Abstract
Increasing demand for food and fibre by the growing human population is driving significant land use (LU) change from forest into intensively managed land systems in tropical areas. But empirical evidence on the extent to which such changes affect the soil-atmosphere exchange of trace gases is still scarce, especially in Africa. We investigated the effect of LU on soil trace gas production in the Mau Forest Complex region, Kenya. Intact soil cores were taken from natural forest, commercial and smallholder tea plantations, eucalyptus plantations and grazing lands, and were incubated in the lab under different soil moisture conditions. Soil fluxes of nitrous oxide (N2O), nitric oxide (NO) and carbon dioxide (CO2) were quantified, and we approximated annual estimates of soil N2O and NO fluxes using soil moisture values measured in situ. Forest and eucalyptus plantations yielded annual fluxes of 0.3–1.3 kg N2O–N ha−1 a−1 and 1.5–5.2 kg NO–N ha−1 a−1. Soils of commercial tea plantations, which are highly fertilized, showed higher fluxes (0.9 kg N2O–N ha−1 a−1 and 4.3 kg NO–N ha−1 a−1) than smallholder tea plantations (0.1 kg N2O–N ha−1 a−1 and 2.1 kg NO–N ha−1 a−1) or grazing land (0.1 kg N2O–N ha−1 a−1 and 1.1 kg NO–N ha−1 a−1). High soil NO fluxes were probably the consequence of long-term N fertilization and associated soil acidification, likely promoting chemodenitrification. Our experimental approach can be implemented in understudied regions, with the potential to increase the amount of information on production and consumption of trace gases from soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) and carbon dioxide (CO2) are important greenhouse gases (GHG) contributing directly to climate change (IPCC 2014), while nitric oxide (NO) is a key substance involved in the tropospheric production of ozone, which is also a potent GHG (Chameides et al. 1992). Soils are the dominating source of atmospheric CO2 (Raich et al. 2002) and N2O (Butterbach-Bahl et al. 2013) and contribute considerably to the atmospheric budget of NO (Conrad 1996; Butterbach-Bahl et al. 2009). The exchange of these gases between soil and atmosphere is influenced by several factors such as land use (LU) and land use change (LUC), temperature and precipitation, N input and soil properties (Butterbach-Bahl et al. 2013).
Land use change from natural forest ecosystems into other more intensively managed LU is driven by the increasing demand for food and fibre, especially in tropical regions, where population growth fosters agriculture encroachment in forested areas (IPCC 2007). In East Africa, where agriculture is the primary LU, tropical montane forests are particularly endangered because they are located in areas highly suitable for agricultural production; thus, the expansion of cropland, grazing areas, and forest plantations at the expense of natural forests and other natural ecosystems is expected to continue in the future (Potting and Bakkes 2004). For Kenya’s montane forests, deforestation was approximately 50,000 ha for the 2000–2010 period, with the encroachment of tea cultivation areas being an important driver for LUC (UNEP 2012; Mutugi and Kiiru 2015).
Kenya is the third largest tea producer and the first black tea exporter worldwide (Monroy et al. 2013). The tea sector is divided in two production systems: the large-scale monoculture tea plantations (also called tea estates) and the local-scale smallholders, who traditionally cultivate tea to supplement subsistence agriculture. The commercial tea plantations have usually higher yields compared to those managed by smallholder producers, as more fertilizers are used and management is optimised (Kenya Human Rights Commision 2008). Following increased tea demand, the area devoted for tea production in Kenya has grown in the past decades, primary by the smallholders (Monroy et al. 2013).
Both land use and land management play a significant role in the C and N cycling, potentially influencing the exchange rates of trace gases between the soil and the atmosphere. For example, N fertilization usually promotes both N2O and NO production in the soil due to enhanced substrate availability for microbial utilization (Stehfest and Bouwman 2006). Changes in micro-climate (moisture, temperate) and soil bulk density, porosity, mineral N content and pH following LUC (Farquharson and Baldock 2008) influence the consumption and production of trace gases in the soil (Davidson et al. 2000b; Saiz et al. 2006; Tang et al. 2006). However, studies of the soil-atmosphere trace gas exchange in tropical ecosystems are still scarce, especially in Africa (Kim et al. 2016). The lack of evidence translates into considerable uncertainty on the impact of LUC on soil trace gas emissions (van Lent et al. 2015).
Understanding LU effects on soil GHG fluxes remains difficult due to high spatio-temporal variations of fluxes. Therefore, the design of experiments and sampling strategies for evaluating LUC effects on soil GHG fluxes is crucial (Arias-Navarro et al. 2017). Unfortunately, in situ measurements with high spatial and temporal resolution in remote tropical ecosystems are constrained by limited infrastructure and therefore high costs. To overcome these constrains, soil samples can be taken to the laboratory for targeted incubation experiments, e.g. for studying spatial and temporal variability of fluxes in dependence of changes of environmental conditions. Fluxes from soil cores determined in the laboratory usually agree well with fluxes determined via field chambers (Otter et al. 1999; Ludwig et al. 2001; Gut et al. 2002; van Dijk 2002; Yao et al. 2010).
In this study, we quantified soil CO2, N2O, and NO fluxes of representative LUs of the Mau Forest Complex in Kenya. We used a fully-automated laboratory incubation and monitoring system to study fluxes from intact soil cores at different soil moisture contents. The objectives of this study were:
-
(1)
To evaluate the effect of LU on the soil N2O, NO and CO2 fluxes.
-
(2)
To analyse effects of tea plantation management (commercial versus smallholder) on the soil N2O, NO and CO2 fluxes.
-
(3)
To quantify the importance of soil moisture as a driver of soil trace gas emissions.
We hypothesised that tea plantations would emit higher amounts of N-trace gases than other LUs and that N fluxes from soils taken from commercial tea plantations are higher than those from soils of smallholder farmers. Moreover, we hypothesized that soil water content could be used for approximating the seasonality of soil trace gas emissions and for calculating annual fluxes.
Materials and methods
Study area
The Mau Forest Complex in Kenya is the largest indigenous Afromontane forest of East Africa covering an area of about 417,000 ha. The study site lies in the Southwest Mau part, east of Kericho town (0°22′3′′S, 35°16′59′′E) (Fig. 1) at approximately 2500 m a.s.l. The climate is cool and humid tropical with a mean annual precipitation between 1800 and 1950 mm (1979–2009) (Omumbo et al. 2011). The region has a bi-modal rainfall pattern, with the “long rains” falling between April and August and “short rains” between October and December, while January and February are generally the driest months. The mean annual temperature ranges from 15.7 to 18 °C (1979–2009) (Omumbo et al. 2011) with modest (approx. 7 °C) seasonal variations. The geology substrate is formed by Tertiary lavas from the mid-Miocene (Blackie and Edwards 1979). The soils are well drained, deeply weathered, dark reddish-brown, clayey, and with an acidic humic topsoil (Krhoda 1988; Jaetzold et al. 2010). Soils are classified as Andic Humic Nitisols (IUSS Working Group WRB 2015).
In the last decades the Mau Forest region has experienced a loss of forest cover of about 25% at the expense of other LUs (Government of Kenya 2010). In the region, tea is produced in both large estates and smallholder farms. Smallholder farms are typically less than half a hectare, with most of the land planted with tea and only 20% of the land reserved for food crops and grazing (Milder et al. 2015). Commercial tea estates grow eucalyptus woodlots in addition to tea, as source of firewood for the tea factories.
