Abstract
Permafrost disturbance is expected to alter nitrogen (N) export in High Arctic watersheds by enhancing loads of dissolved inorganic N (DIN), particularly nitrate (\({\text{NO}}_{3}^{ - }\)), by enabling nitrification and/or the mobilization of N previously sequestered in deeper permafrost soils. Using chemical, isotopic, and hydrologic measurements, we compare the seasonal evolution of concentrations and sources of \({\text{NO}}_{3}^{ - }\) in a stream draining an undisturbed catchment with one that drains a catchment that has been affected by active layer detachments (ALDs) at the Cape Bounty Arctic Watershed Observatory (74°54′N, 109°35′W) on Melville Island, Nunavut. Oxygen stable isotope values of \({\text{NO}}_{3}^{ - }\) (δ18O-\({\text{NO}}_{3}^{ - }\)) from streamwater indicate that \({\text{NO}}_{3}^{ - }\) in runoff predominantly originated from atmospheric sources in both catchments only during the first days of melt. In the undisturbed catchment, low \({\text{NO}}_{3}^{ - }\) concentrations and elevated streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values relative to the disturbed catchment indicate that \({\text{NO}}_{3}^{ - }\) export was suppressed by sink mechanisms. In contrast, low δ18O-\({\text{NO}}_{3}^{ - }\) values and high \({\text{NO}}_{3}^{ - }\) concentrations in runoff from the disturbed catchment indicate that the supply of \({\text{NO}}_{3}^{ - }\) from microbial sources far outweighed sinks in the watershed. This research demonstrates that ALDs enhanced the export of microbially derived \({\text{NO}}_{3}^{ - }\) relative to undisturbed watersheds, and that this is likely a result of limited \({\text{NO}}_{3}^{ - }\) retention and enhanced nitrification in the mineral soils exposed in the scar zones of ALDs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent studies show that permafrost degradation may disrupt nitrogen (N) transport dynamics and lead to greater watershed nitrate (\({\text{NO}}_{3}^{ - }\)) export due to increases in streamwater \({\text{NO}}_{3}^{ - }\) concentrations (Bowden et al. 2008; Harms et al. 2014; Louiseize and Lafrenière 2013). For example, Bowden et al. (2008) reported that \({\text{NO}}_{3}^{ - }\) concentrations immediately downstream of thermokarst gullies and thaw slumps were exceptionally high compared to concentrations upstream of these features. Similar results were documented by Louiseize and Lafrenière (submitted), who show that permafrost disturbances resulted in significantly higher \({\text{NO}}_{3}^{ - }\) concentrations and therefore much larger seasonal fluxes of \({\text{NO}}_{3}^{ - }\) compared to undisturbed watersheds. This is of concern because even minor changes in N export can impact downstream aquatic ecosystem function (McClelland et al. 2007).
Despite the mounting evidence that various types of permafrost disturbance enhance \({\text{NO}}_{3}^{ - }\) concentrations in Arctic rivers (Bowden et al. 2008; Frey and McClelland 2009; Harms and Jones 2012; Harms et al. 2014; Louiseize and Lafrenière submitted), very little is known about the impact of permafrost disturbances on the sources and processes that regulate seasonal \({\text{NO}}_{3}^{ - }\) concentrations in High Arctic streams. Active layer detachments (ALDs) are landslide type slope disturbances that cause the vegetation cover and thawed soils to move downslope along the ice-rich permafrost, thus leading to the exposure of mineral soils in the scar zone as well as amassment of soils at the base of slopes (Lewkowicz and Harris 2005). Hence, ALDs represent a type of permafrost disturbance that can affect hydrologic pathways and response, which are among the main controls of dissolved inorganic N (DIN, including ammonium (\({\text{NH}}_{4}^{ + }\)) and \({\text{NO}}_{3}^{ - }\)) concentrations in streams (Petrone et al. 2006; Cooper et al. 2007). The ensuing changes to subsurface environmental conditions, such as soil temperature, moisture, oxygen availability, and nutrient content, may also stimulate microbial activity (Paulter et al. 2010) and alter the relative importance of productive (e.g., nitrification) and consumptive N cycling processes (e.g., denitrification, vegetative and microbial uptake) in disturbed areas (Harms et al. 2014). ALDs could therefore mobilize relatively older or deeper sources of N that were previously isolated from cycling activities and hydrologic export, as hypothesized for permafrost disturbances in general (Shaver et al. 1992; Levine and Whalen 2001; Jones et al. 2005; McClelland et al. 2007; Frey and McClelland 2009; Woods et al. 2011; Keuper et al. 2012; Harms and Jones 2012; Harms et al. 2014). The occurrence of ALDs and other types of disturbance is expected to increase with regional climate warming (Lamoureux and Lafrenière 2009; Vincent et al. 2013); hence, it is important to understand how these disturbances affect watershed N dynamics and stream \({\text{NO}}_{3}^{ - }\) concentrations.
