Abstract
Urbanization has resulted in the extensive burial and channelization of headwater streams, yet little is known about the impacts of stream burial on ecosystem functions critical for reducing downstream nitrogen (N) and carbon (C) exports. In order to characterize the biogeochemical effects of stream burial on N and C, we measured NO3 − uptake (using 15N-NO3 − isotope tracer releases) and gross primary productivity (GPP) and ecosystem respiration (ER) (using whole stream metabolism measurements). Experiments were carried out during four seasons, in three paired buried and open stream reaches, within the Baltimore Ecosystem Study Long-term Ecological Research site. Stream burial increased NO3 − uptake lengths by a factor of 7.5 (p < 0.01) and decreased NO3 − uptake velocity and areal NO3 − uptake rate by factors of 8.2 (p < 0.05) and 9.6 (p < 0.001), respectively. Stream burial decreased GPP by a factor of 11.0 (p < 0.01) and decreased ER by a factor of 5.0 (p < 0.05). From fluorescence Excitation Emissions Matrices analysis, buried streams were found to have significantly altered C quality, showing less labile dissolved organic matter. Furthermore, buried streams had significantly lower transient storage (TS) and water temperatures. Differences in NO3 − uptake, GPP, and ER in buried streams, were primarily explained by decreased TS, light availability, and C quality, respectively. At the watershed scale, we estimate that stream burial decreases NO3 − uptake by 39 % and C production by 194 %. Overall, our results suggest that stream burial significantly impacts NO3 − uptake, stream metabolism, and the quality of organic C exported from watersheds. Given the large impacts of stream burial on stream ecosystem processes, daylighting or de-channelization of streams, through hydrologic floodplain reconnection, may have the potential to alter ecosystem functions in urban watersheds, when used appropriately.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urbanization has degraded water quality and altered global biogeochemical cycles (Grimm et al. 2005; Walsh et al. 2005). One common symptom of urbanization is the extensive channelization and burial of stream networks, where much of the urban drainage network flows through storm drains and tunnels under developed landscapes (Leopold 1968; Elmore and Kaushal 2008). While the degradation of urban streams has been well documented (Paul and Meyer 2001; Walsh et al. 2005), the often overlooked effect of engineered headwaters such as culverts and storm drains on ecosystem functions may play an important role at broader watershed scales (Meyer et al. 2005; Wild et al. 2011; Kaushal and Belt 2012). Of particular concern are the unknown impacts of stream burial on nitrogen (N) uptake and carbon (C) metabolism and subsequent potential to influence watershed export of N and C to downstream ecosystems (Boesch et al. 2001; Diaz 2001; Walsh et al. 2005). Here, we compare NO3 − uptake and stream metabolism in urbanized open and buried stream reaches to test the hypothesis that stream burial reduces NO3 − uptake and stream metabolism, and therefore increases N and C export from urban watersheds.
Previous work in forested, agricultural, and urban watersheds has shown that N uptake and C processing can be considerable in headwater streams (Peterson et al. 2001; Mulholland et al. 2004; Alexander et al. 2007). Consequently, burial of headwater streams may have a disproportionally large impact on N and C uptake and retention along urban watersheds. We expected that stream burial may decrease hydrologic residence times, hydrologic connectivity, transient storage (TS), and riparian-stream interactions, thereby reducing conditions conducive to N retention (e.g. Valett et al. 1996; Hall et al. 2002; Ensign and Doyle 2005; Bukaveckas 2007; Kaushal et al. 2008b; Klocker et al. 2009). Given that light also controls ecosystem productivity (Mulholland et al. 2001, 2006), decreased light availability as a result of stream burial may also impact stream metabolism and nitrogen uptake. Additionally, decreased light may impact organic matter quantity/quality (i.e. lability), which influences denitrification, food web dynamics, and other ecosystem processes (Burford and Bremner 1975; Groffman et al. 2005; Huguet et al. 2009; Petrone et al. 2011).
Elucidating the impacts of stream burial has implications for improving stream restoration strategies. If stream burial reduces ecosystem functions related to enhancing water quality, then restoration by “daylighting” currently buried streams (Buchholz and Younos 2007) and hydrologically re-connecting streams placed in concrete-lined channels with adjacent floodplains (Sivirichi et al. 2011) may be an effective N management approach, when used appropriately. While some studies have addressed the benefits of stream restoration or stream daylighting (e.g. Buchholz and Younos 2007; Bukaveckas 2007; Kaushal et al. 2008b; Filoso and Palmer 2011; Wild et al. 2011), none have specifically looked at the impact of stream burial on nutrient dynamics. Because channelization and stream burial are pervasive throughout urban areas, understanding the effects of burial on N and C retention capacity can help provide realistic expectations for stream restoration.
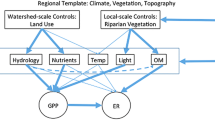
The objectives of this study were to (1) determine the effects of stream burial on NO3 − uptake and metabolism along an urban stream network, (2) identify the mechanisms responsible for the effects of burial on nutrient processing, including hydrologic residence time, reduced light availability, TS, temperature, and carbon quality, and (3) to estimate the potential impact of stream burial on N and C uptake and production, respectively, at the larger watershed scale due to decreased in-stream retention. We hypothesized that buried stream reaches will show significantly reduced NO3 − uptake due to decreased primary production, hydrologic residence time, and TS and reduced stream gross primary production and ER due to reduced light availability and carbon quality. Our work was conducted at the Baltimore Long-term Ecological Research (LTER) site and builds on background research regarding the sources, transport, and transformation of nitrogen in the LTER (Groffman et al. 2004; Kaushal et al. 2008a, 2011), and the influence of stream hydrology, organic carbon availability and geomorphology on denitrification and N uptake (Groffman et al. 2002; Kaushal et al. 2008b; Klocker et al. 2009; Mayer et al. 2010; Newcomer et al. 2012).
Methods
Experimental design
Nitrate uptake rates, whole stream metabolism, and potential explanatory variables including light availability, nutrient concentrations, carbon quality, TS, and hydrology were measured in three headwater streams, each with a paired buried and open reach, within the urbanized Dead Run watershed of the Baltimore LTER site. Each stream contained one buried and one contiguous “open” reach (Fig. 1). The effects of stream burial on metabolism and NO3 − uptake were determined by comparing rates in open and buried reaches of the same stream, which controlled for differences in nitrate concentration between streams that may have otherwise obscured the effect of burial. Nitrate uptake rates and ecosystem metabolism rates were measured once each season for 1 year, using 15N-NO3 − tracer releases and data sonde deployments to measure dissolved oxygen (DO) and temperature. All measurements were made during baseflow conditions.
Locations of the buried and open reaches where nitrate uptake and metabolism measurements where carried out within the DR1, DR2, and DR5 tributaries of the Dead Run watershed. The bold white bars mark the location of open reaches and bold black bars mark the location of buried reaches. The thin black lines represent the buried sections of the stream network (the path of storm drains) and the thicker grey lines represent streams. The two larger scale maps represent the location of the Dead Run watershed within the Gwynn’s Falls watershed (with drainage area in grey) in the Baltimore, MD region and the location of the Baltimore Ecosystem Study within the Chesapeake Bay watershed (with drainage area in grey)
Site description
The Dead Run watershed is a subwatershed of the Gwynn’s Falls and part of the Baltimore Ecosystem Study LTER site. Dead Run is drained by 31 % impervious surface cover, has six USGS stream gauging stations along its stream network, and has had weekly stream chemistry sampling at its mouth since 1998 (Groffman et al. 2004; Kaushal et al. 2008a). The Dead Run watershed has been the focus of numerous eco-hydrological investigations and there exists a rich dataset on nitrogen dynamics (e.g. Groffman et al. 2004; Kaushal et al. 2008a, 2011; Newcomer et al. 2012), hydrologic characteristics, and water mass balances (Klocker et al. 2009; Ryan et al. 2010, 2011; Sivirichi et al. 2011).
Paired open and buried NO3 − uptake and metabolism measurements were conducted in three headwater tributaries of the Dead Run watershed: DR1, DR2, and DR5 (Fig. 1). Each tributary has a USGS stream gauge and was characterized as a first or second order stream, with the buried reach flowing through large cement or steel culverts ranging from 74 to 111 m in length and 3.0 to 3.7 m in width and height. In all three streams the buried reach was downstream of the open reach. The reach length for each open stream site was chosen based on water velocity to provide between 30 and 60 min of travel time from the top to the bottom of the stream reach, to ensure that a detectable amount of 15N-NO3 − was removed from the water column. Due to seasonal changes in stream discharge, the open reach lengths were different each season. The reach lengths for the buried stream sites, however, were the same as the culvert length. DR1 (USGS Gage 01589317) had an open reach that ranged from 76 to 86 m, depending on water velocity and travel-time, and a buried reach that flowed through a 74.1 m long, 3.7 m wide, and 2.4 m high box culvert. DR2 (USGS Gage 01589316) had an open reach that ranged 41.8–73.3 m in length and a cylindrical culvert, 111 m long and 3.0 m in diameter. DR5 (USGS Gage 01589312) had an open reach that ranged from 36 to 224 m and a downstream box culvert 110 m long, 3 m wide, and 1.8 m high. Buried reaches of DR1 and DR2 were clear of sediment, while DR5 had sediment accumulation throughout most of the culvert.