Experimental design
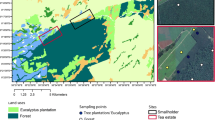
In this experiment, two contrasting tea-growing areas were investigated. The first area is located in Kapkorech Estate, a large tea estate (hereafter, TE) owned by a private company. Nearly 120 ha of commercial plantations of tea (Camelia sinensis var. sinensis L.) were established more than 60 years ago after clearance of the native forest. Approximately 20 ha were designated to grow eucalyptus (Eucalyptus grandis L.). Aerial application of NPK 26:5:5 fertilizer to the tea fields is conducted 2–3 times a year (300–400 kg N ha−1 a−1; personal communication). The second tea-growing area is managed by smallholder farmers (hereafter, SH). Smallholder tea plantations are fertilized with NPK 26:5:5 at an approximate rate of 150 kg N ha−1 a−1 (personal communication). In addition to tea cultivation, a significant share of the land is devoted to livestock grazing.
At the SH area, we monitored plots under tea (T), grazing (G) and the contiguous natural forest (F) (Fig. 1a). At the TE area, we investigated plots under tea (T), eucalyptus plantations (P) and the adjacent natural forest (F) (Fig. 1b). Therefore, the experimental design comprised six experimental sites (SH-T, SH-G, SH-F, TE-T, TE-P and TE-F) each of them replicated three times, making a total of 18 experimental plots, with an area of 0.25 ha each, approximately.
Soil sampling
We used intact soil cores to minimize the disturbance of the soil structure. Five soil cores were collected at three random locations within each plot. At each location, the uppermost layer of litter with visible undecomposed material (leaves, twigs, etc.) was removed before PVC tubes (5 cm inner diameter; 10 cm height) were driven into the soil with the help of a wooden block and rubber hammer. The filled PVC cores were carefully removed and immediately air-dried at 25 °C for three days. After this period, the soils had a water filled pore space (WFPS) of approximately 10–15%. Subsequently, the soils were transported to the laboratory at IMK-IFU (Garmisch-Partenkirchen, Germany) and stored at ambient temperature until laboratory incubations were conducted, approximately four months after sampling.
Soil incubation
The soil cores were incubated using a custom-built, temperature-regulated automatic gas sampling system encompassing 18 incubation chambers. Each chamber consists of a poly-methylmethacrylate cylinder (126 mm inner diameter; 240 mm height) acting as a steady-state dynamic chamber (Pumpanen et al. 2004; Pihlatie et al. 2013). A cylindrical piece of 7 cm height was placed at the bottom of each chamber, on top of which we placed three intact soil cores, one from each of replicate within a plot. The air volume between the cores was filled with quartz sand up to the upper edge of the soil cores. The sand was covered with a metallic sheet (2 mm thick), so that only the soil surface of the cores was directly exposed to the headspace. This design allowed small chamber headspace (374 cm3) without dead volumes. The chamber was then closed with a gas-tight lid equipped with an inlet and an outlet.
During the incubation, background air was continuously supplied through the inlet to all the chambers, allowing for a permanent equilibrium state of the headspace (Pape et al. 2009) (Fig. 2a). The sampling from the incubation chambers and the background air was controlled through electromechanically operated solenoid valves (Bürkert GmbH & Co. KG, Ingelfingen, Germany) in 180-min long cycles in which a measure of the concentration at the outlet of each incubation chamber was gained. Further details on the custom-build system can be found in Zuazo (2016).
Schematic overview of the experiment. a Incubation chamber. b Incubation setup. Dots represent flux measurements for a given gas for an incubation chamber. Blue arrows represent watering events. Temperature (T) and relative humidity (RH) were kept constant at 18 °C and 70%. c Soil analysis. The incubation procedure outlined here was replicated four times for separate soil cores (once for each of the four soil moisture levels, WFPS water-filled pore space)
Nitrous oxide and CO2 concentrations were determined using cavity ring-down spectroscopy (G2508, Picarro, Santa Clara, CA, USA). The gas analyser was calibrated every measuring cycle using a gas blend containing defined concentrations of N2O (408 ppbv) and CO2 (406 ppmv) in synthetic air (Air Liquide GmbH, Düsseldorf, Germany). Nitric oxide concentrations were quantified by a chemiluminescence detector (CLD88p, Eco Physics AG, Duernten, Switzerland) calibrated daily with four different NO concentrations in synthetic air: 0, 50, 200 and 500 ppbv NO. These blends were prepared by mixing a stable concentrated preparation (4 ppm NO in N2; Air Liquide GmbH, Germany) with synthetic air (20% O2 + 80% N2) using a multi-gas calibration system (series 6100; Environics Inc., Tolland, CT, USA).
The soil-headspace exchange rate of each trace gas was calculated from the mass balance between the inlet and outlet concentrations assuming mass flow equilibrium conditions (Pape et al. 2009).
where F chamb stands for the trace gas flux (nmol m2 s−1); A denotes the soil surface of the three soil cores (m2); Q is the headspace air flow rate (m3 s−1). µ cham and µ amb are the trace gas mixing ratios (nmol mol−1) of the inflowing ambient air and of the outflowing chamber air, respectively; and ρ is the molar density of dry air molecules (mol m−3).
Because seasonal fluctuations of mean daily air temperatures at the study area are <7 °C, our experiment focused on the effects of soil moisture changes on the trace gas fluxes. The incubation temperature throughout the experiment was set to the average annual temperature in the study area (i.e. 18 °C). We chose an air relative humidity of 70% to avoid excessive drying of the soil during the measuring cycle. Soil water content of the cores was determined gravimetrically prior to the experiment using a replicate intact soil core. Bulk density (BD) was determined gravimetrically using oven-dry (105 °C) soil weight divided by the core volume. BD was used to calculate the pore volume and consequently the amount of water required to reach 20, 30, 50, 70 and 90% WFPS using the equation:
where W vol is the volumetric water content (g cm−3), BD is soil bulk density (g cm−3) and 2.65 is the soil particle density (g cm−3).
Incubations at different WFPS levels were performed with independent soil cores to avoid potential bias associated with substrate depletion due to sequential incubation. Targeted WFPS was achieved by adding a standard rain solution (Breuer et al. 2002).
Each incubation run (30, 50, 70 and 90% WFPS, respectively), was divided in three periods (Fig. 2b) as follows:
Dry conditions (2 days)
Trace gas fluxes from the soil cores were measured prior to soil re-wetting.
20% WFPS (3 days)
Re-wetting of dry soils usually leads to a short-lasting, over-proportionate increase of emissions (initial pulse, Fig. 2b), with the magnitude of this response being dependent on moisture soil conditions prior to re-wetting (Borken and Matzner 2009; Liang et al. 2015). For this reason, we adjusted the initial moisture content to 20% WFPS to purposely homogenize the soil moisture status before setting the soils to the final targeted WFPS % treatment. Trace gas fluxes were measured for three consecutive days, after which we observed that soil trace gas fluxes stabilized.
Targeted WFPS% (10 days)
Water was added to the soil surface until the targeted WFPS% was achieved and the soil trace gas fluxes were measured for 10 consecutive days. To avoid the short-term interference typically observed as a consequence of the pulse of NO, N2O and CO2 occurring after rewetting of soil we excluded the data of the four days after rewetting (second pulse, Fig. 2b) to analyse the effects of soil moisture on trace gas fluxes for the different LUs (post-pulse area, Fig. 2b).
