study reported that N fertilization increased soil organic matter accumulation without altering its biochemical composition (Zak et al. 2017). Although we used more detailed molecular techniques to investigate the composition of SOC with long-term N fertilization, the disparity in reports also highlights the varying responses of different forest ecosystems. For example, Gallardo and Schlesinger (1994) reported increases in microbial biomass with short-term N fertilization but also identified the importance of other limiting nutrients, such as phosphorus. Additionally, variations in accrual across SOC components may partially explain why SOC from different forests, which are expected to vary in biochemical composition due to differences in tree species composition, forest age and disturbance history, soil characteristics, and climate, display inconsistent total SOC responses to N fertilization (Janssens et al. 2010; Lu et al. 2011; Frey et al. 2014; Yue et al. 2016).
Anthropogenically-accelerated global N deposition in forests will certainly continue in the coming decades (Lamarque 2005), resulting in a suppression of microbial biomass and shifts in microbial community composition that will subsequently hamper microbial processing of SOC and slow down C cycling. Increases in forest soil C might be perceived as a benefit, given the large amount of C stored in forest soils, and that small percentage changes in storage rates will translate to meaningful global C budgetary contributions. However, concomitant with SOC storage, reduced decomposition will alter additional variables. For example, nutrient release from soil organic matter will decrease, which will decrease soil fertility thus altering ecosystem productivity and forest biomass C sequestration. Overall, long-term N deposition shifts the fundamental biogeochemical cycling via not only alteration in SOC quantity but also its molecular composition. Alteration of SOC quality may influence additional biogeochemical processes. For example, accumulation of the antimicrobial and more difficult to degrade SOC components may alter soil microbiota and their ability to process SOC. Furthermore, altered accrual of specific SOC components will make the response of forests to continued N deposition more difficult to predict given that these components respond uniquely and precisely to changes in climate (e.g., temperature and precipitation (Pisani et al. 2015; Greaver et al. 2016)) and may react differently with inorganic soil components (e.g., minerals and nutrients). This work highlights critical linkages between C and N cycles and exemplifies that N deposition can alter the rates of organic matter decomposition, thus influencing a host of important ecological processes that control forest productivity. Additionally, we demonstrate that quantifying global C budgets needs to consider dynamic environmental changes that will alter rates of C processing and storage over time, and that concerted efforts to manage soil C, such as through N fertilization, need to be evaluated more holistically.
References
Aber J, McDowell W, Nadelhoffer K et al (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48:921–934
Adamczyk S, Adamczyk B, Kitunen V, Smolander A (2015) Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil Biol Biochem 87:59–66
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14:1156–1168
Angst G, Heinrich L, Kögel-Knabner I, Mueller CW (2016) The fate of cutin and suberin of decaying leaves, needles and roots—inferences from the initial decomposition of bound fatty acids. Org Geochem 95:81–92
Baldock JA, Oades JM, Waters AG et al (1992) Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy. Biogeochemistry 16:1–42
Bhatnagar JM, Peay KG, Treseder KK (2018) Litter chemistry influences decomposition through activity of specific microbial functional guilds. Ecol Monogr 88:429–444
Bianchi TS (2011) The role of terrestrially derived organic carbon in the coastal ocean: a changing paradigm and the priming effect. Proc Natl Acad Sci 108:19473–19481
Billings SA, Ziegler SE (2008) Altered patterns of soil carbon substrate usage and heterotrophic respiration in a pine forest with elevated CO2 and N fertilization. Glob Chang Biol 14:1025–1036
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bowden RD, Steudler PA, Melillo JM, Aber JD (1990) Annual nitrous oxide fluxes from temperate forest soils in the northeastern United States. J Geophys Res 95:13997–14005