15N-NO3 − tracer experiments
Four seasonal 15N-labeled NO3 − tracer experiments were carried out in each of the 3 buried and 3 open stream reaches, comprising a total of 24 injections (two reaches in three streams across four seasons). The general design for these experiments entailed the release of a solution of 15N-NO3 − tracer at a constant rate into a stream reach and making measurements downstream to determine how rapidly the tracer is removed from the water column. A solution of 99.99 at.% potassium 15N-nitrate (K15NO3 −, reactive tracer) and potassium bromide (KBr, conservative tracer) was prepared and then continuously dripped into each stream reach (at a pump rate of 20 mL per minute) for the duration of the injection experiment. Enough 15N-NO3 − and bromide was added to the stream reaches to enrich the δ15N of nitrate to ~5000 ‰ and increase Br− by 500 μg L−1 [similar to LINX (Lotic Interstate Nitrogen eXperiment) II protocols, Mulholland et al. 2004, 2008; Hall et al. 2009]. Additionally, rhodamine dye was added to the stream reach at a constant rate to a target enrichment of 20 μg L−1. Rhodamine and Br− were used as conservative tracers to account for any changes in discharge between the top and bottom of the experimental reach (Stream Solute Workshop 1990; Hall et al. 2009). Tracers were injected until rhodamine was observed to be at plateau concentrations for 2–4 h. The tracer was added approximately 10–30 m above the top of the reach to ensure that it was completely mixed into the stream water before reaching the top sampling station. Mixing was verified with in situ rhodamine measurements. During each tracer injection, water samples were collected from two stations: at the top (station 1) and bottom (station 2) of each reach before the tracer was injected into the stream (for background samples) and after the level of tracer in the stream reached plateau for 2–4 h (similar to Hanafi et al. 2007). Three replicate samples were taken for each background sampling and 5 replicate samples were taken for each plateau sampling.
The δ15N-NO3 − samples were filtered (0.45 μm), frozen, and shipped to the Colorado Plateau Stable Isotope Facility (CPSIF) for analysis within 1 month. The isotopic composition of nitrate was measured following the denitrifier method (Sigman et al. 2001; Casciotti et al. 2002). Briefly, denitrifying bacteria were used to convert nitrate in samples to N2O, which was analyzed on a mass spectrometer for determination of 15N/14N ratios. Values for 15N-NO3 − are reported as per mil (‰) relative to atmospheric N2 according to δ15N(‰) = [(R)sample/(R)standard − 1] × 1,000, where R denotes the ratio of the heavy to light isotope (15N/14N). Sample replicates for 15N-NO3 − had a mean coefficient of variation of 1.0 ± 0.3 and 0.62 ± 0.3 % for the open and buried reach samples (N = 17 and 11), respectively.
The three NO3 − uptake metrics: (1) uptake length (S W), the distance a NO3 − molecule travels downstream before being removed from water column; (2) uptake velocity (v f), the rate nitrate molecules move vertically through the water column toward the benthos; and (3) areal uptake (U), mass of N removed per area per time, were calculated for each tracer injection experiment (Stream Solute Workshop 1990; Hall et al. 2009). To calculate these NO3 − uptake metrics, the first order nitrate uptake rate constant, k, for each tracer injection was calculated from the slope of the line relating the log of the tracer flux and the distance between sampling stations (Stream Solute Workshop 1990; Hall et al. 2009). The 15N-NO3 − flux at each station was calculated as the product of the average 15N mole fraction, NO3 − concentration, and discharge for the plateau samples minus the background samples. Discharge was calculated at each station using one of the conservative tracers (bromide or rhodamine) and NO3 − uptake length S W (m) was calculated as the inverse of k (Stream Solute Workshop 1990; Hall et al. 2009). Nitrate uptake velocity, v f (mm h−1), which accounts for the effect of discharge and stream depth, was calculated as v f = Q/(S W × w), where w is stream wetted width; NO3 − uptake velocity is considered a measure of biological demand for nitrate relative to its concentration (Stream Solute Workshop 1990; Hall et al. 2009). Areal NO3 − uptake (U, μg N m−2 s−1) was calculated as 15N-NO3 − flux/(S W × w) (Stream Solute Workshop 1990; Hall et al. 2009).
We calculated a minimum detection limit (MDL) for k for two of the buried stream tracer injections (DR1 summer and fall) where the decline in 15N-NO3 − flux across the reach was below our detection limit. The MDL NO3 − uptake value was calculated by sequentially reducing the measured 15N-NO3 − flux values at the bottom station by 0.1 % until the 15N-NO3 − flux at station 2 was lower than that of station 1. This value for the slope, k, was used for calculating the NO3 − uptake metrics as described above. MDL values for these 2 buried stream sites were considered conservative estimates because the true NO3 − uptake rate could not be higher than the MDL value, otherwise we would have detected a measureable decrease in 15N-NO3 − flux across the reach. These MDL estimates therefore overestimate the NO3 − uptake rate in these buried reaches and underestimate the degree to which stream burial reduces NO3 − uptake. Additionally, the NO3 − uptake results for one buried stream tracer injection (DR5 winter) had to be discarded due to improper mixing of 15N-NO3 − injectate solution at station 1. Therefore 11 paired comparisons of NO3 − uptake metrics and 23 total data points were used for analyses involving the NO3 − uptake metrics.
Whole stream metabolism and PAR measurements
Whole stream metabolism, a measure of gross primary production (GPP) and ER within each stream reach, was estimated using a ~36 h record of DO and temperature during the date of the tracer injection. The rates of GPP and ER were determined by the change in the upstream and downstream diurnal DO concentration using the two-station approach, with a DO sonde at the top and bottom of each open and buried reach (Marzolf et al. 1994; with modifications as suggested by Young and Huryn 1998). We directly measured the gas exchange rate using SF6 tracer releases, with 5 replicate gas samples taken at the top and bottom of the study reaches (Marzolf et al. 1994; Mulholland et al. 2001, 2005). Due to a DO sonde malfunction for DR5 buried in the fall and errors in the measured gas-exchange rate for DR5 buried, in winter and spring, the GPP and ER estimates from these three sampling periods were not included in analyses.
We also measured photosynthetically active radiation (PAR, μmol m−2 day−1) using a light sensor logging system (Odyssey, New Zealand). Light meters were deployed in the center of the buried and open reaches for the duration of each experiment. Due to instrument malfunctions, no PAR data was collected during the fall, for DR1 during the summer, or for DR1 and DR2 during the winter.
Transient storage measurements
Transient storage was estimated for each stream reach using rhodamine dye. TS is a measure of the amount of time stream water is temporarily stored in components of the stream that can increase residence time (e.g. pools, backwaters, sediments, hyporheic zone) (D’Angelo et al. 1993; Bukaveckas 2007). Greater TS promotes nutrient removal through greater contact time of nutrients with benthos and greater time for biogeochemical processing (Mulholland et al. 1997; Bukaveckas 2007).
During the 15N-NO3 − tracer injection, a solution of rhodamine was dripped into the stream at 20 mL min−1 to achieve a target stream concentration of 20 μg L−1 rhodamine. The rhodamine concentration in the stream was monitored and recorded at the bottom of the reach using a data sonde equipped with a fluorescence meter (YSI, Yellow Spring, OH, USA). The rising limb of the rhodamine plateau curve was used to calculate TS metrics using OTIS-P, a modified version of the USGS One-Dimensional Transport with Inflow and Storage (OTIS) model (Runkel 1998). The two TS metrics used in this study were F 200med (the proportion of median travel time due to TS, reported as a percentage) and As/A (the proportion of total cross sectional stream area (A) that is TS (As), reported as a percentage).
Hydrologic measurements
Stream velocity was calculated by dividing the length of the reach by the time-to-maximum-slope estimated from the rising limb of the rhodamine plateau curve at the bottom of the reach. Stream width was estimated from averaging 30 evenly spaced measurements of the wetted width taken longitudinally along the stream reach. Effective stream depth was calculated by dividing discharge by stream velocity and stream width. Specific discharge (Q/w) was calculated by dividing the average reach discharge by the average stream reach width. In addition, discharge was measured continuously at four USGS gauging stations: at DR1 (USGS Gage 01589317), DR2 (USGS Gage 01589316), DR5 (USGS Gage 01589312), and near the mouth of the entire Dead Run watershed (USGS Gage 01589330).
Stream chemistry and annual load estimates
Water samples were collected prior to each experiment and analyzed for total Kjeldahl nitrogen (TKN), nitrate + nitrite (NO3 − + NO2 −), bromide (Br−), total phosphorus (TP), orthophosphate (o-P), total organic carbon (TOC), dissolved organic carbon (DOC), and carbon quality characterization via fluorescence spectroscopy (described further below). Samples that required filtration were filtered in the field by passing stream water through a 47 mm filter housing containing a 0.45 µm mixed cellulose ester membrane filter. Water samples were collected during each 15N tracer experiment and bi-weekly for a year at all sites, spanning the dates of the injection experiments to monitor temporal patterns in ambient nutrient and carbon concentrations. All stream chemistry and SF6 samples were shipped to the US EPA, National Risk Management Research Lab in Ada, OK, USA for analysis following standard methods. TKN, NH4 +, NO3 − + NO2 −, o-P and Br− were measured using Lachat flow injection analyses (Lachat Instruments, Loveland, CO USA). Total organic nitrogen (TON) was calculated as TKN of unfiltered water minus ammonium. Dissolved organic nitrogen (DON) was calculated as TKN of filtered water minus ammonium (with TKN being the sum of organic nitrogen plus ammonia/ammonium). Total nitrogen (TN) was calculated as TKN plus NO3 − + NO2 −. TOC and DOC were analyzed using a total organic C analyzer with high-temperature Pt-catalyzed combustion and NDIR detection (Shimadzu TOC-VCPH, Columbia, MD, USA). Samples for carbon quality analysis were analyzed on a Fluoromax-4 spectrofluorometer (Jobin–Yvon Horiba) at the University of Maryland Biogeochemistry Lab. The bi-weekly concentration data, mean daily discharge, and the USGS program LOADEST (Runkel et al. 2004) was used to calculate the annual loads of nitrogen and carbon from the Dead Run watershed and the DR1, DR2, and DR5 subwatersheds.