Because the incubation system allowed to obtain flux measurements for each chamber every 3 h and the incubation run for 15 days in total, approximately 120 individual flux measurements were generated per incubation chamber (N = 18). Cumulative N2O, NO and CO2 emissions were calculated by linear interpolation between two consecutive sampling events.
Determination of soil properties
At the end of the incubation, the soils were air-dried and sieved (2 mm). We mixed 20 g from each replicate soil core to obtain a composite sample, which was sent to a commercial laboratory (Landwirtschaftliches Labor Dr. Janssen GmbH, Gillersheim, Germany) for analysis. Total nitrogen (TN) content was determined by dry combustion (DIN ISO 13878). Carbonates were removed beforehand by acid application, and the organic C content was determined by dry combustion (DIN ISO 10694). Soil pH was determined in water (10 g soil + 25 ml solution) as detailed in the VDLUFA (1991, section A, 5.1, 1). Soil texture was determined according to DIN ISO 18123.
Monitoring of environmental parameters on site
Environmental data were collected from July 2015 to July 2016 from a near weather station located at the Kenya Forest Service Kericho forest station (0°21′5′′S, 35°21′5′′E, 2184 m a.s.l.). Moisture and temperature in 10 cm soil depth were measured using a combined water potential and temperature sensor (Decagon 5TM, Decagon Devices, Inc., Pullman, WA, USA). Rainfall was measured with a rain gauge (Decagon ECRN-50, Decagon Devices, Inc.). Data were logged in 10 min intervals on a digital data logger and downloaded periodically (Decagon Em50 series, Decagon Devices, Inc.). Daily average soil volumetric water content (Wvol) monitored at the weather station was used to calculate the daily average WFPS of each experimental site using the respective BD values (Table 1) assuming that Wvol did not vary across sites.
Because soil temperature does not vary greatly in many tropical forests, soil water content is often found to be a more significant factor affecting temporal variation of soil trace gases (Butterbach-Bahl et al. 2004). Thus, estimated daily values of WFPS were used for approximating the seasonality of soil trace gas emissions and for calculating annual fluxes on basis of the regression curves describing the relationship between soil moisture and the trace gas fluxes observed in our laboratory experiments (See “Relationship between soil properties and trace gas fluxes” section).
Data processing, data analysis
All statistical analyses and plotting were carried out using R 3.1.3 (R Core Team 2016). Downloading and formatting Google Maps images was done with the ggmap package (Kahle and Wickham 2013). One-way ANOVA was used to test differences in soil properties across experimental sites. Trace gas fluxes among different sites and soil moisture levels were compared using a two-way ANOVA. We used the Fisher LSD method to compare individual means. Descriptive statistics are reported as the mean of the three soil core replicates along with the standard deviation. The contribution of soil parameters to the variance of N2O, NO and CO2 gas fluxes was studied using regression analysis. Uncertainty of the curve fitting for linear models was calculated using the function “predict.lm” (R package stats), which produces predicted values, obtained by evaluating the regression function and calculates the standard errors of the predictions. For exponential models we used the function “predictNLS” (R package propagate, Spiess 2014) which propagates the error using Monte Carlo simulation. Significance level was established at p ≤ 0.05.
Results
Soil parameters
All topsoils had a clayey texture, with clay contents in the range of 56–67% (Table 1). Topsoil bulk density (BD) values were below 1 g cm−3, with the lowest values observed for soils sampled at the natural forests (SH-F and TE–F) and at the commercial tea plantations (TE-T). Soil organic carbon (SOC) contents were in the range of 58–79 g kg−1. Soils taken from SH-F had the highest SOC content although differences are not significant from TE-T and SH-G. Nitrogen contents ranged between 5.6 and 8.1 g kg−1. Soils taken from SH-F had as well the highest total nitrogen (TN) content. Soils were acidic (4.2–6.0), with the lowest soil pH value observed for soils from TE-T (Table 1).
Soil trace gas fluxes
Soil N2O fluxes
We measured a very low N2O uptake from dried soil cores (average: −9 ± 31 µg N m−2 h−1). Initial re-wetting of soil cores to 20% WFPS did not cause N2O emission pulses, regardless of the site. After setting the soil cores to the targeted WFPS, substantial N2O fluxes (>50 µg N2O–N m−2 h−1) were measured only when WFPS was finally set to 90%. The peak maximum for these cores was observed after two to four days, depending on the experimental sites (Fig. 3). The only significant differences in soil N2O fluxes were found between 90% WFPS and the other soil moisture levels. With an average post-pulse N2O flux of 131 ± 61 µg N2O–N m−2 h−1, TE–F tended to emit more N2O than soils from other sites, although strong variation precluded significant differences (Table 2).
Temporal evolution of the soil fluxes of nitrous oxide (N2O) for different soil moisture levels (WFPS%) and different sites (SH smallholder, TE tea estate, F forest, T tea, G grazing, P eucalyptus plantations). Vertical bars indicate standard deviations of the three spatial replicates. Water was applied at day 2 and at day 5 to reach 20% WFPS and targeted WFPS, respectively. Grey area indicates measurements used to calculate the mean post-pulse fluxes (day 9 to 12 of the incubation run)
Pulse fluxes contributed to more than half (initial pulse: 22 ± 9%, second pulse: 34 ± 16%) of the total time-weighted average cumulative soil N2O rates during the 15 day-incubation period for the different sites and levels of WFPS (Fig. 4a). Cumulative soil N2O fluxes during the second pulse were significantly higher for soils moistened to 90% WFPS (159 ± 258 g N2O–N ha−1) compared to soils adjusted to other moisture levels (70% WFPS: 27 ± 28 g N2O–N ha−1; 50% WFPS: 27 ± 9 g N2O–N ha−1; 30% WFPS: 27 ± 11 g N2O–N ha−1). The second pulse was not significantly different between sites.
Soil NO fluxes
Dry-soil NO fluxes were very low for all sites (average 5 ± 7 µg NO–N m−2 h−1). Large NO pulses (average 99 ± 100 µg NO–N m−2 h−1) occurred already following the first re-wetting event, when soil moisture was adjusted to 20% WFPS (Fig. 5). The highest peak fluxes following the first initial re-wetting were observed for soils from commercial tea plantations, while for soils from smallholder grazing plots this first pulse was negligible (TE-T: 259 ± 111 µg NO–N m−2 h−1; SH-G: 23 ± 13 µg NO–N m−2 h−1). Soil NO fluxes after the second soil re-wetting to the target moisture were not significantly different across soil moisture levels (Table 3). Nitric oxide emissions were significantly higher for soils from tea plantations (TE-T and SH-T) and natural forests (TE-F and SH-F) compared to those taken from eucalyptus plantations (TE-P) or grazing land (SH-G) (Table 3).
