Abstract
The decomposition of soil organic carbon (SOC) is intrinsically sensitive to temperature. However, the degree to which the temperature sensitivity of SOC decomposition (as often measured in Q10 value) varies with soil depth and labile substrate availability remain unclear. This study explores (1) how the Q10 of SOC decomposition changes with increasing soil depth, and (2) how increasing labile substrate availability affects the Q10 at different soil depths. We measured soil CO2 production at four temperatures (6, 14, 22 and 30 °C) using an infrared CO2 analyzer. Treatments included four soil depths (0–20, 20–40, 40–60 and 60–80 cm), four sites (farm, redwood forest, ungrazed and grazed grassland), and two levels of labile substrate availability (ambient and saturated by adding glucose solution). We found that Q10 values at ambient substrate availability decreased with increasing soil depth, from 2.0–2.4 in 0–20 cm to 1.5–1.8 in 60–80 cm. Moreover, saturated labile substrate availability led to higher Q10 in most soil layers, and the increase in Q10 due to labile substrate addition was larger in subsurface soils (20–80 cm) than in surface soils (0–20 cm). Further analysis showed that microbial biomass carbon (MBC) and SOC best explained the variation in Q10 at ambient substrate availability across ecosystems and depths (R2 = 0.37, P < 0.001), and MBC best explained the variation in the change of Q10 between control and glucose addition treatment (R2 = 0.14, P = 0.003). Overall, these results indicate that labile substrate limitation of the temperature sensitivity of SOC decomposition, as previously shown in surface soils, is even stronger for subsoils. Understanding processes controlling the labile substrate availability (e.g., with rising atmospheric CO2 concentration and land use change) should advance our prediction of the fate of subsoil SOC in a warmer world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in soil carbon, the largest terrestrial carbon pool, regulate the global carbon cycle, atmospheric CO2 levels, and the global climate (Amundson 2001). The decomposition of soil organic carbon (SOC) is intrinsically sensitive to temperature (Davidson and Janssens 2006). Increased decomposition of SOC due to soil warming is an important potential feedback to climate change (Davidson and Janssens 2006; Luo and Zhou 2007; Smith et al. 2008). Therefore, accurate representation of the temperature sensitivity of SOC decomposition (often measured in Q10 value) in Earth System Models (ESMs) is crucial because it affects our predictions of the impact of climate change on soil carbon stock (Holland et al. 2000; Fang et al. 2005; Foereid et al. 2014). However, there is still no consensus on how the temperature sensitivity of SOC decomposition varies with biotic and abiotic factors (Davidson and Janssens 2006; Kirschbaum 2006; Smith et al. 2008; von Lützow and Kögel-Knabner 2009; Subke and Bahn 2010; Conant et al. 2011), such as soil depth (Fierer et al. 2003; Rey et al. 2008; Gillabel et al. 2010) and labile substrate availability (Davidson and Janssens 2006; Gershenson et al. 2009; Fissore et al. 2013).
In well-drained upland soils, the chemical composition and turnover time of SOC change significantly with increasing soil depth (Trumbore 2000; Rumpel et al. 2002; Schrumpf et al. 2013). Soil organic matter (SOM) consists of compounds of vastly different structures and properties. It is commonly perceived that the fraction of less reactive and more recalcitrant organic matter increases with increasing soil depth, meaning that the organic matter at depth is less biodegradable and has more complicated structure than the organic matter at surface (Rumpel et al. 2002; Spielvogel et al. 2008; Hassouna et al. 2010). Kinetic theory predicts that the intrinsic temperature sensitivity of decomposition increases with increasing recalcitrance of the carbon compounds (Bosatta and Ǻgren 1999). Therefore, the intrinsic Q10 value of soil respiration is expected to increase with increasing soil depth. However, previous studies have shown inconsistent patterns of Q10values of soil respiration with soil depth. Increase (Lomander et al. 1998; Fierer et al. 2003; Jin et al. 2008; Karhu et al. 2010), decrease (Winkler et al. 1996; MacDonald et al. 1999; Gillabel et al. 2010), or no changes (Fang et al. 2005; Leifeld and Fuhrer 2005; Rey et al. 2008) in apparent Q10 values with increasing soil depth have been observed in different studies. Much of the variation in the apparent temperature sensitivity of SOC decomposition may be related to the fact that labile substrate availability is often unaccounted for in these studies (Davidson and Janssens 2006; Gershenson et al. 2009; Conant et al. 2011). Therefore, in-depth studies are needed, particularly those in which labile substrates are added to a range of soil types and depths.
A theoretical relationship between labile substrate availability and the temperature sensitivity of SOC decomposition has been described using the Michaelis–Menten model (Michaelis and Menten 1913), which is well tested and widely used in studying enzymatic kinetics (Marx et al. 2005; German et al. 2012). The Michaelis–Menten equation for soil respiration can be written in the form R = (V max[C])/(K m + [C]), where R is soil respiration rate, V max is the maximal rate of enzymatic activity, K m is the dissociation constant (or ‘half-saturation constant’), and [C] is the concentration of organic carbon substrates. Both V max and K m are positively temperature dependent. When substrate concentration is at or above the saturation level, the temperature sensitivity of soil respiration approaches the temperature sensitivity of V max, or the intrinsic temperature sensitivity. When substrate concentration is below the saturation level, the temperature sensitivity of K m becomes important, and the overall temperature sensitivity of soil respiration (also defined as the apparent temperature sensitivity) decreases below that of V max. Applying this equation to soil respiration offers a plausible mechanistic explanation for the current controversy concerning the role of substrate availability in affecting the temperature sensitivity of SOC decomposition (Davidson and Janssens 2006). Although empirical validation of the Michaelis–Menten model for soil respiration using surface soils has been reported (Larionova et al. 2007; Gershenson et al. 2009; Curiel Yuste et al. 2010; Zhu and Cheng 2011b; Fissore et al. 2013), little experimental evidence come from subsurface soils. Subsurface soils contain more carbon than surface soils at the global scale (Jobbágy and Jackson 2000), have a greater amount of mineral-associated or physically-protected organic carbon (Schrumpf et al. 2013), and play a crucial role in the global carbon cycle (Rumpel and Kögel-Knabner 2011). Therefore, in-depth studies of the temperature sensitivity of subsurface soils in general and the mechanistic relationship between the substrate status and the temperature sensitivity of subsoils in particular are needed.
