Abstract
Plant growth in arctic tundra is known to be commonly limited by nitrogen. However, biogeochemical interactions between soil, vegetation and microbial biomass in arctic ecosystems are still insufficiently understood. In this study, we investigated different compartments of the soil-vegetation system of polygonal lowland tundra: bulk soil, inorganic nutrients, microbial biomass and vegetation biomass were analyzed for their contents of carbon, nitrogen, phosphorus and potassium. Samples were taken in August 2011 in the Indigirka lowlands (NE Siberia, Russia) in a detailed grid (4 m × 5 m) in one single ice-wedge polygon. We used a stoichiometric approach, based on the N/P ratios in the vegetation biomass and the investigated soil fractions, to analyze limitation relations in the soil-vegetation system. Plant growth in the investigated polygonal tundra appears to be co-limited by nitrogen and phosphorus or in some cases only limited by nitrogen whereas potassium is not limiting plant growth. However, as the N/P ratios of the microbial biomass in the uppermost soil horizons were more than twice as high as previously reported for arctic ecosystems, nitrogen mineralization and fixation may be limited at present by phosphorus. We found that only 5 % of the total nitrogen is already cycling in the biologically active fractions. On the other hand, up to 40 % of the total phosphorus was found in the biologically active fractions. Thus, there is less potential for increased phosphorus mineralization than for increased nitrogen mineralization in response to climate warming, and strict phosphorus limitation might be possible in the long-term.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth in the arctic tundra is typically limited by nitrogen availability (Mack et al. 2004; Weintraub and Schimel 2005a; Reich et al. 2006) or co-limited by nitrogen and phosphorus (Chapin et al. 1995; Giesler et al. 2012). The supply of bioavailable nutrients primarily depends on the rate of microbial decomposition. Along different ecosystems, nitrogen mineralization rates have been found to be lowest in the arctic tundra (Nadelhoffer et al. 1992). To assess a complete picture of the nutrient relations in the arctic tundra, it is crucial to integrate plant communities and microbial organisms within the analysis of the soil-vegetation system. In this study, we partitioned the macronutrients carbon (C), nitrogen (N), phosphorus (P) and potassium (K) in the soils into inorganic, microbial and residual fractions. Elemental contents of the foliar biomass of the according plant communities were studied as well.
Input of inorganic nitrogen into pristine ecosystems occurs on the one hand by decomposition and mineralization of soil organic matter and on the other hand by microbial fixation of atmospheric nitrogen (N2, Aber and Melillo 2001). In contrast to the nitrogen cycle, the phosphorus cycle lacks a major atmospheric component (Aber and Melillo 2001). As weathering processes are very slow under low temperatures, there is little phosphorus input from geological parent materials in arctic soils (Hill 1961). The pool of labile phosphorus is mainly filled by periodical crashes of microbial communities (Chapin et al. 1978). In contrast, the potassium cycle is quite different from the nitrogen and phosphorus cycles. It is negligibly bound to the organic matter (Scheffer and Schachtschabel 2002) and within dead plant tissues it is not bound to organic compounds but is mobile by diffusion in ionic form. Thus, it is released into the soil by leaching of dead organic matter (Titus and Malcolm 1992).
Increased temperatures in the course of climate change will have major impacts on arctic ecosystems. Higher soil temperatures will probably enhance microbial decomposition and mineralization rates in arctic soils (MacDonald et al. 1995; Rustad et al. 2001). Higher nutrient availability will influence the plant species diversity and composition of arctic ecosystems (Aerts 2006; Eriksson et al. 2010). Experimental studies have found that increased nutrient availability in high latitudes can compensate higher soil CO2 emissions by increased primary production (Natali and E. A. G. Schuur, and R. L. Rubin. 2012). Mack et al. (2004) observed a doubling of primary production after 20 years of fertilization, but also net carbon losses from the ecosystem are possible. Therefore, changes of nutrient availability will indirectly control the carbon balance of arctic ecosystems by influencing changes in the plant community composition (Weintraub and Schimel 2005a). Higher nitrogen availability can lead to higher CH4 production in arctic peatlands (Eriksson et al. 2010) but can also depress microbial respiration in these soils (Schimel and Weintraub 2003). However, higher decomposition rates could lead in the long-term also to phosphorus limitation of arctic ecosystems (Peñuelas et al. 2012). Thus, nutrient pools and their mobility in soils play a key role for the further development of arctic ecosystems.
Many lowland tundra landscapes are characterized by the formation of thermal-contraction-crack polygons (French 2007). Worldwide, polygonal lowland tundra covers about 250.000 km2 of earth´s surface (Minke et al. 2007). In the last decade, these complex tundra wetlands have been in the focus of ecological and biogeochemical arctic research because they are able to accumulate considerable amounts of peat and are significant sources of atmospheric methane (e.g. Kutzbach et al. 2004; Wille et al. 2008; Parmentier et al. 2011; de Klerk et al. 2011). However, it is unclear how sensitively the carbon and greenhouse budget of the polygonal tundra ecosystems will react to the ongoing climatic warming in the Arctic. The long-term net effect of warming on the carbon balance will critically depend on the relative magnitudes of the changes of soil respiration on the one hand and vegetation productivity on the other hand in these heterogeneous and dynamic ecosystems.
The polygonal pattern results from thermal-contraction-cracks which fill with re-freezing snow melt water in spring. Repetition of this process leads to the formation of ice-wedges and the development of a distinct polygonal pattern. As the soil between these ice-wedges extends during summer warming it gets pushed above the ice-wedges and thus forms the elevated polygon ridges above the ice wedges (Chernov and Matveyeva 1997; MacKay 2000). However, the single polygons are not isolated systems but are connected via subsurface hydrological windows (Minke et al. 2007). This subsurface hydrological connectivity is mainly controlled in the short-term by the extend of soil thaw during summer (Helbig et al. 2013). In the long-term, polygonal dynamics controlled by climatic changes regulate the hydrological connectivity and the whole microtopography of this ecosystem—for instance, increased melt water flows may induce rapid collapses of single ice-wedges (de Klerk et al. 2011).
In this study we used a stoichiometric approach to investigate nutrient limitation in different compartments of the soil-vegetation system in the polygonal tundra. Nitrogen and phosphorus, as most limiting macronutrients, can be found in different organisms in a relatively fixed stoichiometry (Redfield 1958; Cleveland and Liptzin 2007). Their ratios can be used—instead of fertilization studies—to identify ecosystem nutrient limitation (Koerselman and Meuleman 1996; Sterner and Elser 2002; Olde Venterink et al. 2003; Güsewell 2004). Also N/K and K/P ratios are indicators for potential potassium limitation (Olde Venterink et al. 2003). Though different elements or factors can limit plant growth or microbial activity at the same time (Saito and a., T. J. Goepfert, and J. T. Ritt. 2008; Harpole et al. 2011), these elemental ratios are useful as first order approximation of the nutrient limitation in the soil-vegetation system (Ågren et al. 2012). We assume that phosphorus plays a key role in the nutrient limitation of the polygonal tundra beyond the well-known role of nitrogen. The goal of this study was to identify mechanisms of nutrient limitation by comparing elemental nutrient ratios in the foliar biomass of plant communities, within the fractions of inorganic nutrient compounds as well as in the microbial biomass within different soils of polygonal tundra.