Dynamics of soil fluxes of nitric oxide (NO) at different water-filled pore space (WFPS%) and for different sites (SH smallholder, TE tea estate, F forest, T tea, G grazing, P eucalyptus plantations). Vertical bars indicate standard deviations of three spatial replicates. Water was applied at day 2 and at day 5 to reach 20% WFPS and targeted WFPS, respectively. Grey area indicates measurements at the post-pulse period (day 9 to 12 of the incubation cycle)
Pulse fluxes contributed to more than 80% of the total time-weighted average cumulative soil NO rates during the 15 day-incubation period for the different sites and levels of % WFPS (initial pulse: 31 ± 14%, second pulse: 51 ± 9%) (Fig. 4b). The highest time-weighted average cumulative NO fluxes during the initial pulse (20% WFPS) were those for soils from TE-T (196 ± 78 g NO–N ha−1) followed by TE-F sites (127 ± 65 g NO–N ha−1). The initial pulse was not significantly different for the rest of the sites (TE-P: 47 ± 19 g NO–N ha−1; SH-T: 44 ± 21 g NO–N ha−1; SH-G: 18 ± 19 g NO–N ha−1; SH-F: 13 ± 9 g NO–N ha−1). After setting the soil cores to the final targeted WFPS, average cumulative NO fluxes during the second pulse followed the same pattern, i.e., significant highest emissions were found for soils from TE-T and TE-F (201 ± 46 g NO–N ha−1 and 200 ± 68 g NO–N ha−1 respectively) although in this case, fluxes were not significant different from SH-T (147 ± 99 g NO–N ha−1). The second pulse at SH-T and at the rest of the sites were no significantly different (TE-P: 78 ± 31 g NO–N ha−1; SH-F: 67 ± 19 g NO–N ha−1; SH-G: 65 ± 55 g NO–N ha−1). Moisture content did not have a significant effect on the magnitude of the second pulse.
Soil CO2 fluxes
The CO2 emissions during the dry-incubation period were on average 2 ± 1 mg C m−2 h−1. Following the initial re-wetting of soils to 20% WFPS small (<40 mg C m−2 h−1) and short-lived pulse fluxes of CO2 were observed, which returned to pre-incubation levels in less than 24 h (Fig. 6). Following rewetting to target moisture levels, the soil CO2 fluxes gradually increased with increasing WFPS for all sites. No significant differences in CO2 fluxes were found between 70 and 90% WFPS. Soils taken from forests had significantly higher CO2 fluxes than the rest of the soils (Table 4). The contribution to the total time-weighted average cumulative soil CO2 rates of the 15 day-incubation period of soil CO2 fluxes from the initial pulse event after re-wetting of soils was 12 ± 10% (Fig. 4c) and no significant differences were found among sites. The second pulse event, on the other hand, contributed with 58 ± 7% (Fig. 4c) and was significantly higher for soils set to 90 and 70% WFPS (92 ± 40 kg CO2–C ha−1 and 79 ± 40 kg CO2–C ha−1 respectively) compared to the pulse obtained when the soils were set to 50 and 30% WFPS (45 ± 24 kg CO2–C ha−1 and 23 ± 16 kg CO2–C ha−1 respectively). The second pulse event was significantly higher for soils from forests (SH-F: 81 ± 53 kg CO2–C ha−1; TE-F: 69 ± 32 kg CO2–C ha−1) compared to TE-T (34 ± 11 kg CO2–C ha−1). No significant differences were found between forests and the rest of the soils (SH-G: 64 ± 60 kg CO2–C ha−1 SH-T: 57 ± 37 kg CO2–C ha−1, TE-P: 53 ± 29 kg CO2–C ha−1).
Dynamics of soil fluxes of carbon dioxide (CO2) at different water-filled pore space (WFPS%) and for different sites (SH smallholder, TE tea estate, F forest, T tea, G grazing, P eucalyptus plantations). Vertical bars indicate standard deviations of three spatial replicates. Water was applied at day 2 and at day 5 to reach 20% WFPS and targeted WFPS, respectively. Grey area indicates measurements at the post-pulse period (day 9 to 12 of the incubation cycle)
Relationship between soil properties and trace gas fluxes
Average post-pulse soil N2O and CO2 fluxes were significantly and positively correlated (R2 = 0.4). There were no correlations between N2O or CO2 soil fluxes with soil properties. Average post-pulse soil NO emissions were negatively correlated with pH (R2 = 0.3), BD (R2 = 0.3) and silt content (R2 = 0.2) and positively correlated with clay content (R2 = 0.2).
Estimates of annual emissions
In this incubation study, N2O emissions increased exponentially with WFPS, while the relationship between WFPS and NO followed a 2th degree polynomial (Fig. 7). In the case of TE-T, no satisfactory model was found for N2O; therefore, the mean N2O flux across WFPS levels was used for calculating the annual soil N2O fluxes.
Relationships between soil WFPS and N2O (upper panel) and NO (lower panel) fluxes determined in laboratory experiments. The curve fits were used for calculating annual flux estimates for different sites (SH smallholder, TE tea estate, F forest, T tea, G grazing, P eucalyptus plantations) using observed in situ daily WFPS values at our meteorological observation site. The grey areas indicate the 95% confidence intervals for the individual curve fits. Vertical bars indicate standard errors of three spatial replicates
Measured rainfall at our weather station in the Mau region showed a bi-modal pattern, with rains falling between April and June and between October and December. The total annual rainfall for the period July 2015–July 2016 was 1956 mm and the mean air temperature was 16.7 °C (min = 13.7 °C, max = 20.9 °C) (Fig. 8).
Soil moisture at 10 cm soil depth ranged from 10.5 to 78.5% WFPS with an average value of 25.3% for SH-F, TE-F, TE-T sites and between 11.6 and 86.6% with a mean value of 28% for SH-T, SH-G and TE-P sites. The daily values of WFPS were finally used to calculate annual emissions on basis of the relationships between WFPS and trace gas fluxes (Fig. 9 shows SH-F as an example).
N2O fluxes through the year were generally below 50 µg N2O–N m−2 h−1 (mean value: 11.2 µg N2O–N m−2 h−1) with peak emissions only when the soil moisture content was above 60% WFPS. Daily soil NO emissions were on average 31.8 µg NO–N m−2 h−1, decreasing when the WFPS levels were below 25%. Daily estimated soil NO and N2O fluxes and WFPS% at the SH-F site is shown in Fig. 9.
Table 5 summarizes calculated annual flux rates for the different sites. Across sites, NO fluxes contributed 60–95% to the total N-oxide losses (N2O+NO losses). Highest annual fluxes of N-oxides were observed for the TE-F and TE-T sites, with emissions of 5.5 ± 0.9 and 5.2 ± 0.5 kg N ha−1 a−1 respectively. At the smallholder site the estimated annual soil N emissions for SH-F and SH-T were 3.9 ± 0.9 and 2.2 ± 0.3 kg N ha−1 a−1, respectively.
In general, lowest annual N2O emissions were estimated at the smallholder tea plantations and grazing sites (SH-T: 0.1 ± 0.2 kg N2O–N ha−1 a−1; SH-G: 0.1 ± 0.3 kg N2O–N ha−1 a−1). Soil from the grazing sites showed also the lowest annual NO emissions (SH-G: 1.1 ± 0.3 kg NO–N ha−1 a−1). Highest annual NO emissions were estimated from the natural forest sites and tea plantations in the TE area (TE-F: 5.2 ± 0.7 kg N ha−1 a−1; TE-T: 4.3 ± 0.2 kg NO–N ha−1 a−1).
Discussion
Soil moisture is a key governing parameter in the production and consumption of N oxides in the soil as it controls both soil gas diffusion and oxygen (O2) availability for microbial use (Davidson et al. 2000a). Whereas both NO and N2O may be produced through the same microbial processes (i.e. nitrification and denitrification), the ratios of the two products may vary strongly depending on O2 availability (Pilegaard 2013). Soil NO production during nitrification requires O2 as electron acceptor, while N2O is more commonly produced by reductive processes –i.e. under O2-limiting environmental conditions- such as denitrification or nitrifier-denitrification (Butterbach-Bahl et al. 2013). Therefore, highest emission rates for NO have been frequently observed at soil moisture contents below field capacity, which for many soils is about 60% WFPS. With regard to N2O, maximum emission rates are reported at values between 50 and 90% WFPS, depending on soil properties (Davidson et al. 1991; Breuer et al. 2002; Werner et al. 2007) and at WFPS >90% dominant soil anaerobiosis favours complete denitrification, yielding N2 as product.