In this study, we aimed to explore (1) how the Q10 of SOC decomposition changes along the soil profile, and (2) how the Q10 of SOC decomposition responds to a saturated labile substrate level (through glucose addition) at different soil depths. We collected soils at four different depths (0–20, 20–40, 40–60, and 60–80 cm) from four ecosystems (farm, redwood forest, ungrazed grassland, and grazed grassland), and measured the temperature sensitivity of SOC decomposition at ambient (without glucose addition) and saturated (with glucose addition) labile substrate availability. We chose glucose because it has been widely demonstrated as a readily available common substrate for most soil microbes (Anderson and Domsch 1978; Cheng et al. 1996). The soil respiration after addition of glucose at or above the saturation level has been called the substrate-induced respiration. Studies (Anderson and Domsch 1978; Lin and Brookes 1999) have shown that substrate-induced respiration remains nearly constant during the initial 4 h period before any microbial growth is noted.
We have two specific hypotheses in this study. First, at ambient substrate condition (without glucose addition), increasing soil depth leads to increasing intensity of physico-chemical protection of SOC and decreasing amount of labile substrate for microbial respiration (Rumpel and Kögel-Knabner 2011; Schrumpf et al. 2013). This increasing substrate limitation causes decreasing apparent temperature sensitivity of SOC decomposition with increasing depth (Gillabel et al. 2010). Second, if the kinetics of soil respiration can be adequately approximated by the Michaelis–Menten equation, Q10 of the substrate-induced respiration should be significantly higher than that of the basal respiration without substrate addition, because the canceling effect of the temperature sensitivity of K m on the temperature sensitivity of soil respiration is negligible when the substrate is saturated and much higher than K m ([C] ≫ K m, Davidson and Janssens 2006; Larionova et al. 2007; Gershenson et al. 2009). Moreover, the change in Q10 after substrate addition is more significant in subsurface soils where organic carbon is more protected and less available for microbial respiration (Rumpel and Kögel-Knabner 2011). In short, we hypothesized that (1) the Q10 of microbial respiration at ambient substrate availability decreases with increasing soil depth, and (2) the Q10 of microbial respiration increases with glucose addition, particularly for subsurface soils.
Materials and methods
Soil sampling and experimental setup
Soil samples were collected from four ecosystems in the Natural Reserve on the campus of University of California, Santa Cruz. Santa Cruz has a Mediterranean climate, with cool wet winters and warm dry summers. The four sites include an organic farm which has been planted with various crops and vegetables (sunflower, strawberry, lettuce, etc.) since its conversion from grassland in 1974, a redwood (Sequoia sempervirens) forest which was logged about 90 years ago and has not experienced fire since then, an ungrazed grassland dominated by non-native annual grasses, and a cow-grazed grassland with more native perennial forbs (Gershenson et al. 2009). We chose these four sites to test whether the depth control of substrate-induced temperature sensitivity of SOC decomposition varies across landuse changes (cropland, forest, ungrazed and grazed grassland). At each site, we collected soils from four randomly chosen locations within 100 m; each of these four locations was considered as a field replicate. At each location, we dug a soil pit and collected soils at four depths (0–20, 20–40, 40–60 and 60–80 cm). All soils were limestone-derived sandy soils in the region, with pH values from 4 to 6 and without detectable carbonates (Gershenson et al. 2009; Zhu and Cheng 2011a). Organic C content ranged from 10.9 to 77.2 mg g−1 dry soil at 0–20 cm layer and gradually dropped to 3.0 to 18.7 mg g−1 dry soil at 60–80 cm layer across the four ecosystems (Table 1).
All soil samples were brought to the laboratory, picked free of roots, hand homogenized, and passed through a 4 mm sieve. Each sample was divided into eight 150 g aliquots; each aliquot was placed in a separate 150 cm3 (5 cm in diameter and 20 cm in length) copper tube, which was covered with white paint to minimize the potential impact of copper on microbial activity (Fig. S1). We closed both ends of the copper tube using synthetic rubber stoppers connected with plastic tubing for air circulation. The stopper at the air inlet end was further connected with a semi-rigid polyethylene tube perforated with many small pin holes. The polyethylene tube was placed at the center of the copper tube for homogenously adding water or glucose solution into the soil sample inside the copper tube before respiration measurement. Separate copper tubes were treated with two labile substrate treatments (Gl+ for added glucose and CK for ambient substrate), and submerged into four temperature-controlled water baths (6, 14, 22 and 30 °C, Fig. S1). The four temperatures are within the natural temperature range experienced by these soils in the region. We added 100 g L−1 glucose solution to Gl+ samples using a 20 ml syringe at a calculated amount (10–15 ml per sample, or 3.69–5.54 mg C g−1 soil) that gave soil water at 100 % holding capacity and saturated carbon substrate concentration (Cheng and Coleman 1989). Deionized water (DI) was added to ambient substrate replicates in the same manner to avoid differences in soil moisture. We took precautions to minimize the disturbance due to soil sampling and processing. In order to best reflect natural differences in substrate availability among soil depths, we (1) gently processed and sieved the fresh soils using 4.0 mm screen which minimally disrupted soil aggregates; (2) carried out respiration measurements within 3 h from the initial collection of the soil samples to minimize the loss of naturally available substrate to respiration, and (3) did one soil pit (or field replicate) of one ecosystem type per day in order to keep soils fresh and finish the above tasks within the required time period. The experiment is a full factorial design, including four ecosystems, four soil depths, four temperatures, two substrate concentrations, and four field replicates.