DOC quality characterization
Sources and lability of dissolved organic matter (DOM) were characterized using fluorescence excitation emission matrices (EEMs) (Coble et al. 1990; Coble 1996; Cory et al. 2010), which can be used to produce indices for distinguishing humic-like, fulvic-like, and protein-like DOM. Water samples were analyzed on a spectrofluorometer with an excitation range of 240–450 nm at a 10 nm increment, and an emission range of 290–600 nm with a 2 nm increment. Fluorescence EEMs were instrument corrected, blank subtracted, and normalized by the water Raman signal following Cory et al. (2010), however the standard inner-filter corrections were not done on samples because no absorbance measurements were attained.
We analyzed fluorescence EEMs for the following indices: the fluorescence index, FI (McKnight et al. 2001), the humification index, HIX (Zsolnay et al. 1999; Huguet et al. 2009), the biological freshness index, BIX (Huguet et al. 2009), and the protein to humic fluorescence intensities ratio (Coble 1996; Stolpe et al. 2010). The FI, which is the ratio of the fluorescence intensity at 450 nm/400 nm when excitation is 370, was used to distinguish aquatic and microbial DOM sources. FI values are ~ 1.9 for microbial derived fulvic acids and ~ 1.4 for terrestrial derived fulvic acids sources (McKnight et al. 2001; Cory et al. 2010). BIX was estimated as the ratio of fluorescence intensity at emission wavelength 380 nm/430 nm for excitation wavelength 310 nm (Huguet et al. 2009). BIX values of < 0.7, 0.8 – 1.0, or > 1.0 are associated with terrestrial sourced DOM, algal sourced DOM, or aquatic bacterial sources, respectively (Huguet et al. 2009). HIX was used to distinguish the humic or autochthonous nature of the organic matter in the sample (Zsolnay et al. 1999; Ohno 2002). HIX is the ratio of the integrated fluorescence intensity within the emission range of 300–345 nm divided by the integrated fluorescence intensity within the emission range 435–480 nm, at excitation 254 nm. Higher HIX values suggest DOM of strong humic character and terrestrial origin, while lower values indicate weaker humic character and higher autochthonous sourced DOM. Fluorescence EEM peak intensities at specific excitation and emission wavelengths were also used to determine the relative contribution of protein (at excitation 275 nm and emission 340 nm) and humic (at excitation 350 nm and emission 480 nm) DOM (Coble 1996; Stolpe et al. 2010) and then used to calculate the protein to humic (P/H) organic matter ratio in each sample.
Statistical analyses
The software R was used for all statistical analyses (R Development Core Team 2011). We used paired t tests to test for differences in NO3 − uptake rates (S W, v f, and U), metabolism metrics (GPP and ER), and explanatory variables (hydrology, stream chemistry, TS, temperature, carbon quality, and stream chemistry variables) between paired open and buried reaches. The paired t tests controlled for differences between stream sites and for seasonal variability that would otherwise obscure the effect of burial. Paired t tests in this study were based on the calculated difference between each paired open and buried reach; as a result, the paired t tests detected significant differences between open and buried reaches even when the overall mean values, plus or minus the standard errors overlapped (due to site and seasonal variability). Similarly, we calculated the magnitude of the effect of stream burial on NO3 − uptake, metabolism and other metrics by finding the paired ratio of the buried reach value to open reach at each site and then averaging the ratios for all sites. We used stepwise multiple linear regression to test for significant relationships between NO3 − uptake or metabolism with their potential explanatory variables. First, we used Pearson’s correlation analysis to check for collinearity among explanatory variables; when two or more variables showed a correlation coefficient above 0.5, we removed all but one of these variables from the model (Booth et al. 1994). We included interactions between reach (e.g. buried or open) and explanatory variables in the initial model to test whether the effect of an explanatory variable on uptake or metabolism differed between the open versus buried reaches. All non-significant interactions were removed from the model first, followed by all non-significant main effects until only significant interactions or main-effects remained in the linear model. To meet the assumption of normal distribution and homogeneous model residuals, we log transformed all dependent variables [NO3 − uptake length, uptake velocity, areal NO3 − uptake, gross primary productivity, and ecosystem respiration and checked the normality of the residuals for each model using the Shapiro–Wilk test (Royston 1982) and the normal Q–Q plot. Once the best model was selected, we calculated the coefficient of partial determination (partial R 2) for each predictor variable left in the model, by partitioning the sums of squares, to estimate the contribution of each predictor variable to the total variance explained by the model. We also tested for the effect of season or stream on NO3 − uptake and metabolism using a 2-way analysis of variance (ANOVA).
Results
Effects of stream burial on hydrology and temperature
Discharge did not differ between open and buried stream reaches (p = 0.24). Similarly, the change in discharge from the top to the bottom of each experimental reach (as measured by a change in conservative tracer concentration) did not differ between open and buried stream reaches (p = 0.9), indicating that on average there was similar groundwater-surface water exchange in both buried and open reaches. For the 3 stream sites, discharge was the lowest during the summer (mean = 2.2 ± 1.4 L s−1), ranging from 0.5 to 3.6 L s−1, and highest in the winter (mean 7.3 ± 3.1 L s−1) ranging from 1.4 to 13.9 L s−1 (Table 1). Stream water velocity (mean 1.5 ± 0.5 m min−1 for open and 4.9 ± 0.6 m min−1 for buried) was a factor of 3.4 ± 0.5 higher in all 3 buried reaches than open reaches, during all 4 seasons (Fig. 2; Table 1, p < 0.001). Stream depth was greater (p < 0.001) for the open reaches than buried (mean 9 ± 1 cm for open reaches and 3 ± 0.2 cm for buried reaches) (Table 1). Stream width for buried and open reaches did not differ (1.7 ± 0.1 m for open reaches and 2.2 ± 0.4 m for buried reaches, p = 0.14) (Table 1). Reach lengths for the buried sites varied only by a meter or less each season, but the open reach lengths were sometimes more variable, depending on the discharge, due to the requirement of having reach travel time be between 30 and 60 min in order to detect NO3 − decline (see Table 1). Water temperature in buried streams was lower than in open reaches by an average of 1.9 ± 0.72 °C (p < 0.05) (Table 2).
Effect of stream burial on water chemistry
Stream water nutrient concentrations did not differ between paired open and buried reaches during the nutrient injection experiments, with the exception of DOC, which were higher in the buried reaches (p < 0.05), with a mean of 2.5 ± 0.3 mg L−1 for the open reaches and 3.6 ± 0.5 mg L−1 for buried reaches (Table 2). The overall mean NO3 −-N concentrations were 1.6 ± 0.2 and 1.4 ± 0.2 mg L−1 for the open and buried reaches, respectively (Table 2).
Bi-weekly concentrations for nitrate varied throughout the year at all three sites, ranging from about 0.5 to 2.3 mg NO3 −-N L−1, with DR2 having the lowest concentrations and both DR1 and DR5 showing similar concentrations (Fig. 3a). DOC concentrations were less variable, though elevated concentrations were observed following storms (Fig. 3b). DR2 generally had the highest biweekly DOC concentration and DR5 generally had the lowest concentration (Fig. 3b). Discharge was similar at all three sites, with DR2 generally having the lowest discharge (Fig. 3c). Annual export of total nitrogen was greatest at DR1 and lowest at DR2 (Table 3). DR1 showed the greatest DOC export, while DR2 showed the lowest (Table 3).
Effects of stream burial on DOC quality
Results from the fluorescence EEMs analysis and paired t tests also indicated that the buried and open sites have statistically different carbon quality types. The BIX was greater (p < 0.05) in the open reaches (0.65 ± 0.09) than the buried reaches (0.63 ± 0.09), with a mean paired difference between open and buried sites of 0.017 ± 0.007 (Fig. 4). The FI was also higher in the open reaches than buried reaches (1.08 ± 0.12, 1.04 ± 0.12, respectively, p < 0.05), with a mean paired difference between open and buried sites of 0.035 ± 0.01 (Fig. 4). The HIX was marginally elevated in the buried reaches compared to open reaches (p = 0.06, mean paired difference 0.014 ± 0.007). The protein to humic-like organic matter (P/H) ratio showed the largest differences observed between open and buried reaches, but paired differences were not significant, in part due to an outlier value (p = 0.19, mean paired difference 0.057 ± 0.04) (Fig. 4). The effect of season on differences in all carbon quality indices was significant (p < 0.05), with greater humic-like and terrestrial sourced DOM found in both open and buried streams during the fall.
Strip plot comparison of the difference between each paired open and buried reach carbon quality index (N = 12). HIX humification index, BIX biological freshness index, FI fluorescence index, and P/H the protein/humic fluorescence intensity ratio. Points above the line (positive values) indicate open > buried, while points below the line (negative values) indicate open < buried. Note one negative outlying point in P/H ratio
Stream burial effects on TS
On average, stream burial decreased TS (as measured by Fmed 200) by a factor of 3.3 (p < 0.01) (Fig. 5a). Mean F 200med was 20 ± 2 % for open stream reaches and 7.8 ± 1.3 % for buried stream reaches. TS size (measured as As/A) was reduced by stream burial by a factor of 2.5 (p < 0.05), with a mean of 28 ± 3 % for open reaches and 15 ± 2 % for buried stream reaches (Fig. 5b).