In our study, N2O fluxes increased exponentially if soil cores were wetted at WFPS higher than 70%, but were relatively low at 30–50% WFPS. These findings are in agreement with many other studies which have reported that N2O production and emission increase exponentially with soil water content (Garcia-Montiel et al. 2001; Arai et al. 2008). We further found high soil CO2 rates and a significant correlation between N2O fluxes and soil respiration rates at 90% WFPS. This correlation has also been described in field studies conducted by Castaldi et al. (2012) in a rainforest in Ghana and by Werner et al. (2007) in a lowland rainforest in Kenya. While soil moisture contents close to water saturation may favour the development of anaerobiosis and therefore reduce organic matter decomposition and the soil CO2 efflux (e.g. Smith 1990), it seems that our soils were predominantly aerobic even at 90% WFPS due to the low bulk density of the topsoils (<1 g cm−3). Similarly, in Chinese montane, subtropical rainforests Zhou et al. (2013) found that soil CO2 fluxes significantly increase with soil moisture at constant temperatures due to improved substrate availability for microbial respiration.
In our study, optimum soil moisture content for NO emission for different sites and LUs varied, which was probably an effect not only of LU but also of varying soil properties. The effect of WFPS on soil NO emissions was markedly lower than on N2O fluxes, leading to a decrease of the NO: N2O ratio with increasing WFPS, suggesting that denitrification or nitrifier-denitrification processes took over nitrification as source process for N trace gases with increasing soil moisture levels (Breuer et al. 2002; Butterbach-Bahl et al. 2004; Werner et al. 2007).
For the forest sites, our approach yielded flux rate estimates of 0.3 ± 0.2 and 1.3 ± 0.2 kg N2O–N ha−1 a−1 for TE-F and SH-F, respectively. This is similar to what has been measured in situ at other tropical montane forests (0.3−1.3 kg N2O–N ha−1 a−1) (Riley et al. 1995; Ishizuka et al. 2002; Purbopuspito et al. 2006; Koehler et al. 2009; Müller et al. 2015), but lower than most of the studies performed in tropical lowland forests, which range between 1.9 and 6.1 kg N2O–N ha−1 a−1 (Keller et al. 1993; Serca et al. 1994; Keller and Reiners 1994; Verchot et al. 1999; Breuer et al. 2000; Melillo et al. 2001; Garcia-Montiel et al. 2003; Werner et al. 2007; Castaldi et al. 2013), with some studies showing exceptionally lower N2O fluxes (e.g. Wieder et al. 2011, ca. 0.75 kg N2O–N ha−1 a−1). Some evidence suggests that in contrast to tropical lowland forests, tropical montane forests may be N limited, as they e.g. show low net N mineralization rates (Purbopuspito et al. 2006; Nottingham et al. 2015; Gütlein et al. 2016). Our results may be a conservative estimate since we removed the litter layer, which may have contributed to additional N2O losses (e.g. Wang et al. 2014) and we only incubated the top 10 cm of soil, while N2O fluxes of tropical soils have been shown to have their N2O production optima at 5–20 cm soil depth (Nobre et al. 2001). On the contrary, no effective plant N uptake in the soil cores may have led to higher microbial N availability and thus, to a modest overestimate of N2O fluxes compared to field conditions (Brumme 1995).
Fluxes of NO from soils of tropical montane forests have so far only rarely been reported, but the annual flux which we estimated (3.9 ± 1.8 kg NO–N ha−1 a−1) seems to be higher than in previous studies in montane (0.03–0.4 kg NO–N ha−1 a−1) (Johansson et al. 1988; Riley et al. 1995; Davidson and Kingerlee 1997; Purbopuspito et al. 2006; Koehler et al. 2009) and in lowland tropical forests (0.7–1.5 kg NO–N ha−1 a−1) (Keller et al. 1993; Serca et al. 1994; Keller and Reiners 1994; Verchot et al. 1999). However, Butterbach-Bahl et al. (2004) found that soils of a tropical lowland rainforest in Australia emitted approx. 3.0 kg NO–N ha−1 within a three-month period following the rewetting of soils after a drought period, suggesting that the emission of NO from tropical rain forest soils may still be underestimated. Our estimate is however very similar to the 3.0 kg NO–N ha−1 a−1 model-estimated by Gharahi Ghehi et al.(2014) for a tropical montane forest in Rwanda. The high NO fluxes we found might be related to the high clay content, the high SOC and the low soil pH. Soil organic C content has been found to be positively correlated with NO emissions (Bouwman et al. 2002), and rates of microbial transformation of N are expected to be higher in soils with high SOC content (Matson et al. 1990; Li et al. 2005). Further, chemodenitrification (abiotic reduction of nitrite) may have contributed to some NO production due to the low soil pH found in our sites, as this process has been observed to occur under strongly acidic conditions, both in temperate (Venterea et al. 2003) and tropical environments (Serca et al. 1994, Gharahi Ghehi et al. 2014).
Annual estimates of soil N2O emissions from eucalyptus plantations (TE-P) were, with 1.0 ± 0.02 kg N2O–N ha−1 a−1, similar, though towards the lower end, to those in other studies covering measurements of N2O fluxes from tropical rainforest forest soils. Forest conversion to pasture influences strongly soil N cycling (mineralization, nitrification, and denitrification) and therefore also soil N2O and NO fluxes (Davidson et al. 2000b). In our study, soils from smallholder grazing sites (SH-G) showed relative low N2O (except at 90% WFPS) and low NO fluxes, in line with previous studies in tropical ecosystems (Keller et al. 1993; Veldkamp et al. 1999; Davidson and Verchot 2000; Garcia-Montiel et al. 2001; Melillo et al. 2001).
Background N2O and NO emissions occurring in non-fertilized control areas are crucial for developing robust national emission inventories of nitrogenous gases and corresponding emission factors (Zheng et al. 2004); nevertheless, direct measurements of background emissions in tea plantations, especially measurements covering an entire year, have been rarely reported (Akiyama et al. 2006). The few studies available from commercial tea plantations are from Asia where large amounts of N fertilizers are applied (up to 2600 kg ha−1 a−1 Fu et al. 2012; Han et al. 2013; Li et al. 2013), while at the commercial tea plantation in our study 300 kg N ha−1 a−1 was applied. The calculated annual N2O emission rate for commercial tea plantations in our study (0.9 ± 0.3 kg N2O–N ha−1 a−1) is relatively low compared to the 4–7 kg N2O–N ha−1 a−1 estimated from “zero N-control” tea plantations in China and Japan (Akiyama et al. 2006; Fu et al. 2012; Yao et al. 2015) although those flux estimates were likely highly affected by the previous application of large amounts of N fertilizer (average of 553 kg N ha−1 a−1). Regarding smallholder tea plantations, our annual N2O estimations (0.1 ± 0.2 kg N2O–N ha−1 a−1) are somewhat lower than those estimates from Rosenstock et al. (2015) who reported annual N2O fluxes of 0.4 ± 0.2 kg N2O–N ha−1 a−1 and 0.7 ± 0.5 kg N2O–N ha−1 a−1, kg N ha−1 a−1 in similar systems in Kenya and Tanzania, respectively. They further suggested that the emission factor from N application in smallholder tea systems would be below 1% of N applied. Beyond N2O, our work shows that tea plantations are a major source for NO. The estimated annual NO flux of 4.3 ± 0.7 kg ha−1 a−1 and 2.1 ± 1.1 kg ha−1 a−1 for TE-T and SH-T, respectively is higher than the annual NO fluxes reported by Yao et al. (2015) for a tea plantation in China (1.6 ± 0.4 kg NO–N ha−1 a−1, no N fertilizer application).