Soil respiration measurement
We measured soil respiration rate using the methodology described by Cheng and Virginia (1993) and Gershenson et al. (2009) with critical improvement for subsurface soils. The use of the copper tube allows a much higher amount of soil sample than the original method (150 vs. 20 g), and significantly improves the sensitivity of the measurement, especially for subsurface soils with much lower respiration rates. The copper tube also maintains better thermal equilibrium with water bath temperature than does a glass flask, and the perforated polyethylene tube inside the copper tube ensures homogeneous distribution and faster diffusion of substrates through the soil column.
Briefly, an air pump forces ambient air through a soda-lime column, thereby producing CO2-free air. The CO2-free air travels through a copper coil submerged in the water bath to equilibrate air temperature with water bath temperature, and then enters a manifold, from which individual tubes lead to individual sample inflow tubes. The flow of air to each sample is controlled by a needle valve to ensure a constant flow rate (80 ± 5 mL min−1). One of the outlets was connected to an empty copper tube without soil and was used as a blank. Prepared copper tubes with soil samples were allowed to acclimate to water bath temperature for 1 h before respiration measurements occurred. Previous studies (Gershenson et al. 2009; Zhu and Cheng 2011b) showed that 1 h was adequate for respiration of these soils to equilibrate at such temperatures. We measured soil respiration by connecting a LiCOR IRGA 6262 (LiCOR Biosciences, Lincoln, NB, USA) and a mass flow meter to the outflow tube of each sample, and recorded the flow rate and the concentration of CO2 in the sample. Respiration measurement for one sample lasted approximately 2 min, which ensures both the accuracy of each measurement and finishing all measurements during the initial stable phase of the substrate-induced respiration (0–5 h after glucose addition).
Four water baths were set up with temperatures of 6, 14, 22 and 30 °C. Temperature in the 6 and 14 °C were automatically maintained by chilled water (about 3 °C) circulated through heat exchangers made of copper tubing and pumps (Fig. S1). The automatic control was accomplished by monitoring water temperature at 1 min intervals using calibrated thermocouples (Omega thermocouple type-T, Stamford, CT, USA) connected to a datalogger (Campbell Scientific CR10X, Logan, UT, USA) which triggers on–off controls of the two circulating pumps. The chilled water was supplied by an automated water chiller (ADVANTAGE M1-.5A, Greenwood, IN, USA) with a temperature control accuracy of ±0.1 °C. Temperature in the 30 °C water bath was maintained using a processor-controlled temperature regulator (VWR 1122 Polyscience 1122 Heating Circulator, Manasquan, NJ, USA). Temperature in the 22 °C treatment (close to the room temperature) was regulated manually by adding cold or warm water as needed. We also verified the temperature in each water bath by manual measurements every 10 min using a mercury thermometer.
Analysis of soil organic C, total N, extractable organic C, and microbial biomass C
We dried (105 °C and 48 h in an oven) and ground a subsample (20 g) of the soils from each replicate. Ground soil samples were analyzed for C and N % on a Carlo Elba 1108 elemental analyzer. We also measured microbial biomass carbon (MBC) using the chloroform fumigation-extraction method with a slight modification (Vance et al. 1987). In brief, two 50 g subsample of each moist soil were weighed to measure microbial biomass carbon. One subsample was fumigated for 48 h at 22 °C with ethanol-free CHCl3 and the other subsample was kept as unfumigated. These two subsamples were then extracted with 60 ml 0.5 M K2SO4 for 2 h by reciprocating shaker and filtered. Organic C in extracts was determined with a Shimadzu TOC-5050A analyzer equipped with a Shimadzu ASI-5000A autosampler. Microbial biomass C was calculated as follows: microbial biomass C = EC/kEC, where EC is the difference between organic C extracted from fumigated soils and that from non-fumigated soils and kEC = 0.45 (Beck et al. 1997). Admittedly, the extraction efficiency, kEC, might vary to some degree across ecosystems and soil depths. But, accurate determination of each kEC value would require excessive amount of work beyond the focus of this study. Extractable organic carbon in the unfumigated samples was used as an indication of the ambient labile carbon substrate availability.
Calculations and statistical analysis
In order to determine the dry weight of samples, we took a sample of each replicate, dried it at 105 °C for mineral soil samples and 70 °C for the forest organic horizon samples, and determined gravimetric water content. The samples were weighed to nearest 0.01 g before and after drying on an analytical balance. We compared soil properties between different soil horizons within each soil type using one-way ANOVA (analysis of variance) and Tukey’s post hoc test (Table 1).
We used the following equation (Gershenson et al. 2009) to calculate the respiration rate of each soil sample (Table S1):
where R r is soil respiration rate in mg CO2-C g−1 SOC h−1, C is the recorded CO2 concentration in µmol CO2 mol−1, R f is the recorded flow rate in mL h−1, and W s is the gram dry weight of the sample.