Material & methods
Site description
The study area is located in the Indigirka lowlands 28 km northwest of the village Chokurdakh near the World Wildlife Fund station Kytalyk in the Republic of Sakha, Russian Federation (70°53′12.1 N, 147°48′29.9 E, Figs. 1, 4). The site is at the bottom of a previous thermokarst lake which was intersected by the Berelekh River, a tributary to the Indigirka River (Tumskoy and Schirrmeister 2012). The nearest weather station is located at Chokurdakh (WMO 21946, ~30 km distant to the study location) and reports for the period from 2001 to 2011 a mean annual air temperature of −12.8 °C. Monthly mean air temperature of the warmest month (July) is +11.8 °C; mean air temperature of the coldest month (February) is −34.6 °C. The annual mean precipitation (period from 2001–2003) does not exceed 250 mm (Russsia´s Weather Server 2013).
Map of the circumpolar arctic permafrost extent. The three permafrost zones (continuous, discontinuous and sporadic permafrost) are shown in grey colors. The location of the study area is marked by a red circle. Modified after Brown (1998)
The polygonal depressions in the study area are characterized by high soil water contents because drainage is impeded by the underlying permafrost and the adjacent ice-wedges. The elevated ridges vary from a dry to a moderately moist water regime. This small-scale hydrological variability causes a heterogeneous plant species composition between the polygon ridges and the polygonal depressions. In the studied polygonal tundra, the wet polygon depressions are mainly characterized by sedges and also mosses (Teltewskoi et al. 2012). The lower soil water contents on the polygon ridges provide the conditions for the growth of lichens, mosses, herbs and dwarf shrubs (van Huissteden et al. 2005; van der Molen et al. 2007; Fig. 2).
Fieldwork
Fieldwork was conducted in the late summer period (July–August) in 2011. In one selected ice-wedge polygon (Lhc11), 23 soil profiles were sampled in a regular grid of 4 m × 5 m across the polygon (Fig. 3). Of each soil profile, all organic horizons of the thawed soil to a depth of up to 50 cm and the overlying vegetation were sampled. As dry elemental contents of peat and mineral material are hardly comparable, due to large differences in their bulk density, the underlying mineral horizons were not included in this study. Characterization of the soil types were conducted following the US Soil Taxonomy (Soil Survey Staff 2010). The vegetation of the selected polygon was continuously mapped in a grid of 1 × 1 m quadrats, covering an area of 26 m × 21 m and resulting in 546 quadrats with information about identity and abundance of occurring plant species. Species coverage was estimated into 15 cover classes after Londo (1976). Vascular plants were identified with Polunin (1959); Tolmachev (1974) and Rothmaler (2002); their nomenclature follows Czerepanov (1995). Sphagnum species were identified and named after (Frey et al. 1995) and Michaelis (2011). Representative samples of the foliar biomass of the plant communities (including mosses) on each plot of the soil profiles were sampled for further analyses.
Measurements of heights of ground surface, frost table and water level were mapped in the same grid as the vegetation was mapped. Ground surface elevation, frost table height (i.e. elevation) and open water level were determined in the centre of each quadrat relative to a horizontal reference level (Fig. 3). Ground surface was defined as the surface of living moss or the surface of unvegetated peat. Frost table position was measured by pushing a rod into the unfrozen soil down to resistance. Thaw depth is the difference between the two measures (Minke et al. 2009).
To ensure representativeness of the results, microtransects of four soil profiles from the polygon rim into the polygonal depressions were sampled in seven additional polygons (KYT 1–7) within the whole study area (~20 km2, Fig. 4). Sampling of these soil profiles were conducted as described before.
Aerial image of the study site in Kytalyk. The location of the investigated polygon Lhc11 is marked by a green circle. Locations of the seven additionally investigated polygons (KYT 1–7) are marked by red circles (GeoEye image from 2010, 0.5 m resolution, by courtesy of Jacobus van Huissteden, Vrije Universiteit, Faculty of Earth and Life Sciences, Amsterdam)
Laboratory work
Initial analyses were conducted directly in the field laboratory on dissolved inorganic nitrogen (DIN), which was considered as the sum of ammonium (NH4 +) and nitrate (NO3 −). Contents of exchangeable ammonium and nitrate in the soils were extracted by 0.0125 M CaCl2 and analyzed by using photometrical test-kits for ammonium and nitrate analyses (HACH-Lange LCK304 & LCK339). These test-kits use colorimetrical reactions, namely the indophenolblue-reaction (ammonium, Selmer-olsen 1971) and the dimethylphenol-reaction (nitrate, Elton-Bott 1977), respectively.
Further analyses were conducted after transport under frozen conditions: total carbon, nitrogen, phosphorus and potassium as well as labile phosphorus (loosely bound inorganic phosphorus) and labile potassium (loosely bound inorganic potassium) in the bulk soil were measured. Dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and dissolved organic phosphorus (DOP) as well as contents of carbon, nitrogen and phosphorus in the microbial biomass were also measured. For each soil profile, a mixed sample of the foliar biomass of the according plant communities was analyzed for contents of total carbon, nitrogen, phosphorus and potassium. After air drying of the samples, all green leaves of vascular plants as well as the mosses were separated and homogenized. With respect to the sampling period at the end of the growing season, all of these leaves were considered as mature leaves.
Total carbon and nitrogen in the soil samples as well as in the vegetation samples have been measured using a C/N analyzer (Variomax elementar CNMS). Total phosphorus and potassium also in the soil samples and in the vegetation samples were extracted with nitric acid using microwave application (1,600 W, 200 °C, and 15 min). Subsequent to this extraction was a digestion with 5.5 M sulphuric acid (H2SO4) and ammonium persulfate ((NH4)2S2O8) at >150 °C (Hedley et al. 1982). Labile phosphorus and potassium were addressed as lactate-soluble phosphorus and potassium (VDLUFA 1991). The content of phosphate in all extracts was determined using the colorimetric molybdenum blue reaction (Murphy and Riley 1962). Potassium in the extracts was measured by an atomic absorption spectrometer (Varian AA280FS).