The higher N2O and NO emissions (N2O+NO) from soils of the commercial tea plantation (5.2 ± 0.5 kg N ha−1 a−1) compared to soils of tea plantations from smallholder farmers (2.2 ± 0.3 kg N ha−1 a−1) are very likely due to the long-term N fertilization and the subsequent soil acidification (Tokuda and Hayatsu 2004; Yamamoto et al. 2014). Soils from the commercial tea plantation (TE-T) in our study showed a mean pH value of 4.2, which was significantly lower than soils from tea plantations of smallholders (SH-T, mean: 5.0). Enhanced soil acidity is an important factor affecting biotic and abiotic processes and consequently promoting N losses, by e.g. inducing chemodenitrification and therefore NO losses but also N2O (Venterea et al. 2003; Kesik et al. 2006; Medinets et al. 2015; Yao et al. 2015). Chemodenitrification has been suspected to be an important source of NO emissions from soils after drying and wetting of soil, and in excessively fertilized soils, as it is the case in the soils from the commercial tea plantations, where nitrite can accumulate (Davidson 1992; Neff et al. 1995; Verchot et al. 1999). Thus, while relevant chemodenitrification may already occur in the forest and in the small holder tea areas, high rates of fertilization in combination with very low soil pH values may have further increased the contribution of chemodenitrification to the total NO efflux in the commercial tea plantations.
Conclusions
Large uncertainties still exist with regard to the magnitude of soil NO and N2O emissions from tropical African terrestrial ecosystems. Our observations contribute to a growing body of empirical evidence on soil trace gas emissions from different LUs in the African tropics and their governing parameters. Temporal upscaling solely based on soil moisture carries additional uncertainty, since we were not able to include spatial variations in soil C and N availability for microbial processes. We present a conservative upscaling of flux rates which do not include the effect of consecutive watering-drying cycles. For a robust understanding of the trace gas exchange processes in tropical ecosystems, long-term observations at multiple sites are strongly required. Our results reveal aspects of control of N2O, NO and CO2 emissions that may assist to the development of baseline information required to develop LU and agricultural practices and management approaches aiming to ensure sustainable increases in productivity while reducing the contribution of agriculture to climate change.
References
Akiyama H, Yan X, Yagi K (2006) Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: summary of available data. Soil Sci Plant Nutr 52:774–787. doi:10.1111/j.1747-0765.2006.00097.x
Arai S, Ishizuka S, Ohta S et al (2008) Potential N2O emissions from leguminous tree plantation soils in the humid tropics. Global Biogeochem Cycles 22:1–9. doi:10.1029/2007GB002965
Arias-Navarro C, Díaz-Pinés E, Klatt S et al (2017) Spatial variability of soil N2O and CO2 fluxes in different topographic positions in a tropical mountain forest in Kenya. J Geophys Res Biogeosci 122:514–527. doi:10.1002/2016JG003667
Blackie JR, Edwards KA (1979) General conclusions from the land use experiments in East Africa. East Afr Agric For J 43:273–277. doi:10.1080/00128325.1979.11662968
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Chang Biol 15:808–824. doi:10.1111/j.1365-2486.2008.01681.x
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilized fields: summary of available measurement data. Global Biogeochem Cycles. doi:10.1029/2001GB001811
Breuer L, Papen H, Butterbach-Bahl K (2000) N2O emission from tropical forest soils of Australia. J Geophys Res 105:26353. doi:10.1029/2000JD900424
Breuer L, Kiese R, Butterbach-Bahl K (2002) Temperature and moisture effects on nitrification rates in tropical rain-forest soils. Soil Sci Soc Am J 66:834. doi:10.2136/sssaj2002.8340
Brumme R (1995) Mechanisms of carbon and nutrient release and retention in beech forest gaps. Plant Soil 2:593–600
Butterbach-Bahl K, Kock M, Willibald G et al (2004) Temporal variations of fluxes of NO, NO2, N2O, CO2, and CH4 in a tropical rain forest ecosystem. Global Biogeochem Cycles. doi:10.1029/2004gb002243
Butterbach-Bahl K, Kahl M, Mykhayliv L et al (2009) A European-wide inventory of soil NO emissions using the biogeochemical models DNDC/Forest-DNDC. Atmos Environ 43:1392–1402. doi:10.1016/j.atmosenv.2008.02.008
Butterbach-Bahl K, Baggs E, Dannenmann M et al (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122. doi:10.1098/rstb.2013.0122
Castaldi S, Bertolini T, Valente A et al (2013) Nitrous oxide emissions from soil of an African rain forest in Ghana. Biogeosci Discuss 9:16565–16588. doi:10.5194/bgd-9-16565-2012
Chameides WL, Fehsenfeld F, Rodgers MO et al (1992) Ozone precursor relationships in the ambient atmosphere. J Geophys Res 97:6037. doi:10.1029/91JD03014
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Davidson EA (1992) Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci Soc Am J 56:95–102. doi:10.2136/sssaj1992.03615995005600010015x
Davidson EA, Kingerlee W (1997) A global inventory of nitric oxide emissions from soils. Nutr Cycl Agroecosystems 48:37–50. doi:10.1023/A:1009738715891
Davidson EA, Verchot LV (2000) Testing the hole in the pipe model of nitric and nitrous oxide emission from soils using the TRAGNET database. Global Biogeochem Cycles 14:1035–1042
Davidson EA, Vitousek PM, Riley R et al (1991) Soil emissions of nitric oxide in a seasonally dry tropical forest of Mexico. J Geophys Res Atmos. doi:10.1029/91JD01476
Davidson EA, Keller M, Erickson HE et al (2000a) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50:667. doi:10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2
Davidson EA, Verchot LV, Cattanio JH, Ackerman IL (2000b) Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48:53–69. doi:10.1023/A:1006204113917
Farquharson R, Baldock J (2008) Concepts in modelling N2O emissions from land use. Plant Soil 309:147–167. doi:10.1007/s11104-007-9485-0
Fu XQ, Li Y, Su WJ et al (2012) Annual dynamics of N2O emissions from a tea field in southern subtropical China. Plant, Soil Environ 58:373–378
Garcia-Montiel DC, Steudler PA, Piccolo MC et al (2001) Controls on soil nitrogen oxide emissions from forests and pastures in the Brazilian Amazon. Global Biogeochem Cycles 15:1021–1030
Garcia-Montiel DC, Steudler PA, Piccolo M et al (2003) Nitrogen oxide emissions following wetting of dry soils in forest and pastures in Rondônia, Brazil. Biogeochemistry 64:319–336. doi:10.1023/A:1024968802018
Gharahi Ghehi N, Werner C, Hufkens K et al (2014) N2O and NO emission from the Nyungwe tropical highland rainforest in Rwanda. Geoderma Reg 2–3:41–49. doi:10.1016/j.geodrs.2014.09.008
Government of Kenya (2010) Rehabilitation of the Mau Forest Ecosystem. A project concept prepared by the Interim Coordinating Secretariat, Office of the Prime Minister, on behalf of the Government of Kenya
Gut A, Van Dijk SM, Scheibe M et al (2002) NO emission from an Amazonian rain forest soil: continuous measurements of NO flux and soil concentration. J Geophys Res D Atmos 107:1–10. doi:10.1029/2001JD000521
Gütlein A, Zistl-Schlingmann M, Becker JN et al (2016) Nitrogen turnover and greenhouse gas emissions in a tropical alpine ecosystem, Mt. Kilimanjaro, Tanzania. Plant Soil. doi:10.1007/s11104-016-3029-4
Han W, Xu J, Wei K et al (2013) Estimation of N2O emission from tea garden soils, their adjacent vegetable garden and forest soils in eastern China. Environ Earth Sci 70:2495–2500. doi:10.1007/s12665-013-2292-4
IPCC (2007) Climate change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Geneva.