We fitted the measured respiration rates for the CK and Gl+ treatments to the van’t Hoff equation (1899):
where α and β are fitted parameters. We determined the Q10 value for each treatment combination using the equation:
we used multi-factor ANOVA to assess the effects of Ecosystem (main factor), Depth (split-plot design), Substrate (repeated-measure), and their interactions on the temperature sensitivity of SOC decomposition (Q10, Table 2). In addition, we used Pearson correlation (Table S2) and multiple linear regression (stepwise, Table S3) to explore the relationships between soil properties and the Q10 at ambient substrate condition (Q10) as well as the change in Q10 with substrate addition (∆Q10) across ecosystems and depths. Curve fitting was performed using Origin 8.0 (Origin Inc.) and statistical analysis was performed using PASW Statistics 18v (SPSS Inc.).
Results
Soil properties among ecosystems and depths
Most of soil properties were significantly different among ecosystems and soil depths (Table 1). Organic C and total N both decreased with soil depth in all four ecosystems, while C:N ratio only declined in the farm soil and did not change with soil depth in the forest and two grassland soils. Extractable organic carbon (EOC, as measured in unfumigated samples) declined slightly in farm and redwood forest soils, but remained stable in two grassland soils with depth. Microbial biomass carbon (MBC) decreased dramatically with soil depth in all four ecosystems. Moreover, the ratio of EOC to SOC showed increasing trend with soil depth (not significant in redwood forest and grazed grassland), while the ratio of MBC to SOC declined progressively with soil depth (not significant in redwood forest).
Soil respiration and Q10 under ambient substrate condition
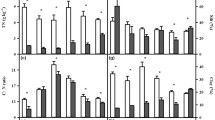
We observed large variations in soil respiration rate among ecosystems and soil depths. Soil respiration rate per unit soil organic carbon (μg CO2-C g−1 C h−1) under ambient substrate condition (CK) at each depth increased exponentially with temperature (Table S1). The temperature sensitivity (Q10) under ambient substrate condition significantly decreased with increasing soil depth (Table 2; Fig. 1). In the farm soil, Q10 was significantly higher in 0–20 cm layer than in both 40–60 cm and 60–80 cm layers (P < 0.05), but was not different between 0–20 cm and 20–40 cm depth (Fig. 1a). In redwood forest soil, Q10 of the Oi organic layer was significantly higher than that of the top 0–20 cm mineral soil (P < 0.001), and Q10 of 0–20 cm layer was significantly higher than that of the following three depths (20–40, 40–60, and 60–80 cm, P < 0.05, Fig. 1b). In the ungrazed grassland soil, the only significant difference in Q10 was between 0–20 and 60–80 cm layers (P < 0.01, Fig. 1c), whereas in the grazed grassland soil, Q10 of the top two depths (0–20 and 20–40 cm) was significantly higher than Q10 of the deeper two layers (40–60 and 60–80 cm, P < 0.001, Fig. 1d).
Difference in Q10 (mean ± SE, n = 4) of soil respiration between ambient substrate (CK) and added substrate (Gl+) treatments in 0–20, 20–40, 40–60 and 60–80 cm soil layers of farm (a), redwood forest (b), ungrazed grassland (c), and grazed grassland (d) ecosystems. Different letters indicate significant (P < 0.05) differences in Q10 at ambient substrate (CK) among different soil layers. Asterisks indicate significant (ns not significant, *P < 0.05, **P < 0.01, ***P < 0.001) differences in Q10 between ambient (CK) and added (Gl+) substrate treatments for each soil layer
Responses of soil respiration and Q10 to labile substrate saturation
Labile substrate saturation strongly affected soil respiration rates. After adding a saturated level of glucose solution (Gl+), soil respiration rate significantly increased, with the magnitude depending on ecosystems and depths (Table S1). Labile substrate saturation also had significant impacts on the temperature sensitivity of soil respiration (Table 2, Fig. 1): it significantly increased Q10 of soil respiration in most soil depths of the four ecosystems (P < 0.001), except for Oi and 0–20 cm layers of the redwood forest soil (Fig. 1b), and 0–20 and 20–40 cm layers of the ungrazed grassland soil (Fig. 1c). In addition, the increase in Q10 after glucose addition was significantly stronger in subsurface soils (20–80 cm) than in surface soils (0–20 cm). For example, adding glucose resulted in 13.2, 11.1, 11.3 and 2.4 % increases in Q10 in the 0–20 cm layer, but 18.7, 9.4, 28.9 and 22.7 % increases in Q10 in the 60–80 cm layer of farm, redwood forest, ungrazed grassland, and grazed grassland soils, respectively (Figs. 1, 2). Within each ecosystem, the glucose-induced Q10 change was not significantly different among the three subsoil layers (20–40, 40–60, and 60–80 cm), but it was significantly higher in all three subsoil layers than in the topsoil (0–20 cm), except for the redwood forest soil (Fig. 2).
Relationships between soil properties and Q10
We used multi-factor ANOVA to assess the effect of ecosystem, depth (split-plot), substrate (repeated-measure), and their interactions on Q10 (Table 2). The effect of depth on Q10 was significant (P < 0.001) and consistent across all ecosystems (no depth by ecosystem interaction, P = 0.627). Additionally, the effect of substrate on Q10 was significant (P < 0.001), but it was stronger at depth than at surface (significant substrate by depth interaction, P < 0.01) and weaker in the forest than in the other ecosystems (significant substrate by ecosystem interaction, P < 0.001). Finally, the insignificant three-way interaction (P = 0.081) indicates that the stronger substrate-induced Q10 at depth (or the substrate-depth interaction on Q10) was observed in all four ecosystems.