Bowden RD, Rullo G, Stevens GR, Steudler PA (2000) Soil fluxes of carbon dioxide, nitrous oxide, and methane at a productive temperate deciduous forest. J Environ Qual 29:268–276
Bowden RD, Deem L, Plante AF et al (2014) Litter input controls on soil carbon in a temperate deciduous forest. Soil Sci Soc Am J 78:S66–S75
Chan ASK, Steudler PA, Bowden RD et al (2005) Consequences of nitrogen fertilization on soil methane consumption in a productive temperate deciduous forest. Biol Fertil Soils 41:182–189
Conte P, Spaccini R, Piccolo A (2004) State of the art of CPMAS 13C-NMR spectroscopy applied to natural organic matter. Prog Nucl Magn Reson Spectrosc 44:215–223
Crow SE, Lajtha K, Filley TR et al (2009) Sources of plant-derived carbon and stability of organic matter in soil: implications for global change. Glob Chang Biol 15:2003–2019
Dria KJ, Sachleben JR, Hatcher PG (2002) Solid-state carbon-13 nuclear magnetic resonance of humic acids at high magnetic field strengths. J Environ Qual 31:393–401
Du E, deVries W, Galloway JN et al (2014) Changes in wet nitrogen deposition in the United States between 1985 and 2012. Environ Res Lett 9:095004
Eswaran H, Vandenberg E, Reich P (1993) Organic-carbon in soils of the world. Soil Sci Soc Am J 57:192–194
Feng X, Simpson MJ (2009) Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol Biochem 41:804–812
Feng X, Simpson AJ, Schlesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob Chang Biol 16:2104–2116
Frey SD, Ollinger S, Nadelhoffer K et al (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26:1409–1415
Gallo ME, Lauber CL, Cabaniss SE et al (2005) Soil organic matter and litter chemistry response to experimental N deposition in northern temperate deciduous forest ecosystems. Glob Chang Biol 11:1514–1521
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Greaver TL, Clark CM, Compton JE et al (2016) Key ecological responses to nitrogen are altered by climate change. Nat Clim Chang 6:836–843
Hatton PJ, Kleber M, Zeller B et al (2012) Transfer of litter-derived N to soil mineral-organic associations: evidence from decadal 15N tracer experiments. Org Geochem 42:1489–1501
Hedges JI, Blanchette RA, Weliky K, Devol AH (1988) Effects of fungal degradation on the CuO oxidation products of lignin: a controlled laboratory study. Geochim Cosmochim Acta 52:2717–2726
Hobbie SE, Eddy WC, Buyarski CR et al (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162
Lamarque J-F (2005) Assessing future nitrogen deposition and carbon cycle feedback using a multimodel approach: analysis of nitrogen deposition. J Geophys Res 110:D19303
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68
Li Y, Schichtel BA, Walker JT et al (2016) Increasing importance of deposition of reduced nitrogen in the United States. Proc Natl Acad Sci 113:5874–5879
Li XG, Jia B, Lv J et al (2017) Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol Biochem 112:47–55
Liu J, Wu N, Wang H et al (2016) Nitrogen addition affects chemical compositions of plant tissues, litter and soil organic matter. Ecology 97:1796–1806
Lu M, Zhou X, Luo Y et al (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–244
Morrison EW, Frey SD, Sadowsky JJ et al (2016) Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecol 23:48–57
Müller K, Marhan S, Kandeler E, Poll C (2017) Carbon flow from litter through soil microorganisms: from incorporation rates to mean residence times in bacteria and fungi. Soil Biol Biochem 115:187–196
NADP (National Atmospheric Deposition Program), University of Wisconsin, Wisconsin State Laboratory of Hygiene, http://nadp.slh.wisc.edu/NADP. Accessed 11 September 2018
Otto A, Simpson MJ (2006) Sources and composition of hydrolysable aliphatic lipids and phenols in soils from western Canada. Org Geochem 37:385–407
Otto A, Simpson MJ (2007) Analysis of soil organic matter biomarkers by sequential chemical degradation and gas chromatography—mass spectrometry. J Sep Sci 30:272–282
Otto A, Shunthirasingham C, Simpson MJ (2005) A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada. Org Geochem 36:425–448
Pan Y, Birdsey RA, Fang J et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Pardo LH, Robin-Abbott MJ, Fenn ME et al (2015) Effects and empirical critical loads of nitrogen for ecoregions of the United States. Ecol Appl 21:129–169
Peng Y, Guo D, Yang Y (2017) Global patterns of root dynamics under nitrogen enrichment. Glob Ecol Biogeogr 26:102–114
Pisani O, Frey SD, Simpson AJ, Simpson MJ (2015) Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level. Biogeochemistry 123:391–409