Microbial carbon, nitrogen and phosphorus were measured using the chloroform fumigation method (Brookes et al. 1985; Ivanoff et al. 1998). After fumigation under chloroform vapor in a desiccator for 24 h at 24 °C, the samples were extracted by 0.5 M K2SO4 (carbon and nitrogen) and 0.5 M H2CO3 (phosphorus), respectively. For each sample a non-fumigated subsample was also extracted by K2SO4 and H2CO3. After shaking, all extracts were filtered (Macherey–Nagel 315; 4–12 µm). All K2SO4-extracts were measured using a TOC/TON analyzer (Shimadzu TNM-L) for carbon and nitrogen. The H2CO3 extracts were treated with sulphuric acid and ammonium persulfate as described before. Phosphorus in these extracts again was measured photometrically. The differences in total extractable carbon, nitrogen and phosphorus between the non-fumigated and fumigated subsamples were calculated. To estimate elemental contents in the microbial biomass correction factors of 0.35 for carbon (Sparling et al. 1990), 0.54 for nitrogen (Brookes et al. 1985) and 0.37 for phosphorus (Hedley and Stewart 1982) were applied. The content of carbon in the non-fumigated subsamples was considered as DOC. The differences between DIN and labile phosphorus, respectively and the contents of nitrogen and phosphorus in the non-fumigated subsamples were considered as DON and DOP (Jones and Willett 2006).
The residual fractions of carbon, nitrogen, phosphorus and potassium were considered as the total values subtracted by the sum of the other analyzed (more mobile) fractions. Elemental contents of the microbial biomass, the dissolved organic fraction as well as labile phosphorus and potassium and dissolved inorganic nitrogen were considered as the biologically active fractions.
Statistical analyses
Although it is common in ecological reports to express elemental stoichiometry as mass ratios (e.g. g N/g P) we used atomic ratios in our reports (e.g. mol N/mol P) as they reflect the actual stoichiometry. The molar based N/P ratios are equal to 2.21 times the mass based N/P ratios. The N/K and K/P ratios in molar units are equal to 2.79 and 0.79 times the ratios in mass units, respectively. All mass-based elemental ratios from the literature were recalculated by these factors to molar ratios.
The mean N/P ratios of the two vegetation communities were compared by Student´s two-sample t test. Differences in the N/K and K/P ratios of the vegetation communities were identified, due to missing homogeneity of variances, by Welch´s two sample t tests. Differences in the chemical composition between the soils in the studied polygon and the soils in the surrounding study area were also analyzed by Welch´s two sample t tests. To analyze relationships between microbial carbon and microbial nitrogen as well as relationships between microbial nitrogen and DIN, we used linear ordinary least squares regression. Nonlinear ordinary least squares regression was used to identify relationships between microbial carbon and total carbon as well as relations between microbial nitrogen and labile phosphorus. All statistical analyses were performed using the program “R” (R Core Team 2013). A vegetation map was constructed using the software Qgis version 1.8.0 “Lisboa” and modified with drawing software. The 546 vegetation quadrates were classified into 2 communities using the function isopam from the R package isopam (Schmidtlein et al. 2010). Indicator values and significance levels of the indicator species for the dry and the wet community respectively were calculated using the function multipatt from the R package indicspecies (De Cáceres and Legendre 2009).
Results
Soil profiles and plant communities
Following the US Soil taxonomy (Soil Survey Staff 2010), most of the 23 soil profiles belonged either to the great groups of Histels or Orthels (Fig. 5). Histels are permafrost soils (Gelisols) that comprise 80 %, by volume, of organic material to a depth of 50 cm. The second great group Orthels (“normal Gelisols”) contains less organic material and shows no features of cryoturbation.
Two soil profiles of the studied polygon Lhc11. Horizon symbols according to the US Soil Taxonomy in yellow. a Soil Profile M1. (polygonal depression)—Typic Hemistel: three layers of peat in different decomposition states (Oi, Oe, Oa). Soil profile is dominated by moderately decomposed peat (Oe). b Soil Profile R1. (polygon ridge)—Typic Historthel: three layers of peat in different states of decomposition (Oi, Oe, Oa) above the frost table. Note the different thaw depths of the two soil profiles
Typical soil profiles in the studied polygon were built up by three organic horizons of different states of decomposition above mineral horizons. The uppermost organic horizons were always least decomposed, thus characterized as “Oi” horizons. Highly decomposed peat was commonly found at the bottom of the accessible (thawed) soil profile and characterized as “Oa” horizons. Moderately decomposed horizons were commonly found between the Oi and Oa horizons and were characterized as “Oe” horizons. Soils on the polygon ridges generally had lower thaw depths (32 ± 11 cm) than soils in the polygonal depressions (47 ± 8 cm; Fig. 6).
Investigated transects in the polygon Lhc11. Thawed soil (measured between 26th of July and 1st of August 2011), frozen soil (stars) and water bodies (wavy lines) are indicated. Locations of soil profiles are marked and the different soil horizons are indicated by different colors (see legend). Soil profiles were investigated between 5th of August and 7th of August 2011. Extrapolated soil horizons are marked by dotted lines
Locations and horizons of the soil profiles within the micro-topography of the investigated polygon are shown in Fig. 6. The microtopography of the polygon Lhc11 showed a clear distinction between the dry polygon ridges and the wet polygonal depressions. Thaw depths below the water body were generally greater than on the elevated polygon ridges. The polygon ridges were considered as the elevated parts of the polygon above the water table. The polygonal depressions were considered as the parts of the polygon below the water table. Most soil profiles contain three horizons of peat in different states of decomposition. In some cases, these peat profiles are underlain by mineral “Bg” horizons. There are only three exceptions of soil profiles with another sequence of soil horizons. (H9, M5, M17). In these profiles, there is at least one horizon of peat missing while the mineral material has been found closer to the top of the profile.
Two vegetation types could be distinguished in the investigated polygon Lhc11: a wet vegetation type, located in the polygonal depressions and a dry vegetation type, located on the polygonal ridges (Fig. 7). Significant indicator species of the wet vegetation type are S. flexuosum, Utricularia ochroleuca and U. vulgaris, Carex chordorrhiza, Carex rotundata and Caltha palustris. Significant indicator species of the dry vegetation type are Ledum decumbens, Rubus chamaemorus, Vaccinium vitis-idaea, Betula exilis, Poaceae, Dicranum acutifolium, Aulacomnium palustre, Sphenolobus.minutus, Ptilidium ciliare, Polytrichum swartzii, P. strictum and several lichens taxa. The species Carex concolor, Eriophorum angustifolium, Sphagnum squarrosum as well as Comarum palustris occur on wet and dry spots but with a clear dominance in the wet depressions. Salix spec. is quite common in the transition between dry and wet spots. Detailed vegetation maps of the polygon Lhc11 are provided in De Klerk et al. (2014).