In order to explore what soil properties drive the variations of Q10 under ambient substrate condition (Q10) and change in Q10 with substrate addition (∆Q10) across ecosystems and depths, we further carried out Pearson correlation analysis and multiple linear regression (stepwise) analysis. Q10 values at ambient substrate condition of all 64 samples were positively correlated to total N (r = 0.47, P < 0.001) and microbial biomass C (r = 0.51, P < 0.001), indicating that these two soil properties are important in modulating the temperature sensitivity of SOC decomposition (Table 3). ∆Q10 values were negatively correlated with total N (r = −0.41, P < 0.001), microbial biomass C (r = 0.37, P = 0.003), C:N ratio (r = 0.31, P = 0.014), and SOC (r = 0.30, P = 0.017). However, when the interactions between soil variables were accounted for in the stepwise multiple linear regression analysis, only microbial biomass C and SOC explained statistically significant portion of the overall variations of Q10 values under ambient substrate condition (R2 = 0.39, P < 0.001), and only microbial biomass carbon was statistically significant in its contribution to the variations of ∆Q10 (R2 = 0.14, P = 0.003) across ecosystems and soil depths.
Discussion
We compared the temperature responses of SOC decomposition with increasing soil depth in four ecosystems with ambient substrate and adding readily available substrate (glucose) at the saturated level. The temperature sensitivity of microbial respiration (Q10) at ambient substrate decreased with soil depth (Fig. 1). This decreasing trend seemed to be related to total soil N and microbial biomass C since both variables were significantly positively correlated with Q10 values at different depths (Table 3). Moreover, glucose addition increased Q10 at most soil depths, and this substrate-induced change in Q10 was higher in subsurface soils (20–80 cm) than in surface soils (0–20 cm) (Fig. 2). Taken together, our results provide strong experimental evidence for the role of labile substrate availability in controlling the temperature sensitivity of SOC decomposition, particularly for subsurface soils.
Q10 of SOC decomposition at ambient substrate condition decreases with depth
Consistent with our first hypothesis, the temperature sensitivity of SOC decomposition under ambient substrate condition significantly decreased with increasing soil depth in all four ecosystems (Fig. 1). Based on the Arrhenius equation, the more complicated chemical structure of a reactant, the higher activation energy the reaction needs (Arrhenius 1889; Bosatta and Ǻgren 1999). The Arrhenius equation also shows that reactants with higher activation energies (i.e., less reactive and more recalcitrant) should have higher temperature sensitivities (Bosatta and Ǻgren 1999; Davidson and Janssens 2006). In general, with increasing soil depth, organic matter becomes increasingly chemically recalcitrant (Rumpel et al. 2002; Lorenz and Lal 2005; Spielvogel et al. 2008). Therefore, the temperature sensitivity of SOC decomposition should theoretically increase with depth (Lomander et al. 1998; Fierer et al. 2003; Karhu et al. 2010). However, our results did not support this prediction, suggesting mechanisms other than chemical recalcitrance are needed to explain our results.
Results in the published literature point to two possible explanations as to why the temperature sensitivity of SOC decomposition decreases with depth in this study: (1) increase in physical protection of SOC and (2) change in microbial community structure with depth. First, the physical protection of SOC due to aggregation or association with soil minerals tends to increase with depth (Kögel-Knabner et al. 2008; Schrumpf et al. 2013), which may lead to a physical disconnect between microbes and substrates and a lower temperature dependence of microbial respiration in subsoils (Gillabel et al. 2010; Conant et al. 2011). Although physical protection of SOC was not directly measured in this study, MBC and total N contents may indicate the intensity of physical protection of SOC for microbial decomposition. These two variables showed declining trends with depth (Table 1) and positive correlations with Q10, and best explained the variations in Q10 across ecosystems and depths (Table 3). Therefore, the increasing substrate limitation for microbes due to physical protection of SOC may be one of the mechanisms driving the decreasing Q10 at ambient substrate condition with depth. Second, distinct microbial communities at different depths (Eilers et al. 2012; Stone et al. 2014) may have different thermal optima due to physiological adaptations to specific temperature regimes (Stark and Firestone 1996; Balser and Wixon 2009), which may partly account for the different temperature sensitivity of microbial respiration at different depths. Our correlation analysis indicate that microbial biomass C is an important variable in modulating Q10 values at different depths. It is likely that the changes in microbial biomass at different depths are also associated with changes in community composition which may directly contribute to the difference in Q10 values. Considering that these two proposed explanations are solely based on results in the literature. Further studies are needed to pinpoint the specific mechanisms (the physical protection of SOC, microbial community structure, or other factors such as soil moisture or nutrients) responsible for the decrease in Q10 values with depth observed in this study.
Glucose-induced increase in Q10 of SOC decomposition with depth
Soil microbial communities are generally carbon-limited (Cheng et al. 1996; Cleveland et al. 2007). We observed not only an increase in overall respiration rates (Table S1), but also an increase in Q10 values in added substrate treatments (Fig. 1). This result has been reported in previous studies using surface soils (e.g., Gershenson et al. 2009; Zhu and Cheng 2011b). Moreover, the effect of glucose addition on Q10 differed among soil depths (Fig. 2). We observed a larger increase of Q10 values in subsurface soil than that in surface soil following addition of a readily available substrate except in the redwood forest soil (Fig. 2). This result supports our second hypothesis, indicating that the temperature sensitivity of soil microbial respiration at deeper depths is more responsive to changes in labile substrate availability than the topsoil. Based on Michaelis–Menten model of soil respiration, adding a readily available carbon substrate at a saturate concentration should eliminate the canceling effect of K m on the measured Q10 values (Davidson and Janssens 2006), and result in an increase of observed Q10 values. As a response to glucose addition, the larger increases in Q10 values for the subsoil than the topsoil in our study (Figs. 1, 2) indicate that the canceling effect of K m on the measured Q10 value was much stronger for the subsoil than for the topsoil. The mechanism responsible for the lack of difference in the glucose-induced Q10 between topsoil and subsoil in the redwood forest ecosystem is unclear and awaits further investigation.