IPCC (2014) Climate Change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Geneva.
Ishizuka S, Tsuruta H, Murdiyarso D (2002) An intensive field study on CO2, CH4, and N2O emissions from soils at four land-use types in Sumatra, Indonesia. Global Biogeochem Cycles. doi:10.1029/2001GB001614
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. Rome
Jaetzold R, Schmidt H, Hornetz B, Shisanya C (2010) Farm management handbook of Kenya Part II/B. In: Farm management handbook of Kenya, 2nd ed. Ministry of Agriculture, Kenya, in Cooperation with the German Agency for Technical Cooperation (GTZ), Nairobi, p 739
Johansson C, Rodhe H, Sanhueza E (1988) Emission of NO in a tropical savanna and a cloud forest during the dry season. J Geophys Res Res 93:7180–7192
Kahle D, Wickham H (2013) ggmap: Spatial visualization with ggplot2. R J 5:144–161. doi:10.1023/A:1009843930701
Keller M, Reiners WA (1994) Soil-atmosphere exchange of nitrous oxide, nitric oxide, and methane under secondary succession of pasture to forest in the Atlantic lowlands of Costa Rica. Global Biogeochem Cycles 8:399–409. doi:10.1029/94GB01660
Keller M, Veldkamp E, Weitz AM, Reiners WA (1993) Effect of pasture age on soil trace-gas emissions from a deforested area of Costa Rica. Nature 365:244–246
Kenya Human Rights Commision (KHRC) (2008) A comparative study of the tea sector in Kenya. A case study of large scale tea estates. http://resource.khrc.or.ke:8181/khrc/handle/123456789/15?show=full
Kesik M, Blagodatsky S, Papen H, Butterbach-Bahl K (2006) Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J Appl Microbiol 101:655–667. doi:10.1111/j.1365-2672.2006.02927.x
Kim D-G, Thomas AD, Pelster D et al (2016) Greenhouse gas emissions from natural ecosystems and agricultural lands in sub-Saharan Africa: synthesis of available data and suggestions for further research. Biogeosciences 13:4789–4809. doi:10.5194/bg-13-4789-2016
Koehler B, Corre MD, Veldkamp E et al (2009) Immediate and long-term nitrogen oxide emissions from tropical forest soils exposed to elevated nitrogen input. Glob Chang Biol 15:2049–2066. doi:10.1111/j.1365-2486.2008.01826.x
Krhoda GO (1988) The impact of resource utilization on the hydrology of the Mau Hills forest in Kenya. Mt Res Dev 8:193–200. doi:10.2307/3673447
Li C, Frolking S, Butterbach-Bahl K (2005) Carbon sequestration in arable soils is likely to increase nitrous oxide emissions, offsetting reductions in climate radiative forcing. Clim Change 72:321–338. doi:10.1007/s10584-005-6791-5
Li Y, Fu X, Liu X et al (2013) Spatial variability and distribution of N2O emissions from a tea field during the dry season in subtropical central China. Geoderma 193:1–12. doi:10.1016/j.geoderma.2012.10.008
Liang LL, Grantz DA, Jenerette GD (2015) Multivariate regulation of soil CO2 and N2O pulse emissions from agricultural soils. Glob Chang Biol . doi:10.1111/gcb.13130
Ludwig J, Meixner FX, Vogel B, Forstner J (2001) Soil-air exchange of nitric oxide: an overview of processes, environmental factors, and modeling studies. Biogeochemistry 52:225–257. doi:10.1023/a:1006424330555
Matson PA, Vitousek PM, Livingston GP, Swanberg NA (1990) Sources of variation in nitrous oxide flux from Amazonian ecosystems. J Geophys Res 95:16789. doi:10.1029/JD095iD10p16789
Medinets S, Skiba U, Rennenberg H, Butterbach-Bahl K (2015) A review of soil NO transformation: associated processes and possible physiological significance on organisms. Soil Biol Biochem 80:92–117. doi:10.1016/j.soilbio.2014.09.025
Melillo JM, Steudler PA, Feigl BJ et al (2001) Nitrous oxide emissions from forests and pastures of various ages in the Brazilian Amazon. J Geophys Res 106:179–188
Milder J, Moroge M, Shames S (2015) Operationalizing climate-smart agricultural landscapes: the case of a tea- producing landscape in Kericho, Kenya. In: Minang PA, van Noordwijk M, Freeman OE et al (eds) Climate-smart landscapes: multifunctionality in practice. World Agroforestry Centre (ICRAF), Nairobi, pp 319–333
Monroy L, Mulinge W, Witwer M (2013) Analysis of incentives and disincentives for tea in Kenya. Technical notes series
Müller AK, Matson AL, Corre MD, Veldkamp E (2015) Soil N2O fluxes along an elevation gradient of tropical montane forests under experimental nitrogen and phosphorus addition. Front Earth Sci 3:1–12. doi:10.3389/feart.2015.00066
Mutugi M, Kiiru W (2015) Biodiversity, local resource, national heritage, regional concern, and global impact: the case of Mau Forest, Kenya. Eur Sci J 1:681–692
Neff JC, Keller M, Holland EA et al (1995) Fluxes of nitric oxide from soils following the clearing and burning of a secondary tropical rain forest. J Geophys Res 100:25913. doi:10.1029/95JD02027
Nobre AD, Keller M, Crill PM, Harriss RC (2001) Short-term nitrous oxide profile dynamics and emissions response to water, nitrogen and carbon additions in two tropical soils. Biol Fertil Soils 34:363–373. doi:10.1007/s003740100396
Nottingham AT, Turner BL, Whitaker J et al (2015) Soil microbial nutrient constraints along a tropical forest elevation gradient: a belowground test of a biogeochemical paradigm. Biogeosciences 12:6071–6083. doi:10.5194/bg-12-6071-2015
Omumbo JA, Lyon B, Waweru SM et al (2011) Raised temperatures over the Kericho tea estates: revisiting the climate in the East African highlands malaria debate. Malar J 10:12. doi:10.1186/1475-2875-10-12
Otter LB, Yang WX, Scholes MC, Meixner FX (1999) Nitric oxide emissions from a southern African savanna. J Geophys Res Atmos 104:18471–18485. doi:10.1029/1999JD900148
Pape L, Ammann C, Nyfeler-Brunner A et al (2009) An automated dynamic chamber system for surface exchange measurement of non-reactive and reactive trace gases of grassland ecosystems. Biogeosciences 6:405–429. doi:10.5194/bg-6-405-2009
Pihlatie MK, Christiansen JR, Aaltonen H et al (2013) Comparison of static chambers to measure CH4 emissions from soils. Agric For Meteorol 171–172:124–136. doi:10.1016/j.agrformet.2012.11.008
Pilegaard K (2013) Processes regulating nitric oxide emissions from soils. Philos Trans R Soc L B Biol Sci 368:20130126. doi:10.1098/rstb.2013.0126
Potting J, Bakkes J (2004) The GEO-3 scenarios 2002–2032: quantification and analysis of environmental impacts. UNEP/DEWA/RS.03-4 and RIVM 402001022. Division