Preston CM, Trofymow JA, Sayer BG, Niu J (1997) 13C nuclear magnetic resonance spectroscopy with cross-polarization and magic-angle spinning investigation of the proximate-analysis fractions used to assess litter quality in decomposition studies. Can J Bot 75:1601–1613
Pries CEH, Castanha C, Porras RC, Torn MS (2017) The whole-soil carbon flux in response to warming. Science 355:1420–1422
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927
Schmidt MWI, Knicker H, Hatcher PG, Kögel-Knabner I (1997) Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. Eur J Soil Sci 48:319–328
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Simpson MJ, Simpson AJ (2012) The chemical ecology of soil organic matter molecular constituents. J Chem Ecol 38:768–784
Smania EFA, Delle Monache F, Smania A et al (2003) Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare. Fitoterapia 74:375–377
Sollins P, Swanston C, Kleber M et al (2006) Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324
Sollins P, Kramer MG, Swanston C et al (2009) Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96:209–231
Theuerl S, Dorr N, Guggenberger G et al (2010) Response of recalcitrant soil substances to reduced N deposition in a spruce forest soil: integrating laccase-encoding genes and lignin decomposition. FEMS Microbiol Ecol 73:166–177
Thomas DC, Zak DR, Filley TR (2012) Chronic N deposition does not apparently alter the biochemical composition of forest floor and soil organic matter. Soil Biol Biochem 54:7–13
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Tritton LM, Hornbeck JW (1982) Biomass equations for major tree species of the Northeast. US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Upper Darby, PA
Vet R, Artz RS, Carou S, Shaw M, Ro CU, Aas W, Baker A, Bowersox VC, Dentener F, Galy-Lacaux C, Hou A, Pienaar JJ, Gillett R, Forti MC, Gromov S, Hara H, Khodzher T, Mahowald NM, Nickovic S, Rao PSP, Reid NW (2014) A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos Environ 93:3–100. https://doi.org/10.1016/j.atmosenv.2013.10.060
Waldner P, Marchetto A, Thimonier A, Schmitt M, Rogora M, Granke O, Mues V, Hansen K, Pihl Karlsson G, Žlindra D, Clarke N, Verstraeten A, Lazdins A, Schimming C, Iacoban C, Lindroos AJ, Vanguelova E, Benham S, Meesenburg H, Nicolas M, Kowalska A, Apuhtin V, Napa U, Lachmanová Z, Kristoefel F, Bleeker A, Ingerslev M, Vesterdal L, Molina J, Fischer U, Seidling W, Jonard M, O’Dea P, Johnson J, Fischer R, Lorenz M (2014) Detection of temporal trends in atmospheric deposition of inorganic nitrogen and sulphate to forests in Europe. Atmos Environ 95:363–374. https://doi.org/10.1016/j.atmosenv.2014.06.054
Wang J-J, Pisani O, Lin LH et al (2017) Long-term litter manipulation alters soil organic matter turnover in a temperate deciduous forest. Sci Total Environ 607–608:865–875
Watzinger A (2015) Microbial phospholipid biomarkers and stable isotope methods help reveal soil functions. Soil Biol Biochem 86:98–107
Wu NN, Filley TR, Bai E et al (2015) Incipient changes of lignin and substituted fatty acids under N addition in a Chinese forest soil. Org Geochem 79:14–20
Xia M, Talhelm AF, Pregitzer KS (2017) Chronic nitrogen deposition influences the chemical dynamics of leaf litter and fine roots during decomposition. Soil Biol Biochem 112:24–34
Yue K, Peng Y, Peng C et al (2016) Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: a meta-analysis. Sci Rep 6:19895
Zak DR, Freedman ZB, Upchurch RA et al (2017) Anthropogenic N deposition increases soil organic matter accumulation without altering its biochemical composition. Glob Chang Biol 23:933–944
Zelles L, Bai QY, Rackwitz R et al (1995) Determination of phospholipid- and lipopolysaccharide-derived fatty acids as an estimate of microbial biomass and community structures in soils. Biol Fertil Soils 19:115–123
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada via a Discovery Grant (#2015-05760) and a Discovery Accelerator Supplement (#478038-15) to M.J.S. We sincerely thank Lori vandenEnden, Zhangliu Du, Sam Reese, Ivy Ryan, and Allegheny College for field, laboratory, and financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Stuart Grandy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, JJ., Bowden, R.D., Lajtha, K. et al. Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter. Biogeochemistry 142, 299–313 (2019). https://doi.org/10.1007/s10533-018-00535-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-00535-4