A number of emprical studies (Larionova et al. 2007; Gershenson et al. 2009; Curiel Yuste et al. 2010; Zhu and Cheng 2011b; Fissore et al. 2013) have identified the control of temperature sensitvitiy of microbial respiration by labile substrate availability (Davidson and Janssens 2006; Davidson et al. 2006). Larionova et al. (2007) showed that the temperature dependence of microbial respiration is dependent on the concentration of readily decomposable carbon (including native soil carbon and added glucose carbon) by adding increasing levels of glucose to two surface soils (forest and cropland). Three following studies (Gershenson et al. 2009; Curiel Yuste et al. 2010; Zhu and Cheng 2011b) found a rise in the temperature dependence of microbial respiration from the control (no glucose addition) to the saturated-glucose-addition treatment for various surface soils (bulk soil, rhizosphere soil, labile substrate-depleted soil) from a number of ecosystems (forest, grassland, cropland). Fissore et al. (2013) further reported lower temperature dependence of SOC decomposition for labile substrate-depleted soils through extended incubation than for non-depleted soils or substrate-depleted soils amended with saturated glucose. All these studies provide strong evidence for the role of labile substrate supply in controlling the temperature sensitivity of microbial respiration in surface soils (top 20 or 30 cm). Our current study moves a step forward by showing that the decomposition of subsurface SOC is even more responsive to labile substrate supply than that of surface SOC. To best refect natural differences in labile substrate availability along the soil profile, we incubated freshly sampled and carefully processed soils in this study. However, any processing of soil, no matter how careful, will tend to release labile carbon, particularly for subsurface soils with higher intensity of physico-chemical protection. Therefore, labile substrate limitation for the temperature sensitivity of SOC decomposition is likely underestimated in this study.
Soil carbon stocks are comprised of pools with turnover times varying from days to decades, centuries and beyond. There has been apparently contradictory results on the relative temperature sensitivity of fast- vs. slow-cycling SOC (reviewed in Conant et al. 2011). For example, two field studies (Trumbore et al. 1996; Fissore et al. 2009) have used 14C methods to show that the turnover of older carbon is less sensitive to warming than kinetic theory would predict (or the intrinsic temperature sensitivity). In contrast, some incubation methods have shown the opposite (Conant et al. 2008; Karhu et al. 2014). Recent syntheses (e.g., Davidson and Janssens 2006; Conant et al. 2011) highlighted that environmental constraints on decomposition can reduce substrate availability and dampen the intrinsic temperature sensitivity of SOC decomposition. This study illustrated the impact of labile substrate availability on the short-term response of microbial respiration to warming, particularly for subsurface soils. Taken together, it has been increasingly recognized that the persistence of SOC in a warmer world is largely determined by processes influencing SOC availability for decomposition (Conant et al. 2011; Schmidt et al. 2011).
Implications for soil carbon dynamics under global change
Results from our study indicate that SOC in subsoils is vulnerable to warming, particularly when labile substrate availability also increases. This vulnerability may stem from the distinctive substrate kinetics associated with deeper soils (i.e., K m of microbial metabolism and its temperature sensitivity). Therefore, any global environmental change (e.g., elevated CO2, land use change) that increases the labile substrate availability of deep soils may result in potential loss of carbon from soils to the atmosphere in a warming world. Our finding is based on the short-term response of microbial respiration to warming with and without labile substrate addition. Ultimate change in soil carbon storage in response to warming in combination with substrate alteration awaits further investigation which may consider whether priming (enhanced native SOC decomposition by substrate-induced microbial activity) occurs and persists (Kuzyakov 2010), how added substrate is stabilized in soil (Fissore et al. 2013), and the temperature dependence of these two processes.
Given the fact that more than half of SOC is stored in subsurface soils globally (Jobbágy and Jackson 2000) and the entire soil profile is likely to experience the similar magnitude of warming (but different diurnal and season regimes of temperature) as the surface soil in this century (Jungqvist et al. 2014), there is an urgent need to investigate the vulnerability of subsurface soil carbon to global environmental change in future experimental and modeling studies (Rumpel and Kögel-Knabner 2011). A number of empirical studies have found contrasting patterns of the temperature sensitivity of SOC decomposition (Q10) along the soil profile (Winkler et al. 1996; Fierer et al. 2003; Rey et al. 2008). This study showed that labile substrate availability may help explain the variation in Q10 along the soil profile. Further in-depth studies on the processes controlling labile substrate availability, as well as the downstream processes such as priming of native SOC and stabilization of added C, could improve our understanding of the long-term fate of soil carbon in a warming world.
Conclusion
This study provided new empirical evidence illustrating the importance of microbial substrate availability in controlling the temperature sensitivity of SOC decomposition along the soil profiles in four contrasting ecosystems. We showed that the Q10 at ambient substrate availability significantly decreased with increasing soil depth. Moreover, for the first time, we found that saturated C substrate addition led to significantly higher Q10 in most soil depths, and the increase in Q10 as a response to substrate addition was larger in deeper soils than in the surface soils. These results clearly indicated that substrate availability plays an important role in controlling the temperature sensitivity of SOC decomposition, especially in subsoils. In order to improve our prediction of the fate of the large carbon stock stored in subsoils in a warming world, future studies need to focus on key mechanisms controlling substrate availability and its response to temperature change.