Stream burial effects on nitrate uptake rates
Across all sites and seasons, stream burial consistently increased NO3 − uptake length and decreased NO3 − uptake velocity, and areal NO3 − uptake rate (Figs. 6, 7; Table 4). On average, stream burial increased NO3 − uptake length (S W), by a factor of 7.5 (p < 0.01) (Figs. 6a, 7a; Table 4), with a mean NO3 − uptake length for all open reach sites of 1,104 ± 298 m (range: 83 m to 2.9 km) and mean buried reach sites of 3,975 ± 853 m (range: 421 m – 8.7 km). Stream burial decreased uptake velocity (v f) by a factor of 8.2 (p < 0.01) (Figs. 6b, 7b; Table 4), with mean NO3 − uptake velocity of 15.2 ± 2.9 mm h−1 (range: 4.97–37.2 mm h−1) for open reaches and 3.6 ± 1.4 mm h−1 (range: 0.44–16.8 mm h−1) for buried reaches. On average, stream burial decreased areal NO3 − uptake rate (U) by a factor of 9.6 (p < 0.001) (Figs. 6c, 7c; Table 4). Mean areal NO3 − uptake rate was 5.0 ± 0.7 μg N m2 s−1 (range: 2.6–9.7 μg N m2 s−1) for open reaches and 1.1 ± 0.4 μg N m2 s−1 (range: 0.15–4.1 μg N m2 s−1) for buried reaches. There were no significant seasonal trends in NO3 − uptake length, uptake velocity, or areal uptake (p = 0.73, 0.90, and 0.44, respectively). Nitrate uptake length, however, varied among the three stream sites (p < 0.01), but there was no significant effect of stream sites on uptake velocity or areal NO3 − uptake (p = 0.06, 0.47, respectively).
Stream burial effects on stream metabolism
Both GPP and ER were reduced within buried stream reaches compared to the open reaches, and there was consistently no measureable PAR in the middle of the buried reaches. On average, burial reduced GPP by a factor of 11.0 (p < 0.01) (Fig. 8a, c; Table 4). Mean GPP was 3.5 ± 0.86 g O2 m−2 day−1 (range: 0.57–9.9 g O2 m−2 day−1) and 0.36 ± 0.08 g O2 m−2 day−1 (range: 0.07–0.92 g O2 m−2 day−1) in the open and buried stream reaches, respectively (Fig. 8a, c; Table 4). On average, stream burial reduced ER by a factor of 5.0 (p < 0.05) (Fig. 8b, d). Mean ER was 4.2 ± 0.71 g O2 m−2 day−1 (range: 0.93–9.8 g O2 m−2 day−1) and 1.8 ± 0.44 g O2 m−2 day−1 (range: 0.14–5.2 g O2 m−2 day−1) in the open and buried stream reaches, respectively (Fig. 8b, d; Table 4). There was no effect of season on differences in gross primary production and ecosystem respiration (p > 0.1) (Fig. 8a, b). However, there was an effect of stream site on differences in GPP (p < 0.01), but not on ER (p > 0.1).
Effects of stream burial on N uptake: potential mechanisms
Based on stepwise multi-linear regression (MLR) analysis, log transformed NO3 − uptake length (S W) was positively related to NO3 − concentration (R 2 = 0.46, p < 0.001) and TS (As/A) (R 2 = 0.23, p < 0.01), with the overall model explaining 69 % of variation in log S W (p < 0.001). Neither stream velocity nor specific discharge (Q/w) were retained in the MLR model; however S W was positively related to stream velocity when using single linear regression (R 2 = 0.22 p < 0.05), while specific discharge was not (p = 0.08). Log transformed NO3 − uptake velocity (v f ) was positively related to TS (As/A) (R 2 = 0.22, p < 0.01), GPP (R 2 = 0.16, p < 0.05), and DOC (R 2 = 0.13, p = 0.052), with the overall model explaining 51 % of variation in log v f (p < 0.01). Log transformed areal NO3 − uptake rate (U) was also positively related to TS (As/A) (R 2 = 0.38, p < 0.001).
Effects of stream burial on ecosystem metabolism: potential mechanisms
Log transformed GPP was positively related to PAR (R 2 = 0.61 p < 0.001). Log transformed ER was negatively related to HIX (R 2 = 0.30 p = 0.03), positively related to TS, As/A (R 2 = 0.13, p = 0.053), and negatively related to NO3 − concentration (R 2 = 0.12, p = 0.051), with the overall model explaining 55 % of variation in ER (p < 0.01).
Discussion
We found that stream burial significantly reduces both NO3 − uptake and whole stream metabolism, while increasing carbon concentrations and decreasing carbon quality/lability. The dominant control on N uptake metrics was TS, with GPP and specific discharge also affecting N uptake. The dominant control on stream GPP was light availability, while stream ER was primarily controlled by TS and carbon quality. This study in the Chesapeake Bay watershed, along with a paired study in Cincinnati, OH, USA demonstrate that stream burial significantly alters stream ecosystem function relevant to N and C cycling (Beaulieu et al. in review). Additionally, although our measurements were made at the scale of stream reaches (i.e. ~ 100 m), the cumulative impact of extensive burial in the headwaters of a stream network may contribute to increased watershed scale NO3 − fluxes to downstream ecosystems (discussed further below). Given that urbanization is increasing globally (Grimm et al. 2008), and that stream burial and channelization are extensive in urbanizing landscapes (Leopold 1968; Elmore and Kaushal 2008), buried streams should be explicitly considered as part of the stream network and their potential impacts on watershed scale biogeochemical processes should be further examined (Kaushal and Belt 2012).
Nitrate uptake: water velocity and NO3 − concentration are poor predictors
While burial did not affect nitrate concentrations, some of the variation in NO3 − uptake was explained by nitrate concentration, as found in previous studies. For example, nitrate concentration was positively related to uptake length, similar to Hall et al. (2009) and negatively related to uptake velocity (Fig. 9), consistent with the LINX II study (Mulholland et al. 2008) and others which reported reduced N uptake efficiency at higher concentrations in urban streams (e.g. Dodds et al. 2002; Earl et al. 2006; O’Brien et al. 2007), though the buried reaches show some of the lowest v f values reported (Fig. 9). Despite these relationships, because our experiment was designed to minimize differences in nitrate concentrations among reaches, nitrate concentration was a poor predictor for differences in NO3 − uptake between buried and open reach sites.
Relationship between nitrate concentration and uptake velocity, plotted with the 69 LINX sites where NO3 − uptake was measured across the US. Results from the present study are compared to Mulholland et al. (2008)
Unlike studies which encompass a broad range of hydrologic conditions (Peterson et al. 2001; Hall et al. 2002; Grimm et al. 2005; Alexander et al. 2007; Hall et al. 2009), we did not find a relationship between NO3 − uptake length and stream velocity or specific discharge in a multivariate analysis, though uptake length (S W) was weakly related to stream velocity in a univariate analysis. Consequently, the poor relationship between hydrological variables and NO3 − uptake metrics in this study suggests that uptake dynamics were under biological, rather than hydrologic control.
Nitrate uptake: GPP and TS are strong predictors
Nitrate uptake velocity, considered an index for the biological demand of nitrate (Hall et al. 2009), was positively related to gross primary production (GPP), similar to other studies that found ecosystem metabolism to be a strong predictor of NO3 − uptake rates (e.g. Hall and Tank 2003; Fellows et al. 2006; Mulholland et al. 2006, 2008; Sobota et al. 2012). This relationship indicates that the lower NO3 − uptake rates in buried reaches were partially due to reduced autotrophic nutrient assimilation, which is corroborated by the significantly lower GPP measured in the buried reaches. Also, because uptake velocity measures the rate of vertical movement of NO3 − molecules from the water column to benthos, geomorphic factors in the stream that increase residence time and promote a stable environment for autotrophic growth may contribute to greater assimilation and removal of NO3 − from the water column (e.g. Biggs et al. 1999; Uehlinger 2000). ER, an index for heterotrophic assimilation, was not significantly related to NO3 − uptake, which may indicate that heterotrophic NO3 − assimilation was not as important as autotrophic assimilation. While primary productivity was important in controlling NO3 − uptake, TS and interactions with the hyporheic zone of the streambed, were found to be even more important in explaining variation in NO3 − uptake (discussed below).
We found a positive relationship between nitrate uptake rates and TS at our sites, similar to previous studies (Valett et al. 1996; Mulholland et al. 1997; Hall et al. 2002; Gucker and Boechat 2004; Ensign and Doyle 2005). Given that TS is influenced by stream burial and channelization, this suggests that the channelization of buried streams can reduce NO3 − uptake. One mechanism that may account for this relationship is that greater rates of denitrification are typically found in hyporheic sediments (e.g. Duff and Triska 1990; Hill et al. 1998; Groffman et al. 2009; Roley et al. 2012), which buried and channelized streams lack. Another is that higher surface TS likely increased NO3 − uptake in open reaches by providing longer residence times, associated with pools and eddies, allowing for greater opportunities to remove stream water NO3 − via assimilatory and dissimilatory uptake mechanisms (Valett et al. 1996; Hall et al. 2002; Bukaveckas 2007). The strong effect of TS on NO3 − uptake rate may also be due to channelized streams lacking in-stream structures to retain allochthonous organic carbon that can promote “hot spots” of denitrification and assimilatory uptake (e.g. McClain et al. 2003; Groffman et al. 2004; Groffman et al. 2009; Hines and Hershey 2011; Hoellein et al. 2012). Previous work in Baltimore LTER streams (Kaushal et al. 2008b; Klocker et al. 2009) and the study by Bukaveckas (2007) have shown that hydrologic residence time and hydrologic connectivity at the riparian-stream interface is related to denitrification and N uptake. Overall, our results, along with previous studies, clearly demonstrate how channelized streams that lack TS (i.e. hyporheic exchange or pools and eddies) have lower nitrogen retention. This suggests that N retention in buried streams could benefit from the restoration or preservation of hyporheic flow paths (Lawrence et al. 2013).