Distribution of the two vegetation communities in the studied polygon. The grey colors indicate the elevation of the ground. The green and blue circles show the vegetation communities of the dry polygon ridges and the wet polygonal depressions, respectively. The investigated soil profiles are marked by red squares
Soil chemistry and nutrient limitation
All soils had low pH-values between 4.0 and 6.0. The uppermost and lowermost Oi and Oa horizons on the polygon ridges were slightly more acidic than the horizons in the polygon depressions. However, there were no significant differences in the pH-values between the polygon ridges and the polygon depressions. Electrical conductivity was low with values between 9 and 60 µS cm−1 (Table 1).
N/P ratios of the vegetation on the polygon ridges (24.9 ± 2.8) were slightly higher than in the polygon depressions (21.6 ± 4.3). N/P ratios of all samples were below 30 (Fig. 8b). N/K ratios were mostly below 6 and K/P ratios of almost all samples were above 2 (Fig. 8c–d). The results of the t-tests showed that there were only significant differences in the N/P ratios between the two vegetation communities (C/N ratio: p = 0.54; N/P ratio: p = 0.05; N/K ratio: p = 0.61, K/P ratio: p = 0.25).
Molar-based elemental stoichiometry in the polygon depressions and on the polygon ridges of the aboveground foliar biomass of the plant communities. a C/N ratio b N/P ratio c N/K ratio d K/P ratio; n = 22. The thick line shows the median of the data. The boxes indicate the upper and lower quartiles. Outliers are marked by points. The grey colors indicate areas of N/P co-limitation and K limitation, respectively
The analyses of macronutrients in the bulk soil materials showed that, overall, there were no significant differences between the soils on the polygon ridges and the soils in the polygon depressions. Only the content of nitrate (NO3 −) was twice as high in the Oi horizons of the polygon ridge as in the Oi horizons of the polygon depressions (NO3 − ridge: 0.86 ± 0.96 mg kg−1; NO3 − depression: 0.44 ± 0.38 mg kg−1; p < 0.05). Despite of larger variability in the Oi horizons of the polygonal depressions there were no significant differences in the water contents between the polygonal ridges and the polygonal depressions (Table 1). Dissolved inorganic nitrogen contents (DIN; NH4 + & NO3 −) were generally low. There were no differences between DIN contents in the different horizons (Fig. 9b). In contrast, DON in the uppermost horizons was about 15 times higher than DIN and in the lower horizons on average seven times higher. Highest contents of labile phosphorus and potassium were found in the uppermost horizons (Fig. 9c–d). Microbial carbon and nitrogen decreased with depth. However, there were no significant differences with depth in the contents of microbial phosphorus (Fig. 9a–c). An exponential relationship between total carbon and the microbial carbon over all soil horizons was observed (R2 = 0.54, data not shown). Highest contents of total carbon and therefore of microbial carbon as well, were found in the uppermost Oi horizons. Microbial nitrogen content was related to the content of microbial carbon in a linear way (R2 = 0.56; data not shown). In contrast, microbial phosphorus was neither related to microbial carbon (R2 = 0.09) nor to microbial nitrogen (R2 = 0.24; data not shown). Thus, highest contents of microbial biomass were found in the uppermost Oi horizons, with no differences with depth in the contents of microbial phosphorus.
Nutrients of different compartments of the soil-vegetation system in all soil horizons. Inorganic fractions are shown in yellow, dissolved organic fractions are shown in green, microbial fractions are shown in blue and the residual fractions are shown in red. a Carbon as DOC, microbial carbon (Cmic) and residual carbon (Cres). The X-axis is scaled logarithmically. b Nitrogen as DIN (NH4 +–N + NO3−–N), DON, microbial nitrogen (Nmic) and residual nitrogen (Nres). The X-axis is scaled logarithmically. c Phosphorus as labile phosphorus (Plabile), DOP, microbial phosphorus (Pmic) and residual phosphorus (Pres). d Potassium as labile potassium (Klabile) and residual potassium (Kres). The thick line shows the median of the data. The boxes indicate the upper and lower quartiles. Outliers are marked by points n = 50
The contents of the residual-fractions of carbon and nitrogen were orders of magnitudes higher than the contents of the residual-fractions of phosphorus and potassium (Fig. 9a–d). There were no significant differences between the different horizons in the contents of the residual fractions of nitrogen and phosphorus (Fig. 9b–c). However, in contrast to the decreasing contents of labile potassium, there was an increase with depth in the content of residual potassium (Fig. 9d).
Regarding total phosphorus, labile phosphorus, ammonium, nitrate as well as the water contents there were no significant differences between the soils in the studied polygon (Lhc11) and the average properties of the soils in the surrounding study area (KYT). Total carbon and nitrogen as well as labile potassium differed only marginally between the studied polygon and the surrounding polygons. The studied polygonal depressions appeared to have significantly lower contents of total potassium than the polygon depressions of the surrounding area. The largest differences between the studied polygon and the polygons in the surrounding study area were found in the pH values. All horizons in the depressions as well as on the ridges of the study area were more acidic than the soils in the studied polygon (see Table A1 in ESM Appendix). However, as these two groups of samples were measured at different times we suspect a methodological bias behind the investigated differences in the pH values.
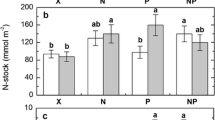
In the uppermost Oi horizons, 6 % of the total nitrogen was found in the biologically active fractions on average. In the lower Oe and Oa horizons, this part was decreasing to 2 and 1 %, respectively. In contrast, the amount of phosphorus in the biologically active fractions accounted for 40 % of the total phosphorus in the Oi horizons and for 24 and 20 % in the Oe and Oa horizons, respectively. Labile potassium accounted even for 43 % of the total potassium in the Oi horizons. In the lower Oe and Oa horizons, labile potassium accounted for 13 and 2 %, respectively (Table 2). Thus, only small percentages of the total contents of carbon and nitrogen were found in the biologically active fractions, whereas large percentages of the total phosphorus and potassium contents were found in the biologically active fractions.
The lowest N/P ratios in the inorganic fractions were found in the uppermost horizons. On average, the N/P ratios in the inorganic fraction were in the uppermost horizons below 1 (0.9 ± 0.6) and increased in the lower Oe and Oa horizons to 6.0 ± 7.1 and 6.7 ± 4.0, respectively (Fig. 10). The N/P ratios of the dissolved organic fractions had similar low values as the N/P ratios of the inorganic fraction in the Oi horizons (1.9 ± 1.4). There was no significant increase in the lower Oe and Oa horizons within the N/P ratios of the dissolved organic fractions (Oe: 1.4 ± 0.6; Oa: 2.0 ± 1.3). There was a large variability of the microbial N/P ratios in the uppermost Oi Horizon (19.6 ± 17.8). However, approximately half of the investigated samples had a microbial N/P ratio below 10 (median = 9.7; Fig. 10). In the Oe and Oa horizons the microbial N/P ratio was much lower than in the Oi horizons (6.8 ± 7.8 and 7.0 ± 10.2). The residual fractions of nitrogen and phosphorus (which are shown in Fig. 9) showed a large excess in nitrogen, which was reflected by the high N/P ratios of approximately 40 and above (Fig. 10).