References
Amundson R (2001) The carbon budget in soils. Annu Rev Earth Planet Sci 29(1):535–562. doi:10.1146/annurev.earth.29.1.535
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Arrhenius S (1889) Über die Reaktionsgeschwindigkeit bei der inversion von Rohzucker durch Säuren. Z Phys Chem 4:226–248
Balser TC, Wixon DL (2009) Investigating biological control over soil carbon temperature sensitivity. Global Change Biol 15(12):2935–2949. doi:10.1111/j.1365-2486.2009.01946.x
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29(7):1023–1032. doi:10.1016/s0038-0717(97)00030-8
Bosatta E, Ǻgren GI (1999) Soil organic matter quality interpreted thermodynamically. Soil Biol Biochem 31(13):1889–1891. doi:10.1016/s0038-0717(99)00105-4
Cheng WX, Coleman DC (1989) A simple method for measuring CO2 in a continuous air-flow system—modifications to the substrate-induced respiration technique. Soil Biol Biochem 21(3):385–388. doi:10.1016/0038-0717(89)90148-x
Cheng W, Virginia RA (1993) Measurement of microbial biomass in arctic tundra soils using fumigation-extraction and substrate-induced respiration procedures. Soil Biol Biochem 25(1):135–141. doi:10.1016/0038-0717(93)90251-6
Cheng W, Zhang Q, Coleman DC, Carroll CR, Hoffman CA (1996) Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol Biochem 28(10–11):1283–1288. doi:10.1016/s0038-0717(96)00138-1
Cleveland C, Nemergut D, Schmidt S, Townsend A (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82(3):229–240. doi:10.1007/s10533-006-9065-z
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, Six J, Steinweg JM (2008) Sensitivity of organic matter decomposition to warming varies with its quality. Global Change Biol 14(4):868–877. doi:10.1111/j.1365-2486.2008.01541.x
Conant RT, Ryan MG, Agren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvönen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Megan Steinweg J, Wallenstein M, Wetterstedt JAM, Bradford MA (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Global Change Biol 17(11):3392–3404. doi:10.1111/j.1365-2486.2011.02496.x
Curiel Yuste J, Ma S, Baldocchi DD (2010) Plant-soil interactions and acclimation to temperature of microbial-mediated soil respiration may affect predictions of soil CO2 efflux. Biogeochemistry 98(1–3):127–138. doi:10.1007/s10533-009-9381-1
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440(7081):165–173. doi:10.1038/nature04514
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Global Change Biol 12(2):154–164. doi:10.1111/j.1365-2486.2005.01065.x
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65. doi:10.1016/j.soilbio.2012.03.011
Fang CM, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433(7021):57–59. doi:10.1038/nature03138
Fierer N, Allen AS, Schimel JP, Holden PA (2003) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Global Change Biol 9(9):1322–1332. doi:10.1046/j.1365-2486.2003.00663.x
Fissore C, Giardina CP, Swanston CW, King GM, Kolka RK (2009) Variable temperature sensitivity of soil organic carbon in North American forests. Global Change Biol 15(9):2295–2310. doi:10.1111/j.1365-2486.2009.01903.x
Fissore C, Giardina CP, Kolka RK (2013) Reduced substrate supply limits the temperature response of soil organic carbon decomposition. Soil Biol Biochem 67:306–311. doi:10.1016/j.soilbio.2013.09.007
Foereid B, Ward DS, Mahowald N, Paterson E, Lehmann J (2014) The sensitivity of carbon turnover in the Community Land Model to modified assumptions about soil processes. Earth Syst Dynam 5(1):211–221. doi:10.5194/esd-5-211-2014
German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis-Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Global Change Biol 18(4):1468–1479. doi:10.1111/j.1365-2486.2011.02615.x
Gershenson A, Bader NE, Cheng W (2009) Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Global Change Biol 15(1):176–183. doi:10.1111/j.1365-2486.2008.01827.x
Gillabel J, Cebrian-Lopez B, Six J, Merckx R (2010) Experimental evidence for the attenuating effect of SOM protection on temperature sensitivity of SOM decomposition. Global Change Biol 16(10):2789–2798. doi:10.1111/j.1365-2486.2009.02132.x
Hassouna M, Massiani C, Dudal Y, Pech N, Theraulaz F (2010) Changes in water extractable organic matter (WEOM) in a calcareous soil under field conditions with time and soil depth. Geoderma 155(1–2):75–85. doi:10.1016/j.geoderma.2009.11.026
Holland EA, Neff JC, Townsend AR, McKeown B (2000) Uncertainties in the temperature sensitivity of decomposition in tropical and subtropical ecosystems: implications for models. Global Biogeochem Cycle 14(4):1137–1151. doi:10.1029/2000GB001264
Jin X, Wang S, Zhou Y (2008) Microbial CO2 production from surface and subsurface soil as affected by temperature, moisture, and nitrogen fertilisation. Aust J Soil Res 46(3):273–280. doi:10.1071/SR07144
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10(2):423–436. doi:10.2307/2641104
Jungqvist G, Oni SK, Teutschbein C, Futter MN (2014) Effect of climate change on soil temperature in Swedish boreal forests. PLoS One. doi:10.1371/journal.pone.0093957
Karhu K, Fritze H, Tuomi M, Vanhala P, Spetz P, Kitunen V, Liski J (2010) Temperature sensitivity of organic matter decomposition in two boreal forest soil profiles. Soil Biol Biochem 42(1):72–82. doi:10.1016/j.soilbio.2009.10.002
Karhu K, Auffret MD, Dungait JAJ, Hopkins DW, Prosser JI, Singh BK, Subke J-A, Wookey PA, Agren GI, Sebastia M-T, Gouriveau F, Bergkvist G, Meir P, Nottingham AT, Salinas N, Hartley IP (2014) Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 513(7516):81–84. doi:10.1038/nature13604
Kirschbaum MUF (2006) The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem 38(9):2510–2518. doi:10.1016/j.soilbio.2006.01.030