Stream burial impacts metabolism: effects of light, carbon quality, and TS
Similar to previous research (e.g. Mulholland et al. 2001; Roberts et al. 2007), our study found that light (PAR) was the main factor in controlling GPP. While there should be no GPP measured in the absence of light, low levels of GPP were measured in the buried streams, likely due to both the propagation of measurement error and because of some light penetration into the ends of each culvert (the culverts were relatively wide, usually >2.4 m in diameter, and relatively short in length ~100 m). The effect of light availability on GPP was also noticed in the open reaches, where the stream with the most shade (DR1) consistently had lower GPP. This finding is similar to other studies, where shading was found to have a strong effect on GPP (e.g. Mulholland et al. 2001; Bott et al. 2006; Roberts et al. 2007). Besides light availability, no other variable significantly affected GPP.
Ecosystem respiration rates were lower in the buried reaches than in the open reaches, and this may be partially attributable to differences in DOM quality (i.e. the extent of organic matter biolability or biodegradability). Supporting this, we found buried streams to have a higher index for recalcitrant humic-like organic matter (HIX), but lower indices for labile organic matter (BIX and FI). Stream burial may have affected DOM quality by suppressing autochthonous C production (through reduced light availability) and altering the input and retention of allochthonous C in the channel. DOM derived from autochthonous production tends to be of higher quality (more labile) than that derived from the leaching of more recalcitrant terrestrial organic matter that is exported to stream ecosystems (McKnight et al. 2001; Huguet et al. 2009; Petrone et al. 2011). Additionally, smaller, more labile organic molecules are more readily used as an energy source by aquatic organisms and may thus increase respiration rates (Kalbitz et al. 2003; Marschner and Kalbitz 2003; Fellman et al. 2008; Griffiths et al. 2009; Leifeld et al. 2012). We specifically found that ER was negatively related to the HIX, an index of terrestrial derived DOM. This suggested that less labile organic matter (as indicated by higher HIX and lower BIX) in buried reaches may have contributed to reduced respiration. We also speculate that the higher levels of respiration in the open reaches may be attributed to greater organic matter standing stocks, more hyporheic habitat for heterotrophic microbes, and significantly higher temperatures, which would increase biological activity compared to buried reaches. Overall, the relationship between stream burial, carbon quality, and ER has important implications for management and deserves further study.
Reduced TS or lack of hyporheic exchange can also negatively impact ER, and our results demonstrated a positive relationship between TS zones (As/A) and ER, similar to previous studies (Pusch 1996; Mulholland et al. 1997; Fellows et al. 2001; Mulholland et al. 2001). This indicates that channelization of the streambed may directly affect ER by reducing hyporheic exchange, hydraulic residence time, and biogeochemical activity. Hyporheic zones are widely recognized as important sites for nutrient cycling and denitrification (Duff and Triska 1990; Triska et al. 1993; Findlay 1995; Mayer et al. 2010) and terrestrial aquatic interfaces, including hyporheic zones, are known to be “hot spots” for biogeochemical activity due to the convergence of chemically distinct flowpaths (McClain et al. 2003). The hyporheic zone may also be an important site for ER due to the potential build up, processing, and production of organic matter in the benthic/hyporheic zone (Schindler and Krabbenhoft 1998; Mayer et al. 2010; Wong and Williams 2010) and other environmental conditions conducive to heterotrophic processes (Stanford and Ward 1988; Marmonier et al. 2012). Overall, our study indicates that the elimination of hyporheic habitat in buried and channelized reaches is a profound disturbance to the ecosystem, resulting in reduced ER and nutrient retention.
Differences in carbon quantity and quality in open vs. buried streams
The differences in carbon quantity and quality between open and buried reaches were likely driven by differences in light, DOM sources (allochthonous vs. autochthonous), and temperature between open and buried reaches. The higher BIX and FI in open reaches is likely due to the presence of light in the open reaches, which promotes autotrophic growth and is associated with more biologically labile DOM (McKnight et al. 2001; Huguet et al. 2009; Petrone et al. 2011). However, the elevated HIX in the buried reaches may be due to the biota in buried reaches preferentially consuming labile organic matter and leaving the more recalcitrant terrestrial OM behind (e.g. Kalbitz et al. 2003; Fellman et al. 2008; Griffiths et al. 2009; Leifeld et al. 2012). Additionally, the higher DOC concentrations and higher humic-like organic matter in the buried stream reaches may be accounted for by temperatures that were 2 degrees (on average) lower in buried than open reaches. Lower stream temperatures could result in lower respiration and organic matter breakdown of dissolved and particulate organic matter (Burton and Likens 1973; Conant et al. 2008, 2011; Duan and Kaushal 2013). However, because the temperature difference is relatively small, these differences in DOC and carbon quality could also be attributed to differences in the sediment that builds up in the buried reaches vs. open reaches or the effects of light availability on greater photodegradation of organic matter in open reaches (e.g. Larson et al. 2007). More work is necessary to elucidate the role of light, DOM sources, and temperature in influencing the quantity and quality of organic carbon in urban streams (Kaushal et al. in press; Kaushal et al. in review).
Potential watershed-scale impacts
Given that cities can have over 70 % of their headwater streams buried (Elmore and Kaushal 2008), we attempted to use the results of this study to estimate the potential impact of stream burial on N and C uptake and production, respectively, at the larger watershed scale. Using stream and storm drain length, average stream width, estimates for the amount of stream burial, and the quantified effect of stream burial on areal NO3 − uptake rate and metabolism, we estimated the decrease in N uptake and C production due to stream burial for each of our stream sites in the Dead Run watershed, during each season. Additionally, because of the need to manage and reduce watershed N and C export, we also estimated the potential effects of “daylighting” the buried streams in the Dead Run watershed, where daylighting is the process of uncovering a buried stream and converting it to a more natural stream (Pinkham 2000; Buchholz and Younos 2007; Trice 2013). These estimates were calculated for each stream (DR1, DR2, and DR5) on each day we carried out injection experiments, and then averaged for each season and for all four seasons together (Tables 5, 6). For nitrogen, we calculated areal NO3 − uptake (μg N m−2 s−1) in open and buried reaches and then estimated the additional nitrate (based on difference) that would be removed if the buried streams were daylighted (Table 5). For carbon, we estimated the net carbon produced through GPP or consumed by ER (g C m−2 day−1) by daylighting (Table 6). We calculated the buried stream lengths and total stream lengths of the DR1, DR2, and DR5 tributaries (Tables 5, 6) using GIS analysis (Esri 2009).
We estimated that stream burial in the DR1, DR2, and DR5 watersheds, on average, reduces NO3 − uptake by 39 ± 5 % during baseflow. Daylighting 100 m of stream (approximately the size of our buried stream reaches) could result in a 3.1 ± 0.7 % increase in NO3 − uptake, and daylighting 100 % of the buried streams could increase NO3 − uptake 185 ± 38 % during baseflow conditions (Table 5). We estimated that stream daylighting would have the greatest effect in spring and smallest in winter, while stream burial has the smallest impact on nitrate uptake in the summer (Table 5). This may be due to the higher rates of autotrophy and heterotrophy during the summer months, but lower biogeochemical rates and higher discharges during the winter months. Because our experiments were carried out during baseflow and there is considerable variability in nitrate export and retention with streamflow in urban watersheds (Kaushal et al. 2008a), it is unclear whether storm events would reduce the effect of daylighting. Specifically, because most of the total annual N flux occurs during stormflow, not baseflow (e.g. Boynton et al. 2008; Kaushal et al. 2008a; Shields et al. 2008), it is likely that the increase in baseflow N retention due to daylighting would have a small effect on total annual retention, unless the restoration can also enhance stormflow retention, such as through hydrologic connection with the floodplain or use of riffle-pool sequences, etc. (e.g. Palmer et al. 2005; Hammersmark et al. 2008; Kaushal et al. 2008b). Additionally, because our study did not distinguish between the permanent removal of nitrate via denitrification or temporary removal via assimilation to organic matter, it may be difficult to accurately estimate the potential change in NO3 − export due to stream daylighting. Also, the possibility that nitrogen is being remineralized and nitrified in the stream at the same time as it is being removed during each injection experiment, suggests that our N uptake estimates are likely maximum uptake estimates, which may mean we are over estimating N uptake at our sites. However, the relative difference between uptake in open and buried channels might still be the same and thus the estimated effect of burial on N uptake may also be the same regardless of remineralization or nitrification. Nevertheless, our results indicate that stream burial has the potential to considerably impair N retention at the watershed scale, especially when natural flood attenuation is lost through urban stream burial and channelization.
Unlike nitrogen, we estimated that carbon production would likely increase if buried streams were daylighted, due to a net increase in primary productivity. While some of the sites and seasons supported net carbon consumption, the majority of the sites had net carbon production (Table 6, individual site data not shown). We estimated that, on average, stream burial decreases GPP by 45 ± 1 % and reduces ER by 27 ± 11 %, with an overall reduction in C production by 194 ± 112 %. On average, daylighting 100 m of buried stream could result in a modest 0.22 ± 1.0 % increase in C export, while daylighting 100 % of buried stream could increase C production by 42 ± 81 % (Table 6). The increased C production from daylighting is likely the result of elevated light, which results in greater GPP and more autochthonous C production (e.g. Mulholland et al. 2001); daylighting would also likely increase inputs from terrestrial C sources. Daylighting appears to have the greatest increase in C production in the summer, but decreases C production in the fall and spring. In fact, due to the variability observed in GPP and ER at each site, we found that at certain stream sites (data not shown), daylighting could decrease C production. Despite this variability, it is apparent that stream daylighting could have significant impacts on stream carbon loads. Additionally, while daylighting will likely increase C production, it will also likely increase C quality/lability (via increased autochthonous production) due to more light availability, and also increase terrestrial carbon sources to the stream. The impact of burial on carbon quality also likely influences important processes like ER and biological oxygen demand. While our results show stream burial affects carbon processing at the watershed scale, more work is necessary to fully examine the impacts of stream burial on the carbon cycle of urban streams (Kaushal and Belt 2012).