Molar-based N/P ratios in different fractions of the soil-vegetation system in all organic horizons (in mmol*kg−1/mmol*kg−1). N/Pveg shows the average N/P ratio of the overlying vegetation and is marked in light green. N/Pinorg describes the ratio of dissolved inorganic nitrogen to plant-available phosphorus. N/Porg shows the ratio of dissolved organic nitrogen (DON) to dissolved organic phosphorus (DOP) and is marked in dark green. The N/P ratio of the microbial organisms is marked in blue. The red boxplots show the total N/P ratios. Thick lines show the medians of the data. The boxes indicate the upper and lower quartiles. Outliers are marked by points. The dotted blue line indicates an average microbial N/P ratio in the arctic tundra (Xu et al. 2013), the dotted red line indicates the average N/P ratio of soil elements in the arctic tundra (Cleveland and Liptzin 2007). n = 50
There was an exponential dependence of labile phosphorus to microbial carbon and nitrogen, respectively. However, the exponential model relating microbial nitrogen and labile bound inorganic phosphorus (\({\text{R}}^{ 2} { = 0} . 6 9\); Fig. 11) had much higher explanatory power than the exponential model relating microbial carbon content and labile phosphorus \(\left( {{\text{R}}^{ 2} { = 0} . 4 4 , {\text{ data not shown}}} \right)\). However, no relationship has been found between DIN and microbial carbon or microbial nitrogen, respectively \(\left( {{\text{R}}^{ 2}_{{{\text{Cmic}} \sim {\text{DIN}}}} { = 0} . 0 0 1 ; {\text{ R}}^{ 2}_{{{\text{Nmic}} \sim {\text{DIN}}}} { = 0} . 0 2 ; {\text{ data not shown}}} \right)\).
Discussion
Based on the N/P ratios of the aboveground foliar biomass, we can exclude that plant growth in our study site is only limited by phosphorus. Critical molar-based N/P ratios for sole nitrogen limitation of plant communities have been determined to be below 22 (mass-based N/P ratio of 10; Güsewell 2004) or 31 (mass-based N/P ratio of 14; Koerselman and Meuleman 1996; Olde Venterink et al. 2003). N/P ratios between 22 and 44 indicate co-limitation by nitrogen and phosphorus (mass-based N/P ratios of 10 and 20; Güsewell 2004). The N/P ratios of the foliar biomass were all below 31 and in some cases also below 22. They were also much lower than N/P ratios reported for temperate or tropical forests (48–95; McGroddy et al. 2004) which are known to be limited by phosphorus, at least on highly weathered, old soils (Vitousek and Farrington 1997). Thus, our results indicate that our system is at least co-limited by nitrogen and phosphorus and in some cases only limited by nitrogen.
The N/P ratios of the vegetation on the polygon ridges were slightly higher than in the polygon depressions, possibly indicating higher nitrogen limitation in the polygon depressions. However, woody plants—which occur mainly on the polygon ridges—have been shown to have higher N/P ratios than herbaceous plants (Sardans et al. 2012). Furthermore, ericoid mycorrhizae have been shown to have access to nitrogen bound in proteins of the soil organic matter (Bending and Read 1996). Therefore, the plant-species composition could account for the different N/P ratios between the polygon depressions and the polygon ridges.
Considering molar-based N/K ratios below 5.9 and molar-based K/P ratios above 2.7 in the foliar biomass, we can exclude potential potassium limitation of the vegetation (mass-based N/K and K/P ratios of 2.1 and 3.4; Olde Venterink et al. 2003). Considering also high contents of labile potassium our results indicate, that potassium is not a limiting factor for plant growth in the polygonal tundra.
As we suggest that plant-growth in the investigated polygon is most or only limited by nitrogen, microbial nitrogen fixation and mineralization appear to be key processes for plant growth in the polygonal tundra. Microbial nitrogen mineralization rates have been shown to be lowest in the arctic tundra in comparison with other ecosystems (Nadelhoffer et al. 1992) but are expected to increase in the course of climate change (Rustad et al. 2001). On the other hand, microbial nitrogen fixation is already a major nitrogen input in arctic ecosystems (Hobara et al. 2006).
Considering the present knowledge about nutrient limitation of arctic plant communities, these results are consistent with the common picture of arctic nutrient limitation. However, our detailed results on the distribution of macronutrients across pools of different availability allow some enhanced understanding of nutrient limitation and element cycle processes in the soil-vegetation system. The N/P ratios of the inorganic fraction in all samples were below the N/P ratios of the vegetation. This implies that plant growth is limited by nitrogen. We found only low contents of DIN and labile phosphorus. Only in the uppermost Oi horizons, we found higher contents of labile phosphorus. Considering the higher nitrogen demand of plants, lower contents of phosphorus than nitrogen in the Oe and Oa horizons do not imply phosphorus limitation. There is at least deficient input of inorganic nitrogen into the soils. Probably most of the mineralized nitrogen is immediately taken up by the vegetation. However, as the DON fraction dominates over the DIN fraction, it can be assumed that amino acids are used by plants rather than becoming mineralized to ammonium (Schimel and Bennett 2004). Thus, the present plant communities may sufficiently be supplied by nitrogen, but there are little nutrient resources to support further productivity increases of the vegetation.
The contents of nitrate were twice as high in the Oi horizons of the polygon ridges as in the Oi horizons in the polygonal depressions. As aerobic conditions are required to oxidize ammonium to nitrate, we could expect lower water contents on the polygon ridges. Though there were no significant differences in the gravimetrical water contents between the polygon ridges and the polygonal depressions during the sampling, the polygon ridges were clearly dryer on a volumetrical basis than the soils in the polygonal depression. As aerobic conditions are commonly found only on the polygon ridges (e.g. Fiedler et al. 2004) measurement of volumetric water contents or O2 measurements probably could have shown differences between the polygon ridges and polygonal depressions. Therefore, the microtopography of the polygon probably accounts for the different nitrate contents on the polygon ridges and in the polygonal depressions.