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171(1):61–82. doi:10.1002/jpln.200700048
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42(9):1363–1371. doi:10.1016/j.soilbio.2010.04.003
Larionova AA, Yevdokimov IV, Bykhovets SS (2007) Temperature response of soil respiration is dependent on concentration of readily decomposable C. Biogeosciences 4(6):1073–1081. doi:10.5194/bg-4-1073-2007
Leifeld J, Fuhrer J (2005) The temperature response of CO2 production from bulk soils and soil fractions is related to soil organic matter quality. Biogeochemistry 75(3):433–453. doi:10.1007/s10533-005-2237-4
Lin Q, Brookes PC (1999) Comparison of substrate induced respiration, selective inhibition and biovolume measurements of microbial biomass and its community structure in unamended, ryegrass-amended, fumigated and pesticide-treated soils. Soil Biol Biochem 31(14):1999–2014. doi:10.1016/S0038-0717(99)00122-4
Lomander A, Katterer T, Andren O (1998) Carbon dioxide evolution from top- and subsoil as affected by moisture and constant and fluctuating temperature. Soil Biol Biochem 30(14):2017–2022. doi:10.1016/s0038-0717(98)00076-5
Lorenz K, Lal R (2005) The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv Agron 88:35–66. doi:10.1016/s0065-2113(05)88002-2
Luo Y, Zhou X (2007) Soil respiration and the environment. Academic Press, Oxford
MacDonald NW, Randlett DL, Zak DR (1999) Soil warming and carbon loss from a lake states spodosol. Soil Sci Soc Am J 63:211–218
Marx M-C, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol Biochem 37(1):35–48. doi:10.1016/j.soilbio.2004.05.024
Michaelis L, Menten ML (1913) Die kinetik der invertinwirkung. Biochem Zeitung 49:333–369
Rey A, Pegoraro E, Jarvis PG (2008) Carbon mineralization rates at different soil depths across a network of European forest sites (FORCAST). Eur J Soil Sci 59(6):1049–1062. doi:10.1111/j.1365-2389.2008.01065.x
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 338(1–2):143–158. doi:10.1007/s11104-010-0391-5
Rumpel C, Kögel-Knabner I, Bruhn F (2002) Vertical distribution, age, and chemical composition of organic carbon in two forest soils of different pedogenesis. Org Geochem 33(10):1131–1142. doi:10.1016/s0146-6380(02)00088-8
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478(7367):49–56. doi:10.1038/nature10386
Schrumpf M, Kaiser K, Guggenberger G, Persson T, Kogel-Knabner I, Schulze ED (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10(3):1675–1691. doi:10.5194/bg-10-1675-2013
Smith P, Fang C, Dawson JJC, Moncrieff JB (2008) Impact of global warming on soil organic carbon. Adv Agron 97:1–43. doi:10.1016/S0065-2113(07)00001-6
Spielvogel S, Prietzel J, Kogel-Knabner I (2008) Soil organic matter stabilization in acidic forest soils is preferential and soil type-specific. Eur J Soil Sci 59(4):674–692. doi:10.1111/j.1365-2389.2008.01030.x
Stark JM, Firestone MK (1996) Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem 28(10–11):1307–1317. doi:10.1016/s0038-0717(96)00133-2
Stone MM, DeForest JL, Plante AF (2014) Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol Biochem 75:237–247. doi:10.1016/j.soilbio.2014.04.017
Subke JA, Bahn M (2010) On the ‘temperature sensitivity’ of soil respiration: can we use the immeasurable to predict the unknown? Soil Biol Biochem 42(9):1653–1656. doi:10.1016/j.soilbio.2010.05.026
Trumbore S (2000) Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol Appl 10(2):399–411. doi:10.2307/2641102
Trumbore SE, Chadwick OA, Amundson R (1996) Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science 272(5260):393–396. doi:10.1126/science.272.5260.393
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707. doi:10.1016/0038-0717(87)90052-6
van’t Hoff JH (1899) Lectures on theoretical and physical chemistry. E. Arnold, London
von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition-what do we know? Biol Fert Soils 46(1):1–15. doi:10.1007/s00374-009-0413-8
Winkler JP, Cherry RS, Schlesinger WH (1996) The Q10 relationship of microbial respiration in a temperate forest soil. Soil Biol Biochem 28(8):1067–1072. doi:10.1016/0038-0717(96)00076-4
Zhu B, Cheng W (2011a) Constant and diurnally-varying temperature regimes lead to different temperature sensitivities of soil organic carbon decomposition. Soil Biol Biochem 43(4):866–869. doi:10.1016/j.soilbio.2010.12.021
Zhu B, Cheng W (2011b) Rhizosphere priming effect increases the temperature sensitivity of soil organic matter decomposition. Global Change Biol 17(6):2172–2183. doi:10.1111/j.1365-2486.2010.02354.x
Acknowledgments
We thank Johanna Pausch, Amy Concilio, and Lindsey Kelly for laboratory assistance, and anonymous reviewers and associate editors for their insightful comments on earlier versions of our manuscript. This study was supported by grants from the U.S. Department of Energy’s Office of Science through the Midwestern Regional Center of the National Institute for Climatic Change Research at Michigan Technological University (DE-FC02-06ER64158), the U.S. National Science Foundation, Division of Environmental Biology’s Ecosystem Studies Program (DEB-1354098), the Overseas Foundation of the Chinese Academy of Sciences, National Science Foundation of China (31270492), and the Office of 985 Project at Peking University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xueyong Pang and Biao Zhu contributed equally to this work.
Responsible Editor: Marc Kramer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pang, X., Zhu, B., Lü, X. et al. Labile substrate availability controls temperature sensitivity of organic carbon decomposition at different soil depths. Biogeochemistry 126, 85–98 (2015). https://doi.org/10.1007/s10533-015-0141-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0141-0