A case for stream restoration involving daylighting or creating bottomless culverts
The results of this study, and other similar research, indicate the potential for both ecological and economic benefits of stream daylighting, as well as the potential benefits of creating “bottomless” culverts (defined below) to increase hyporheic exchange, when full stream daylighting is not feasible. Stream daylighting involves converting an underground culvert to an open, un-channelized natural stream (Pinkham 2000; Buchholz and Younos 2007), while bottomless culverts are defined as a 3-sided culvert with a natural stream bottom instead of a concrete or metal bottom (Resh 2005; Norman et al. 2009). Stream daylighting projects have been increasing worldwide (Wild et al. 2011); yet, while numerous studies have focused on the impacts of stream daylighting on fish habitat (e.g. Pinkham 2000; Jones 2001; Benjamin et al. 2003; Purcell 2004), or the sociological, aesthetic, or economic reasons for daylighting (e.g. Pinkham 2000; Jones 2001; Shin and Lee 2006; Buchholz and Younos 2007; Kang and Cervero 2009; Sinclair 2012), no studies have measured the effects of daylighting on stream biogeochemistry. Similarly, studies show that bottomless culverts can be more beneficial to certain fish and other aquatic biota than regular channelized culverts (e.g. Resh 2005; Norman et al. 2009), but there are no known studies on the impacts of bottomless culverts on nutrient dynamics.
Evidence from our research, as well as a growing body of studies on the biogeochemical implications of restoring degraded urban streams also provides support for the benefits of future stream daylighting projects or installation of bottomless culverts. For example, de-channelizing and restoring streams has been found to increase N retention (Bukaveckas 2007; Klocker et al. 2009), increase denitrification (Kaushal et al. 2008b; Harrison et al. 2011) and enhance carbon processing (Lepori et al. 2005; Millington and Sear 2007; Sivirichi et al. 2011). However, despite evidence for improved nutrient retention with stream restoration, a number of studies point out that when there is not an adequate use of ecological principles in stream restoration, the efforts to restore streams do not always improve ecological function (e.g. Palmer 2009; Doyle and Shields 2012). Nonetheless, daylighting a buried stream is such a radical transformation of stream ecosystems that there is likely to be improved biogeochemical processing when buried streams are open to light and de-channelized. More specifically, daylighting streams and restoring channels to more natural conditions without concrete bottoms, may increase the potential for N retention and C processing in watersheds by increasing autotrophic related N uptake and processing (Mulholland et al. 2001; Hall and Tank 2003; Fellows et al. 2006; Mulholland et al. 2006; Sobota et al. 2012), and by restoring hydrologic retention time and hyporheic exchange (e.g. Bukaveckas 2007; Kaushal et al. 2008b; Klocker et al. 2009; Mayer et al. 2010; Harrison et al. 2012; Mayer et al. 2013). Previous studies also show that open stream channels with a mature riparian zone and tree canopy is important for shading and providing carbon for denitrification in stream restoration strategies (e.g. Groffman et al. 2005; Mayer et al. 2007; Harms and Grimm 2012). Additionally, daylighting to increase hydrologic connectivity between streams and floodplains in channelized and buried urban streams may have some potential to influence N uptake (e.g. Kaushal et al. 2008b).
Overall, while creating bottomless culverts will likely improve N retention through increased TS, stream restoration via daylighting is expected to provide the greatest biogeochemical benefits through increased hyporheic exchange, as well as increased GPP, ER, and organic matter inputs. The feasibility and success of these restoration projects will likely depend on the location (whether or not the culverts are under roads or buildings), management objectives, and funding (Pinkham 2000; Jencks and Leonardson 2004; Hotchkiss and Frei 2007; Smith 2007). Further research is necessary to test the ecological and economic benefits of daylighting, de-channelization, or installation of bottomless culverts in urban streams and to determine the context and situations where de-channelization and daylighting approaches are appropriate strategies for urban stream restoration.
Conclusion
Our results demonstrate that stream burial significantly reduced NO3 − uptake, stream metabolism, TS, DOC quality, and increased stream velocity. Longer NO3 − uptake lengths, and lower NO3 − uptake rates in buried streams were caused primarily by reduced TS and GPP. The significant reduction in GPP associated with stream burial was controlled by light, while ER was primarily controlled by TS and carbon quality.
The results of this study indicate that restoration to daylight streams or increase the TS/hyporheic exchange of buried streams may be effective management approaches for reducing N transport in buried and channelized streams. In particular, our study demonstrated that increasing TS is critical for enhancing N retention in channelized streams. Scaling our results to the watershed suggested that daylighting buried streams may have the potential to significantly reduce N loads, while the effect of stream burial on C loads is more variable. Further research is necessary to improve our understanding of ecosystem-scale impacts of engineered headwaters and infrastructure throughout stream networks (Kaushal and Belt 2012).
References
Alexander RB, Boyer EW, Smith RA, Schwarz GE, Moore RB (2007) The role of headwater streams in downstream water quality. J Am Water Resour Assoc 43(1):41–59
Beaulieu JJ, Mayer PM, Kaushal SS, Pennino MJ, Arango CP, Balz DA, Fritz KM, Hill BH, Elonen CM, Santo Domingo JW, Ryu H, Canfield TJ (in review) Effects of urban stream burial on organic matter dynamics and reach scale nitrate retention. Biogeochemistry (this issue)
Benjamin TS, Green AM, Deshais K (2003) The Neponset river restoration (daylighting) project. American Society of Landscape Architects, Washington
Biggs BJF, Smith RA, Duncan MJ (1999) Velocity and sediment disturbance of periphyton in headwater streams: biomass and metabolism. J N Am Benthol Soc 18(2):222–241
Boesch DF, Brinsfield RB, Magnien RE (2001) Chesapeake Bay eutrophication: scientific understanding, ecosystem restoration, and challenges for agriculture. J Environ Qual 30(2):303–320
Booth GD, Niccolucci MJ, Schuster EG (1994) Identifying proxy sets in multiple linear-regression—an aid to better coefficient interpretation. USDA Forest Service Intermountain Research Station Research Paper 470, pp 1–13
Bott TL, Newbold JD, Arscott DB (2006) Ecosystem metabolism in piedmont streams: reach geomorphology modulates the influence of riparian vegetation. Ecosystems 9(3):398–421
Boynton WR, Hagy JD, Cornwell JC, Kemp WM, Greene SM, Owens MS, Baker JE, Larsen RK (2008) Nutrient budgets and management actions in the Patuxent River estuary, Maryland. Estuaries Coasts 31:623–651
Buchholz T, Younos T (2007) Urban stream daylighting: case study evaluations. Virginia Water Resources Research Center Special Report SR35-2007. Virginia Polytechnical Institute, Blacksburg
Bukaveckas PA (2007) Effects of channel restoration on water velocity, transient storage, and nutrient uptake in a channelized stream. Environ Sci Technol 41(5):1570–1576
Burford JR, Bremner JM (1975) Relationships between denitrification capacities of soils and total, water-soluble and readily decomposable soil organic-matter. Soil Biol Biochem 7(6):389–394
Burton TM, Likens GE (1973) Effect of strip-cutting on stream temperatures in Hubbard Brook Experimental Forest, New-Hampshire. Bioscience 23(7):433–435
Casciotti KL, Sigman DM, Hastings MG, Bohlke JK, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74(19):4905–4912
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar Chem 51(4):325–346
Coble PG, Green SA, Blough NV, Gagosian RB (1990) Characterization of dissolved organic-matter in the black-sea by fluorescence spectroscopy. Nature 348(6300):432–435
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, Six J, Steinweg JM (2008) Sensitivity of organic matter decomposition to warming varies with its quality. Glob Change Biol 14(4):868–877
Conant RT, Ryan MG, Agren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvonen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Steinweg JM, Wallenstein MD, Wetterstedt JAM, Bradford MA (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob Change Biol 17(11):3392–3404
Cory RM, Miller MP, McKnight DM, Guerard JJ, Miller PL (2010) Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol Oceanogr Methods 8:67–78
D’Angelo DJ, Webster JR, Gregory SV, Meyer JL (1993) Transient storage in Appalachian and Cascade Mountain streams as related to hydraulic characteristics. J N Am Benthol Soc 12(3):223–235
Diaz RJ (2001) Overview of hypoxia around the world. J Environ Qual 30(2):275–281
Dodds WK, Lopez AJ, Bowden WB, Gregory S, Grimm NB, Hamilton SK, Hershey AE, Marti E, McDowell WH, Meyer JL, Morrall D, Mulholland PJ, Peterson BJ, Tank JL, Valett HM, Webster JR, Wollheim W (2002) N uptake as a function of concentration in streams. J N Am Benthol Soc 21(2):206–220
Doyle MW, Shields FD (2012) Compensatory mitigation for streams under the Clean Water Act: reassessing science and redirecting policy. J Am Water Resour Assoc 48(3):494–509