The highest contents of microbial biomass (as measured as Cmic and Nmic) were found in the uppermost, organic-rich horizons. This reflects the results of previous studies (e.g. Fierer et al. 2003). In contrast to linear dependencies of microbial carbon to total organic carbon (Anderson and Domsch 1989), we found an exponential relationship between microbial carbon and total carbon. The uppermost horizons of permafrost soils provide the best hydrological and thermal conditions. So these horizons offer the best habitat for microbial activity. Also microbial nitrogen depended on the amount of microbial carbon. However, the amount of microbial phosphorus was neither related to microbial carbon nor to microbial nitrogen. N/P ratios of microbial communities are known to be relatively independent from the nutrient supply ratios which is known as metabolic homeostasis (Cleveland and Liptzin 2007; Hartman and Richardson 2013). By maintaining a homeostatic metabolism, the average N/P ratio of microbial organisms in soils is between 6 and 7 (Cleveland and Liptzin 2007; Xu et al. 2013). Also in the lower horizons (Oe, Oa) of our investigation site, the average N/P ratio of the microbial biomass was in this range. Only in the Oi horizons it was significantly higher, and also the variability of the microbial N/P ratios was much greater.
As microbial biomass (carbon and nitrogen)—unlike microbial phosphorus—was decreasing with depth, these high N/P ratios could indicate phosphorus limitation of the microbial communities in the uppermost horizons. The uppermost soil horizon provides the interface between soils and atmosphere, and most nitrogen fixation might happen in this area. However, as we have shown before, these horizons are the zone of the highest microbial activity and thus also for mineralization processes. According to the growth rate hypothesis (Elser et al. 1996; Sterner and Elser 2002; Makino et al. 2003), organismal growth rate is dependent on the phosphorus content as a measure of ribosomal RNA. Hence, further development and growth of microbial populations in the polygonal tundra might be limited by phosphorus.
The mineralized labile phosphorus was most related to the content of nitrogen in the microbial biomass. Decomposition processes are mainly catalyzed by enzymes which are built of proteins. Hence, microbial nitrogen appears to be more important for nutrient mineralization than microbial carbon. However, there was no relationship between the content of DIN and the microbial biomass. Additionally and quite similar to microbial growth, phosphorus is most needed in the early phase of plant growth (Ågren 2004). As the field campaign was conducted in the later vegetation period, some accumulation of labile phosphorus in the zone of highest microbial activity can be assumed. Also low contents of DIN are consistent with the sampling period: Though there is continuous nitrogen mineralization throughout the growing season (Chapin 1996), there is large seasonal variability in the availability of inorganic nitrogen and phosphorus. Depletion of inorganic nitrogen is associated with plant growth in July (Weintraub and Schimel 2005b).
Potassium contents were not related to the microbial biomass. It easily leaches from the litter without microbial decomposition (Titus and Malcolm 1992). Since potassium is not limiting the plant growth in the polygonal tundra, there is a large accumulation of plant-available labile potassium in the uppermost horizons in comparison to phosphorus and nitrogen. Also in comparison to other ecosystems, labile potassium in our study was as high as total potassium in boreal peatlands (Wind-Mulder et al. 1996) and twice as high as in tropical peatlands (Sjögersten et al. 2010). Overall, there were no differences in the amount of total potassium between the different soil horizons. However, the ratio of bioavailable potassium to residual potassium was changing greatly with depth. The cation exchange capacity (CEC) of soil organic matter increases with decomposition (Scheffer and Schachtschabel 2002; Oorts et al. 2003). Thus, in the lower horizons most of the potassium appears to be adsorbed to the surfaces of the organic matter.
Contrarily to the biologically active fractions (DIN, DON and microbial), the residual “passive fraction” showed a large excess in nitrogen. On average, the global ratio of nitrogen to phosphorus in the bulk soil is about 13 (Cleveland and Liptzin 2007). In contrast, the average N/P ratio of the bulk soil in our samples was between 33 and 40. The amount of “passive” nitrogen, which is less accessible for microorganisms, was more than 20-fold higher than the amount of “passive” phosphorus. Up to 40 % of the total phosphorus is already part of the active cycling in the investigated tundra soils but only 5 % of the total nitrogen was found in the biologically active fractions. Thus, higher decomposition rates of the soil organic matter in the course of climate change (Weintraub and Schimel 2003; Aerts 2006) could eventually result in an excess of bioavailable nitrogen.
Additionally, hydrologic properties have large implications for the further progression of arctic peatlands. Under dry conditions, decomposition and CO2 production is favored, while water-saturated conditions promote methane production (Tveit et al. 2013). Hydrologic properties following permafrost degradation show large spatial and small-scale variability (O’Donnell and a., M. T. Jorgenson, J. W. Harden, a. D. McGuire, M. Z. Kanevskiy, and K. P. Wickland. 2011), but a large decrease of arctic wetlands is projected in the course of climate change (Avis et al. 2011). Thus, increased temperatures and enhanced mineralization rates could lead to a switchover from nitrogen limitation to phosphorus limitation as the pool of potentially mineralizable phosphorus is comparatively restricted.
Conclusions
Presently, nitrogen mineralization and fixation appear to be crucial for plant nutrition in the soil-vegetation system of the polygonal tundra. Possibly, nitrogen fixation and mineralization as key processes for plant nutrition could already be limited by phosphorus. Our data suggest that higher mineralization rates can lead in the long term to strict phosphorus limitation of the plant-available nutrients in the polygonal tundra.
However, we cannot present data for the plant litter stoichiometry in this paper. The stoichiometry of the plant litter influences the stoichiometry of the microbial communities (Wang et al. 2014), controls soil organic matter decomposition rates (Güsewell and Freeman 2005) and thus is important for the nutrient resorption efficiency. Detailed analyses of litter stoichiometry should be done in follow-up studies to show the whole stoichiometric cascade from foliar biomass over litter and microbial biomass to soil nutrients allowing to clearly define the nutrient limitation in this ecosystem. Also, it remains unclear whether the residual fractions of phosphorus are potentially mineralizable or not. Thus, further analyses on the soil phosphorus are needed to distinguish the amount of phosphorus which is bound in more mobile forms (e.g. calcium phosphates) from the amount of phosphorus in stable forms (e.g. occluded Fe and Al phosphates, humic acids) and even the amount of phosphorus in resistant and inaccessible forms.