Pumpanen J, Kolari P, Ilvesniemi H et al (2004) Comparison of different chamber techniques for measuring soil CO2 efflux. Agric For Meteorol 123:159–176
Purbopuspito J, Veldkamp E, Brumme R, Murdiyarso D (2006) Trace gas fluxes and nitrogen cycling along an elevation sequence of tropical montane forests in Central Sulawesi, Indonesia. Glob Biogeochem Cycles 20:1–11. doi:10.1029/2005GB002516
Raich JW, Potter CS, Bhagawati D (2002) Interannual variability in global soil respiration, 1980–1994. Glob Chang Biol 8:800–812. doi:10.1046/j.1365-2486.2002.00511.x
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org/
Riley RH, Vitousek PM, Ecology S, Jan N (1995) Nutrient dynamics and nitrogen trace gas flux during ecosystem development in montane rain forest. Ecology 76:292–304
Rosenstock T, Mpanda M, Pelster D et al (2015) Greenhouse gas fluxes from agricultural soils of Kenya and Tanzania. J Geophys Res Biogeosciences 121:707–723. doi:10.1002/2016JG003341
Saiz G, Green C, Butterbach-Bahl K et al (2006) Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 287:161–176. doi:10.1007/s11104-006-9052-0
Serca D, Delmas R, Jambert C, Labroue L (1994) Emissions of nitrogen oxides from equatorial rain forest in central Africa: origin and regulation of NO emission from soils. Tellus 46:243–254
Smith KA (1990) Anaerobic zones and denitrification in soil: modelling and measurement. In: Revsbech NP, Sørensen J (eds) Denitrification in soil and sediment. Springer, Boston, pp 229–244
Spiess A-N (2014) Propagate: propagation of uncertainty. R package version 1.0-4. http://CRAN.R-project.org/package=propagate
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosystems 74:207–228. doi:10.1007/s10705-006-9000-7
Tang X, Liu S, Zhou G et al (2006) Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob Chang Biol 12(546):560. doi:10.1111/j.1365-2486.2006.01109.x
Tokuda S, Hayatsu M (2004) Nitrous oxide flux from a tea field amended with a large amount of nitrogen fertilizer and soil environmental factors controlling the flux. Soil Sci Plant Nutr 50:365–374. doi:10.1080/00380768.2004.10408490
UNEP (2012) The role and contribution of montane forests and related ecosystem services to the Kenyan economy
van Dijk SM (2002) Biogenic NO emissions from forest and pasture soils: relating laboratory studies to field measurements. J Geophys Res 107:8058. doi:10.1029/2001JD000358
van Lent J, Hergoualc HK, Verchot LV (2015) Reviews and syntheses: soil N2O and NO emissions from land use and land-use change in the tropics and subtropics: A meta-analysis. Biogeosciences 12:7299–7313. doi:10.5194/bg-12-7299-2015
Veldkamp E, Davidson E, Erickson H et al (1999) Soil nitrogen cycling and nitrogen oxide emissions along a pasture chronosequence in the humid tropics of Costa Rica. Soil Biol Biochem 31:387–394. doi:10.1016/S0038-0717(98)00141-2
Venterea RT, Groffman PM, Verchot LV et al (2003) Nitrogen oxide gas emissions from temperate forest soils receiving long-term nitrogen inputs. Glob Chang Biol 9:346–357. doi:10.1046/j.1365-2486.2003.00591.x
Verchot LV, Davidson EA, Cattfinio JH et al (1999) Land use change and biogeochemical controls of nitrogen oxide emissions from soils in eastern Amazonia. Global Biogeochem Cycles 13:31–46. doi:10.1029/1998GB900019
Wang Y, Wang H, Wang ZL et al (2014) Effect of litter layer on soil-atmosphere N2O flux of a subtropical pine plantation in China. Atmos Environ 82:106–112. doi:10.1016/j.atmosenv.2013.10.028
Werner C, Kiese R, Butterbach-Bahl K (2007) Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. J Geophys Res 112:D03308. doi:10.1029/2006JD007388
Wieder WR, Cleveland CC, Townsend AR (2011) Throughfall exclusion and leaf litter addition drive higher rates of soil nitrous oxide emissions from a lowland wet tropical forest. Glob Chan Biol 17:3195–3207. doi:10.1111/j.1365-2486.2011.02426.x
Yamamoto A, Akiyama H, Naokawa T et al (2014) Lime-nitrogen application affects nitrification, denitrification, and N2O emission in an acidic tea soil. Biol Fertil Soils 50:53–62. doi:10.1007/s00374-013-0830-6
Yao Z, Wolf B, Chen W et al (2010) Spatial variability of N2O, CH4 and CO2 fluxes within the Xilin River catchment of Inner Mongolia, China: a soil core study. Plant Soil 331:341–359. doi:10.1007/s11104-009-0257-x
Yao Z, Wei Y, Liu C et al (2015) Organically fertilized tea plantation stimulates N2O emissions and lowers NO fluxes in subtropical China. Biogeosciences 12:5915–5928. doi:10.5194/bg-12-5915-2015
Zheng X, Han S, Huang Y et al (2004) Re-quantifying the emission factors based on field measurements and estimating the direct N2O emission from Chinese croplands. Global Biogeochem Cycles 18:1–19. doi:10.1029/2003GB002167
Zhou Z, Jiang L, Du E et al (2013) Temperature and substrate availability regulate soil respiration in the tropical mountain rainforests, Hainan Island, China. J Plant Ecol 6:325–334. doi:10.1093/jpe/rtt034
Zuazo P (2016) Development of a fully automated soil incubation and gas sampling system for quantifying trace gas emission pulses from soils at high temporal resolution. Dissertation, Albert-Ludwigs-Universität Freiburg im Breisgau, Germany
Acknowledgements
This work was funded by the Consultative Group on International Agricultural Research (CGIAR) Research program on Climate Change, Agriculture, and Food Security (CCAFS), which is carried out with support from CGIAR Fund Donors and through bilateral funding agreements. CAN acknowledges financial support by The Climate Food and Farming Research Network (CLIFF) and by The Helmholtz Research School MICMoR. EDP and KBB received additional funding from the German Federal Ministry of Education and Research (Förderzeichnen 01DG13012). Authors are grateful to the technical support received by the Mazingira Centre, Environmental Research and Educational facility (https://mazingira.ilri.org). We greatly thank the Kenya Forest Service (KFS) for access to the sites and field assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stephen Porder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arias-Navarro, C., Díaz-Pinés, E., Zuazo, P. et al. Quantifying the contribution of land use to N2O, NO and CO2 fluxes in a montane forest ecosystem of Kenya. Biogeochemistry 134, 95–114 (2017). https://doi.org/10.1007/s10533-017-0348-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-017-0348-3