Duan S-W, Kaushal SS (2013) Warming increases carbon and nutrient fluxes from sediments in streams across land use. Biogeosciences 10:1193–1207
Duff JH, Triska FJ (1990) Denitrification in sediments from the hyporheic zone adjacent to a small forested stream. Can J Fish Aquat Sci 47(6):1140–1147
Earl SR, Valett HM, Webster JR (2006) Nitrogen saturation in stream ecosystems. Ecology 87(12):3140–3151
Elmore AJ, Kaushal SS (2008) Disappearing headwaters: patterns of stream burial due to urbanization. Front Ecol Environ 6(6):308–312
Ensign SH, Doyle MW (2005) In-channel transient storage and associated nutrient retention: evidence from experimental manipulations. Limnol Oceanogr 50(6):1740–1751
Esri (2009) ArcMap 9.2. Environmental Systems Resource Institute, Redlands
Fellman JB, D’Amore DV, Hood E, Boone RD (2008) Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 88(2):169–184
Fellows CS, Valett HM, Dahm CN (2001) Whole-stream metabolism in two montane streams: contribution of the hyporheic zone. Limnol Oceanogr 46(3):523–531
Fellows CS, Valett HM, Dahm CN, Mulholland PJ, Thomas SA (2006) Coupling nutrient uptake and energy flow in headwater streams. Ecosystems 9(5):788–804
Filoso S, Palmer MA (2011) Assessing stream restoration effectiveness at reducing nitrogen export to downstream waters. Ecol Appl 21(6):1989–2006
Findlay S (1995) Importance of surface-subsurface exchange in stream ecosystems—the hyporheic zone. Limnol Oceanogr 40(1):159–164
Griffiths NA, Tank JL, Royer TV, Rosi-Marshall EJ, Whiles MR, Chambers CP, Frauendorf TC, Evans-White MA (2009) Rapid decomposition of maize detritus in agricultural headwater streams. Ecol Appl 19(1):133–142
Grimm NB, Sheibley RW, Crenshaw CL, Dahm CN, Roach WJ, Zeglin LH (2005) N retention and transformation in urban streams. J N Am Benthol Soc 24(3):626–642
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu JG, Bai XM, Briggs JM (2008) Global change and the ecology of cities. Science 319(5864):756–760
Groffman PM, Boulware NJ, Zipperer WC, Pouyat RV, Band LE, Colosimo MF (2002) Soil nitrogen cycle processes in urban Riparian zones. Environ Sci Technol 36(21):4547–4552
Groffman PM, Law NL, Belt KT, Band LE, Fisher GT (2004) Nitrogen fluxes and retention in urban watershed ecosystems. Ecosystems 7(4):393–403
Groffman PM, Dorsey AM, Mayer PM (2005) N processing within geomorphic structures in urban streams. J N Am Benthol Soc 24(3):613–625
Groffman PM, Butterbach-Bahl K, Fulweiler RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93(1–2):49–77
Gucker B, Boechat IG (2004) Stream morphology controls ammonium retention in tropical headwaters. Ecology 85(10):2818–2827
Hall RO, Tank JL (2003) Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol Oceanogr 48(3):1120–1128
Hall RO, Bernhardt ES, Likens GE (2002) Relating nutrient uptake with transient storage in forested mountain streams. Limnol Oceanogr 47(1):255–265
Hall RO Jr, Tank JL, Sobota DJ, Mulholland PJ, O’Brien JM, Dodds WK, Webster JR, Valett HM, Poole GC, Peterson BJ, Meyer JL, McDowell WH, Johnson SL, Hamilton SK, Grimm NB, Gregory SV, Dahm CN, Cooper LW, Ashkenas LR, Thomas SM, Sheibley RW, Potter JD, Niederlehner BR, Johnson LT, Helton AM, Crenshaw CM, Burgin AJ, Bernot MJ, Beaulieu JJ, Arango CP (2009) Nitrate removal in stream ecosystems measured by N-15 addition experiments: total uptake. Limnol Oceanogr 54(3):653–665
Hammersmark CT, Rains MC, Mount JF (2008) Quantifying the hydrological effects of stream restoration in a montane meadow, northern California, USA. River Res Appl 24(6):735–753
Hanafi S, Grace M, Webb JA, Hart B (2007) Uncertainty in nutrient spiraling: sensitivity of spiraling indices to small errors in measured nutrient concentration. Ecosystems 10(3):477–487
Harms TK, Grimm NB (2012) Responses of trace gases to hydrologic pulses in desert floodplains. J Geophys Res 117:GO1035
Harrison MD, Groffman PM, Mayer PM, Kaushal SS, Newcomer TA (2011) Denitrification in Alluvial Wetlands in an Urban Landscape. J Environ Qual 40(2):634–646
Harrison MD, Groffman PM, Mayer PM, Kaushal SS (2012) Microbial biomass and activity in geomorphic features in forested and urban restored and degraded streams. Ecol Eng 38(1):1–10
Hill AR, Labadia CF, Sanmugadas K (1998) Hyporheic zone hydrology and nitrogen dynamics in relation to the streambed topography of a N-rich stream. Biogeochemistry 42(3):285–310
Hines SL, Hershey AE (2011) Do channel restoration structures promote ammonium uptake and improve macroinvertebrate-based water quality classification in urban streams? Inland Waters 1(2):133–145
Hoellein TJ, Tank JL, Entrekin SA, Rosi-Marshall EJ, Stephen ML, Lamberti GA (2012) Effects of benthic habitat restoration on nutrient uptake and ecosystem metabolism in three headwater streams. River Res Appl 28(9):1451–1461
Hotchkiss RH, Frei CM (2007) Design for fish passage at roadway-stream crossings: synthesis report. Federal Highway Administration, Office of Infrastructure Research and Development, McLean
Huguet A, Vacher L, Relexans S, Saubusse S, Froidefond JM, Parlanti E (2009) Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org Geochem 40(6):706–719
Jencks R, Leonardson R (2004) Daylighting Islais Creek: a feasibility study. Restoration of rivers and Streams (LA 227). Water Resources Collections and Archives, University of California Water Resources Center, UC Berkeley
Jones SW (2001) Planning for wildlife: Evaluating creek day-lighting as a means of urban conservation. Dalhousie University, Halifax
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113(3–4):273–291
Kang CD, Cervero R (2009) From elevated freeway to urban greenway: land value impacts of the CGC project in Seoul, Korea. Urban Stud 46(13):2771–2794
Kaushal SS, Belt KT (2012) The urban watershed continuum: evolving spatial and temporal dimensions. Urban Ecosyst 15(2):409–435
Kaushal SS, Groffman PM, Band LE, Shields CA, Morgan RP, Palmer MA, Belt KT, Swan CM, Findlay SEG, Fisher GT (2008a) Interaction between urbanization and climate variability amplifies watershed nitrate export in Maryland. Environ Sci Technol 42(16):5872–5878
Kaushal SS, Groffman PM, Mayer PM, Striz E, Gold AJ (2008b) Effects of stream restoration on denitrification in an urbanizing watershed. Ecol Appl 18(3):789–804
Kaushal SS, Groffman PM, Band LE, Elliott EM, Shields CA, Kendall C (2011) Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environ Sci Technol 45(19):8225–8232
Kaushal SS, Mayer PM, Vidon, GP, Smith RM, Pennino MJ, Duan S, Newcomer TA, Welty C, Belt KT (in press) Land use and climate variability amplify carbon, nutrient, and contaminant pulses: a review with management implications. J Am Water Resour Assoc
Kaushal SS, Delaney-Newcomb K, Findlay SEG, Newcomer TA, Duan S, Pennino MJ, Sivirichi GM, Sides-Raley AM, Walbridge MR, Belt KT (in review) Longitudinal changes in carbon and nitrogen fluxes and stream metabolism along an urban watershed continuum. Biogeochemistry
Klocker CA, Kaushal SS, Groffman PM, Mayer PM, Morgan RP (2009) Nitrogen uptake and denitrification in restored and unrestored streams in urban Maryland, USA. Aquat Sci 71(4):411–424
Larson JH, Frost PC, Lodge DM, Lamberti GA (2007) Photodegradation of dissolved organic matter in forested streams of the northern Great Lakes region. J N Am Benthol Soc 26(3):416–425
Lawrence JE, Skold ME, Hussain FA, Silverman DR, Resh VH, Sedlak DL, Luthy RG, McCray JE (2013) Hyporheic zone in urban streams: a review and opportunities for enhancing water quality and improving aquatic habitat by active management. Environ Eng Sci 30(8):480–501
Leifeld J, Steffens M, Galego-Sala A (2012) Sensitivity of peatland carbon loss to organic matter quality. Geophys Res Lett 39:6
Leopold LB (1968) Hydrology for urban land planning: a guidebook on the hydrologic effects of urban land use. U.S. Geological Survey Circular 554, US Geological Survey, Washington
Lepori F, Palm D, Malmqvist B (2005) Effects of stream restoration on ecosystem functioning: detritus retentiveness and decomposition. J Appl Ecol 42(2):228–238
Marmonier P, Archambaud G, Belaidi N, Bougon N, Breil P, Chauvet E, Claret C, Cornut J, Datry T, Dole-Olivier MJ, Dumont B, Flipo N, Foulquier A, Gerino M, Guilpart A, Julien F, Maazouzi C, Martin D, Mermillod-Blondin F, Montuelle B, Namour P, Navel S, Ombredane D, Pelte T, Piscart C, Pusch M, Stroffek S, Robertson A, Sanchez-Perez JM, Sauvage S, Taleb A, Wantzen M, Vervier P (2012) The role of organisms in hyporheic processes: gaps in current knowledge, needs for future research and applications. Ann Limnol Int J Limnol 48(3):253–266
Marschner B, Kalbitz K (2003) Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113(3–4):211–235
Marzolf ER, Mulholland PJ, Steinman AD (1994) Improvements to the diurnal upstream-downstream dissolved-oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 51(7):1591–1599
Mayer PM, Reynolds SK, McCutchen MD, Canfield TJ (2007) Meta-analysis of nitrogen removal in riparian buffers. J Environ Qual 36(4):1172–1180
Mayer PM, Groffman PM, Striz EA, Kaushal SS (2010) Nitrogen Dynamics at the Groundwater-Surface Water Interface of a Degraded Urban Stream. J Environ Qual 39(3):810–823
Mayer PM, Schechter SP, Kaushal SS, Groffman PM (2013) Effects of stream restoration on nitrogen removal and transformation in urban watersheds: lessons from Minebank Run, Baltimore, Maryland. Watershed Sci Bull 4(1). http://www.cwp.org/effects-of-stream-restoration-on-nitrogen
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6(4):301–312