References
Aber JD, Melillo JM (2001) Terrestrial ecosystems, 2nd edn. Academic Press, San Diego
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Ågren GI (2004) The C:N:P stoichiometry of autotrophs—theory and observations. Ecol Lett 7:185–191
Ågren GI, Wetterstedt JÅM, Billberger MFK (2012) Nutrient limitation on terrestrial plant growth—modeling the interaction between nitrogen and phosphorus. New phytol 194:953–960
Anderson T, Domsch KH (1989) Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol Biochem 21:471–479
Avis CA, Weaver AJ, Meissner KJ (2011) Reduction in areal extent of high-latitude wetlands in response to permafrost thaw. Nat Geosci 4:444–448
Bending GD, Read DJ (1996) Nitrogen mobilization from protein-polyphenol complex by ericoid and ectomycorrhizal fungi. Soil Biol Biochem 28:1603–1612
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brown J, OJ. Ferrians JA, Heginbottom, ES Melnikov (1998) Circum-arctic map of permafrost and ground ice conditions. Boulder, CO: National Snow and Ice Data Center, Digital media, 1998, revised February 2001
Chapin DM (1996) Nitrogen mineralization, nitrification and denitrification in a high arctic lowland ecosystem, Devon Island, N.W.T, Canada. Arctic Alp Res 28:85–92
Chapin FS, Barsdate RJ, Barèl D (1978) Phosphorus cycling in Alaskan Coastal Tundra: a hypothesis for the regulation of nutrient cycling. Oikos 31:189–199
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Chernov YI, Matveyeva NV (1997) Arctic Ecosystems in Russia. In: Wielgolaski FE (ed) Ecosystems of the World, part 3: polar and alpine tundra. Elsevier, Amsterdam, pp 361–507
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Czerepanov SK (1995) Vascular plants of Russia and adjacent states (the former USSR). Cambridge University Press, Cambridge
De Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
De Klerk P, Donner N, Karpov NS, Minke M, Joosten H (2011) Short-term dynamics of a low-centred ice-wedge polygon near Chokurdakh (NE Yakutia, NE Siberia) and climate change during the last ca 1250 years. Quatern Sci Rev 30:3013–3031
De Klerk P, Teltewskoi A, Theuerkauf M, Joosten H (2014) Vegetation patterns, pollen deposition and distribution of non-pollen palynomorphs in an ice-wedge polygon near Kytalyk (NE Siberia), with some remarks on Arctic pollen morphology. Polar Biology 37:1393-1412
Elser JJ, Dobberfuhl DR, Mackay NA, Schampel JH (1996) Organism size, life history and N:P stoichiometry. Bioscience 46:674–684
Elton-Bott RR (1977) A modified spectrophotometric method for nitrate plants, soils and water by nitration of 3,4-dimethylphenol. Anal Chim Acta 90:215–221
Eriksson T, Öquist MG, Nilsson MB (2010) Production and oxidation of methane in a boreal mire after a decade of increased temperature and nitrogen and sulfur deposition. Glob Change Biol 16:2130–2144
Fiedler S, Wagner D, Kutzbach L, Pfeiffer E-M (2004) Element redistribution along hydraulic and redox gradients of low-centered polygons, Lena Delta, Northern Siberia. Soil Sci Soc Am J 68:1002–1011
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
French HM (2007) The periglacial environment. Wiley, West Sussex
Frey W, Frahm JP, Fischer E, Lobin W (1995) Die Moos- und Farnpflanzen Europas. Fischer, Stuttgart
Giesler R, Esberg C, Lagerström A, Graae BJ (2012) Phosphorus availability and microbial respiration across different tundra vegetation types. Biogeochemistry 108:429–445
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S, Freeman C (2005) Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N:P ratios. Funct Ecol 19:582–593
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken MES, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
Hartman WH, Richardson CJ (2013) Differential nutrient limitation of soil microbial biomass and metabolic quotients (qCO2): is there a biological stoichiometry of soil microbes? PLoS One 8:e57127
Hedley MJ, Stewart JWB (1982) Method to measure microbial phosphate in soil. Soil Biol Biochem 14:377–385
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Helbig M, Boike J, Langer M, Schreiber P, Runkle BRK, Kutzbach L (2013) Spatial and seasonal variability of polygonal tundra water balance: Lena River Delta, northern Siberia (Russia). Hydrogeol J 21:133–147
Hill DE, Tedrow JCF (1961) Weathering and soil formation in the Arctic environment. Am J Sci 259:84–101
Hobara S, Mccalley C, Koba K, Giblin AE, Weiss MS, Gettel GM, Shaver GR (2006) Nitrogen fixation in surface soils and vegetation in an Arctic tundra watershed: a key source of atmospheric nitrogen. Arct Antarct Alp Res 38:363–372
Ivanoff DB, Reddy KR, Robinson S (1998) Chemical fractionation of organic phosphorus in selected histosols. Soil Sci 163:36–45
Jones D, Willett V (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Koerselman W, Meuleman FM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Kutzbach L, Wagner D, Pfeiffer E-M (2004) Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, northern Siberia. Biogeochemistry 69:341–362
Londo G (1976) The decimal scale for releves of permanent quadrats. Vegetatio 33:61–64
MacDonald NW, Zak DR, Pregitzer KS (1995) Temperature effects on kinetics of microbial respiration and net nitrogen and sulfur mineralization. Soil Sci Soc Am J 59:233–240
Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS (2004) Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443
MacKay JR (2000) Thermally induced movements in ice-wedge polygons, western arctic coast: a long-term study. Géog Phys Quatern 54:41
Makino W, Cotner JB, Sterner RW, Elser JJ (2003) Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C:N:P stoichiometry. Funct Ecol 17:121–130
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85:2390–2401
Michaelis M (2011) Die Sphagnum-Arten der Welt. Bibl Bot 160:1–408
Minke M, Donner N, Karpov NS, De Klerk P, Joosten H (2007) Distribution, diversity, development and dynamics of polygon mires: examples from northeast Yakutia (Siberia). Peatl Int 1:36–40
Minke M, Donner N, Karpov N, De Klerk P, Joosten H (2009) Patterns in Vegetation Composition, Surface Height and Thaw Depth in Polygon Mires in the Yakutian Arctic (NE Siberia): A Microtopographical Characterisation of the Active Layer. Permafrost and Periglacial Processes 20:357–368.