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46(1):38–48
Meyer JL, Poole GC, Jones KL (2005) Buried alive: potential consequences of burying headwater streams in drainage pipes. In: Proceedings of the 2005 Georgia Water Resources Conference, University of Georgia
Millington CE, Sear DA (2007) Impacts of river restoration on small-wood dynamics in a low-gradient headwater stream. Earth Surf Process Landf 32(8):1204–1218
Mulholland PJ, Marzolf ER, Webster JR, Hart DR, Hendricks SP (1997) Evidence that hyporheic zones increase heterotrophic metabolism and phosphorus uptake in forest streams. Limnol Oceanogr 42(3):443–451
Mulholland PJ, Fellows CS, Tank JL, Grimm NB, Webster JR, Hamilton SK, Marti E, Ashkenas L, Bowden WB, Dodds WK, McDowell WH, Paul MJ, Peterson BJ (2001) Inter-biome comparison of factors controlling stream metabolism. Freshw Biol 46(11):1503–1517
Mulholland PJ, Valett HM, Webster JR, Thomas SA, Cooper LW, Hamilton SK, Peterson BJ (2004) Stream denitrification and total nitrate uptake rates measured using a field N-15 tracer addition approach. Limnol Oceanogr 49(3):809–820
Mulholland PJ, Houser JN, Maloney KO (2005) Stream diurnal dissolved oxygen profiles as indicators of in-stream metabolism and disturbance effects: fort Benning as a case study. Ecol Ind 5(3):243–252
Mulholland PJ, Thomas SA, Valett HM, Webster JR, Beaulieu J (2006) Effects of light on NO3- uptake in small forested streams: diurnal and day-to-day variations. J N Am Benthol Soc 25(3):583–595
Mulholland PJ, Helton AM, Poole GC, Hall RO Jr, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452(7184):U202–U246
Newcomer TA, Kaushal SS, Mayer PM, Shields AR, Canuel EA, Groffman PM, Gold AJ (2012) Influence of natural and novel organic carbon sources on denitrification in forest, degraded urban, and restored streams. Ecol Monogr 82(4):449–466
Norman JR, Hagler MM, Freeman MC, Freeman BJ (2009) Application of a Multistate Model to Estimate Culvert Effects on Movement of Small Fishes. Trans Am Fish Soc 138(4):826–838
O’Brien JM, Dodds WK, Wilson KC, Murdock JN, Eichmiller J (2007) The saturation of N cycling in Central Plains streams: n-15 experiments across a broad gradient of nitrate concentrations. Biogeochemistry 84(1):31–49
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36(4):742–746
Palmer MA (2009) Reforming watershed restoration: science in need of application and applications in need of science. Estuaries Coasts 32(1):1–17
Palmer MA, Bernhardt ES, Allan JD, Lake PS, Alexander G, Brooks S, Carr J, Clayton S, Dahm CN, Shah JF, Galat DL, Loss SG, Goodwin P, Hart DD, Hassett B, Jenkinson R, Kondolf GM, Lave R, Meyer JL, O’Donnell TK, Pagano L, Sudduth E (2005) Standards for ecologically successful river restoration. J Appl Ecol 42:208–217
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol Syst 32:333–365
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Marti E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory S, Morrall DD (2001) Control of nitrogen export from watersheds by headwater streams. Science 292(5514):86–90
Petrone KC, Fellman JB, Hood E, Donn MJ, Grierson PF (2011) The origin and function of dissolved organic matter in agro-urban coastal streams. J Geophys Res. doi:10.1029/2010JG001537
Pinkham R (2000) Daylighting: New Life for Buried Streams. Rocky Mountain Institute, Old Snowmass
Purcell A (2004) A long-term post-project evaluation of an urban stream restoration project (Baxter Creek, El Cerrito, California). University of California, Water Resources Center Archives, Berkeley
Pusch M (1996) The metabolism of organic matter in the hyporheic zone of a mountain stream, and its spatial distribution. Hydrobiologia 323(2):107–118
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Resh VH (2005) Stream crossings and the conservation of diadromous invertebrates in South Pacific island streams. Aquat Conserv Mar Freshw Ecosyst 15(3):313–317
Roberts BJ, Mulholland PJ, Hill WR (2007) Multiple scales of temporal variability in ecosystem metabolism rates: results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 10(4):588–606
Roley SS, Tank JL, Williams MA (2012) Hydrologic connectivity increases denitrification in the hyporheic zone and restored floodplains of an agricultural stream. J Geophys Res 117:16
Royston JP (1982) An extension of Shapiro and Wilk-W test for normality to large samples. Appl Stat J R Stat Soc Ser C 31(2):115–124
Runkel RL (1998) One-dimensional transport with inflow and storage (OTIS): a solute transport model for streams and rivers. U.S. Geological Survey Water-Resources Investigations Report 98-4018, pp 1–73
Runkel RL, Crawford CG, Cohn TA (2004) Load Estimator (LOADEST): a FORTRAN program for estimating constituent loads in streams and rivers. In: Techniques and methods book 4. U.S. Geological Survey, Reston
Ryan RJ, Welty C, Larson PC (2010) Variation in surface water-groundwater exchange with land use in an urban stream. J Hydrol 392(1–2):1–11
Ryan RJ, Larson PC, Welty C (2011) The logistics and mechanics of conducting tracer injection experiments in urban streams. Urban Ecosyst 14(1):87–117
Schindler JE, Krabbenhoft DP (1998) The hyporheic zone as a source of dissolved organic carbon and carbon gases to a temperate forested stream. Biogeochemistry 43(2):157–174
Shields CA, Band LE, Law N, Groffman PM, Kaushal SS, Savvas K, Fisher GT, Belt KT (2008) Streamflow distribution of non-point source nitrogen export from urban–rural catchments in the Chesapeake Bay watershed. Water Resour Res. doi:10.1029/2007wr006360
Shin JH, Lee IK (2006) Cheong Gye Cheon restoration in Seoul, Korea. Proc Inst Civ Eng Civ Eng 159(4):162–170
Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Bohlke JK (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73(17):4145–4153
Sinclair C (2012) An exploration of stream daylighting and urban attitudes towards the environment. Trail Six 6:68–79
Sivirichi GM, Kaushal SS, Mayer PM, Welty C, Belt KT, Newcomer TA, Newcomb KD, Grese MM (2011) Longitudinal variability in streamwater chemistry and carbon and nitrogen fluxes in restored and degraded urban stream networks. J Environ Monit 13(2):288–303
Smith BR (2007) Assessing the feasibility of creek daylighting in San Francisco, Part II: a preliminary analysis of Yosemite Creek. Restoration of rivers and streams (LA 227). Water Resources Collections and Archives, University of California Water Resources Center, UC Berkeley
Sobota DJ, Johnson SL, Gregory SV, Ashkenas LR (2012) A stable isotope tracer study of the influences of adjacent land use and riparian condition on fates of nitrate in streams. Ecosystems 15(1):1–17
Stanford JA, Ward JV (1988) The hyporheic habitat of river ecosystems. Nature 335(6185):64–66
Stolpe B, Guo L, Shiller AM, Hassellov M (2010) Size and composition of colloidal organic matter and trace elements in the Mississippi River, Pearl River and the northern Gulf of Mexico, as characterized by flow field-flow fractionation. Mar Chem 118(3–4):119–128
Stream Solute Workshop (1990) Concepts and methods for assessing solute dynamics in stream ecosystems. J N Am Benthol Soc 9(2):95–119
Trice AE (2013) Daylighting streams: breathing life into urban streams and communities. American Rivers, Washington
Triska FJ, Duff JH, Avanzino RJ (1993) Patterns of hydrological exchange and nutrient transformation in the hyporheic zone of a gravel-bottom stream—examining terrestrial aquatic linkages. Freshw Biol 29(2):259–274
Uehlinger U (2000) Resistance and resilience of ecosystem metabolism in a flood-prone river system. Freshw Biol 45(3):319–332
Valett HM, Morrice JA, Dahm CN, Campana ME (1996) Parent lithology, surface-groundwater exchange, and nitrate retention in headwater streams. Limnol Oceanogr 41(2):333–345
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24(3):706–723
Wild TC, Bernet JF, Westling EL, Lerner DN (2011) Deculverting: reviewing the evidence on the ‘daylighting’ and restoration of culverted rivers. Water Environ J 25(3):412–421
Wong JCY, Williams DD (2010) Sources and seasonal patterns of dissolved organic matter (DOM) in the hyporheic zone. Hydrobiologia 647(1):99–111
Young RG, Huryn AD (1998) Comment: improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 55(7):1784–1785
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38(1):45–50
Acknowledgments
This research was supported by EPA NNEMS Award 2010-309, the NSF Graduate Research Fellowship Program under Grant No. DGE-1144243, NSF Awards DBI 0640300 and CBET 1058502, NASA grant NASA NNX11AM28G, Maryland Sea Grant Awards SA7528085-U, R/WS-2 and NA05OAR4171042, and Baltimore Ecosystem Study LTER project (NSF DEB-0423476). We thank Kevin Danaher, Kevin Magerr, Evan Smith and Benjamin Wu for field assistance. Tammy Newcomer and Rose Smith provided help in the field and helpful comments on a previous version of the manuscript. The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed, or partially funded and collaborated in, the research described herein. It has been subjected to the Agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the author (s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Wilfred M Wollheim
Rights and permissions
About this article
Cite this article
Pennino, M.J., Kaushal, S.S., Beaulieu, J.J. et al. Effects of urban stream burial on nitrogen uptake and ecosystem metabolism: implications for watershed nitrogen and carbon fluxes. Biogeochemistry 121, 247–269 (2014). https://doi.org/10.1007/s10533-014-9958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-9958-1