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nadelhoffer KJ, Giblin AE, Shaver GR, Linkins AE (1992) Microbial processes and plant nutrient availability in Arctic soils. In: Chapin FS, Jefferies RL, Reyonolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate. Academic Press, San Diego, pp 281–300
Natali SM, Schuur EAG, Rubin RL (2012) Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. J Ecol 100:488–498
O’Donnell JA, Jorgenson MT, Harden JW, McGuire AD, Kanevskiy MZ, Wickland KP (2011) The effects of permafrost thaw on soil hydrologic, thermal, and carbon dynamics in an Alaskan peatland. Ecosystems 15:213–229
Olde Venterink H, Wassen JM, Verkroost AWM, De Ruiter PC (2003) Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84:2191–2199
Oorts K, Vanlauwe B, Merckx R (2003) Cation exchange capacities of soil organic matter fractions in a Ferric Lixisol with different organic matter inputs. Agric Ecosyst Environ 100:161–171
Parmentier FJW, van Huissteden J, van der Molen MK, Schaepman-Strub G, Karsanaev SA, Maximov TC, Dolman AJ (2011) Spatial and temporal dynamics in eddy covariance observations of methane fluxes at a tundra site in northeastern Siberia. J Geophys Res 116:G03016
Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA (2012) The human-induced imbalance between C, N and P in Earth’s life system. Glob Change Biol 18:3–6
Polunin N (1959) Circumpolar Arctic flora. Clarendon Press, Oxford
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Rothmaler W (2002) Exkursionsflora von Deutschlands Gefäßpflanzen: Grundband. Spektrum Akademischer Verlag, Heidelberg
Russsia´s Weather Server 2013. http://meteo.infospace.ru/wcarch/html/index.sht
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Saito MA, Goepfert TJ, Ritt JT (2008) Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnol Oceanogr 53:276–290
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111:1–39
Scheffer F, Schachtschabel P (2002) Lehrbuch der Bodenkunde, 15th edn. Spektrum Akademischer Verlag, Heidelberg
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schmidtlein S, Tichý L, Feilhauer H, Faude U (2010) A brute-force approach to vegetation classification. J Veg Sci 21:1162–1171
Selmer-olsen AR (1971) Determination of ammonium in soil extracts by an automated indophenol method. Anaylst 96:565–568
Sjögersten S, Cheesman AW, Lopez O, Turner BL (2010) Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry 104:147–163
Soil Survey Staff (2010) Keys to soil taxonomy, 11th edn. USDA-Natural Resources Conservation Service, Washington, D.C
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial c by a fumigation-extraction method: use on soils of high organic matter content, and a reassessment of the kec-factor. Soil Biol Biochem 22:301–307
Sterner RW, Elser JJ (2002) Ecological stoichiometry, 1st edn. Princeton University Press, Princeton
Teltewskoi A, Seyfert J, Joosten H (2012) Records from the model polygon Lhc11 for modern and palaeoecological studies. In: Schirrmeister L, Pestryakova LA, Wetterich S, Tumskoy VE (eds) Reports on polar and marine research—joint Russian–German polygon project east Siberia 2011–2014: The expedition Kytalyk 2011. Alfred-Wegener-Institute, Bremerhaven, pp 51–60
Titus BD, Malcolm DC (1992) Nutrient leaching from the litter layer after clearfelling of sitka spruce stands on peaty gley soils. Forestry 65:389–416
Tolmachev AI (1974) Opredelitel´vysshikh rasteniy Yakutii. Nauka, Yakutsk
Tumskoy VE, Schirrmeister L (2012) Study area, geological and geographical characteristics. In: Schirrmeister L, Pestryakova LA, Wetterich S, Tumskoy VE (eds) Reports on polar and marine research—joint Russian–German polygon project east Siberia 2011–2014: The expedition Kytalyk 2011. Alfred-Wegener-Institute, Bremerhaven, pp 5–10
Tveit A, Schwacke R, Svenning MM, Urich T (2013) Organic carbon transformations in high-Arctic peat soils: key functions and microorganisms. ISME J 7:299–311
Van der Molen MK, van Huissteden J, Parmentier FJW, Petrescu AMR, Dolman AJ, Maximov TC, Kononov AV, Karsanaev SV, Suzdalov DA (2007) The growing season greenhouse gas balance of a continental tundra site in the Indigirka lowlands, NE Siberia. Biogeosciences 4:985–1003
Van Huissteden J, Maximov TC, Dolman AJ (2005) High methane flux from an arctic floodplain (Indigirka lowlands, eastern Siberia). J Geophys Res 110:1–8
VDLUFA (1991) Methodenbuch. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, Darmstadt
Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Wang M, Moore TR, Talbot J, Richard PJH (2014) The cascade of C:N:P stoichiometry in an ombrotrophic peatland: from plants to peat. Environ Res Lett 9:024003
Weintraub MN, Schimel JP (2003) Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in Arctic tundra soils. Ecosystems 6:129–143
Weintraub MN, Schimel JP (2005a) Nitrogen cycling and the spread of shrubs control changes in the carbon balance of Arctic tundra ecosystems. Bioscience 55:408–415
Weintraub MN, Schimel JP (2005b) The seasonal dynamics of amino acids and other nutrients in Alaskan Arctic tundra soils. Biogeochemistry 73:359–380
Wille C, Kutzbach L, Sachs T, Wagner D, Pfeiffer E-M (2008) Methane emission from Siberian Arctic polygonal tundra: eddy covariance measuring and modeling. Glob Change Biol 14:1395–1408
Wind-Mulder HL, Rochefort L, Vitt DH (1996) Water and peat chemistry comparisons of natural and post-harvested peatlands across Canada and their relevance to peatland restoration. Ecol Eng 7:161–181
Xu X, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr 22:737–749
Acknowledgments
The study presented here is part of the German-Russian joint project “Polygons in tundra wetlands: state and dynamics under climate variability in tundra regions (POLYGON)” (Russian Foundation of Basic Research, RFBR grant no. 11-04-91332-NNIO-a and German Research Foundation, DFG grant no. KU 1418/3-1 to Lars Kutzbach and JO 332/14-1 to Hans Joosten). F. Beermann, E.-M. Pfeiffer and L. Kutzbach were supported through the Cluster of Excellence “CliSAP” (EXC177), University of Hamburg, funded by the German Research Foundation (DFG). F. Beermann was also supported through a doctoral fellowship of the University of Hamburg in accordance with the Hamburg Act for the Promotion of Young Researchers and Artists (HmbNFG). We thank our colleagues who helped during the expedition in 2011, especially Lyudmillia A. Pestryakova from the North Eastern Federal University in Yakutsk, Hans Joosten and Juliane Seyfert from the Ernst Moritz Arndt University of Greifswald as well as Lutz Schirrmeister from AWI Potsdam. The analytical work in the laboratories of the University of Hamburg was greatly supported by Susanne Kopelke. Determination of the vegetation communities as well as the indicator plant species for each community was generously conducted by Michael Manthey from the Ernst Moritz Arndt University of Greifswald. We thank Stefan Goen and Dierk Michaelis (Ernst Moritz Arndt University of Greifswald) for identifying the moss species. Martin Schrön (Ernst Moritz Arndt University of Greifswald) and Pim de Klerk (Staatliches Museum für Naturkunde, Karlsruhe) assisted in the preparation of Fig. 3 and Fig. 7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Colin Bell
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beermann, F., Teltewskoi, A., Fiencke, C. et al. Stoichiometric analysis of nutrient availability (N, P, K) within soils of polygonal tundra. Biogeochemistry 122, 211–227 (2015). https://doi.org/10.1007/s10533-014-0037-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0037-4