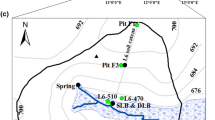
The aim of this research is to investigate how ALDs influence the processes that control streamwater \({\text{NO}}_{3}^{ - }\) concentrations in High Arctic watersheds. We approached this question by comparing the seasonal evolution of the stable isotope compositions of species of \({\text{NO}}_{3}^{ - }\), including nitrogen in nitrate (δ15N-\({\text{NO}}_{3}^{ - }\)) and oxygen in nitrate (δ18O-\({\text{NO}}_{3}^{ - }\)), as well as major ion chemistry in precipitation and runoff from two small headwater catchments, one that is undisturbed and one that has recently been affected by ALDs, at the Cape Bounty Arctic Watershed Observatory (CBAWO) on Melville Island, Nunavut, Canada (Fig. 1). These data are supplemented by measurements of precipitation and stream discharge. To our knowledge, this is the first study that uses stable isotopes of \({\text{NO}}_{3}^{ - }\) to investigate the seasonality and sources of \({\text{NO}}_{3}^{ - }\) in runoff from a disturbed catchment in a continuous permafrost watershed. This is an important step towards understanding how disturbances such as ALDs affect fluvial N export and catchment N dynamics in an understudied region that is experiencing significant environmental change.
Methods
Study site
This study was conducted in two small hillslope catchments within the West watershed at the Cape Bounty Arctic Watershed Observatory (74°54′N, 109°35′W) on Melville Island, Nunavut (Fig. 1). Continuous permafrost underlies the study area and active layer depths in hill slopes are typically between 0.60 and 1 m during the peak growing season. Vegetation communities in the catchments vary according to moisture conditions (Atkinson and Treitz 2012). Low moisture areas host polar desert communities such as Thamnolia subliformis spp. (Worm Lichen) and Cetraria nivalis spp. (Snow lichen) but primarily consist of rock or till. Areas with moderately wet soils are predominantly colonized by mesic heath type vegetation such as Nostoc commune spp. (nitrogen fixing cyanobacteria) and Salix arctica spp., while areas that remain thoroughly saturated throughout the growing season are totally vegetated with wet sedge community type, including Eriophorium spp. and Sphagnum spp. (Atkinson and Treitz 2012).
The disturbed Ptarmigan (PT, unofficial name) catchment encompasses an area of 213,000 m2 and contains three disturbances that formed in late July 2007. These slides were triggered by a significant rainfall event that followed an extended period of unusually warm temperatures (Lamoureux and Lafrenière 2009). Collectively, the ALDs occupy approximately 12 % of the total catchment area (Lafrenière and Lamoureux 2013). These include an isolated, compact ALD (3,774 m2), which was actively retrogressing during the field season, and two elongate ALDs (19,587 and 676 m2) (Ashley Rudy, unpublished data), whose scar zones expose the underlying mineral soils and remain completely free of vegetation. In undisturbed areas of the catchment, the dominant vegetation classes are mesic heath and polar semi-desert. The stream draining PT is well-incised in the mineral soils along most of its length, and flows through the length of the two elongate disturbances. Due to the stream’s linkage to these disturbances, the water exported from PT provides a good representation of the potential impacts of ALDs on water quality and on biogeochemical processes occurring within disturbed soils. The reference catchment, Goose (GS, unofficial name), covers an area of 179,000 m2 and remains completely free of any physical disturbances. The catchment is drained by a poorly incised, vegetated channel or water track. Although GS is the smaller catchment, its topography allows for greater snow accumulation and thus an extended runoff period compared to PT. The dominant vegetation communities in this catchment are mesic heath and wet sedge.
The snowmelt season typically lasts from early to mid-June until mid-July, when river discharge is primarily sustained by snowmelt from the thick snow packs in channels and depressions. Beyond early or mid July, hill slope streams typically dry up, and flow only resumes following rainfall (Lafrenière and Lamoureux 2013). Rainfall events are typically infrequent and of low magnitude (Lafrenière and Lamoureux 2008), but mean precipitation amounts in the summer have ranged between 2.5 and 66.8 mm from 2003 to 2012 (Favaro and Lamoureux submitted).
Hydrological and environmental measurements
Instantaneous discharge was obtained by converting measurements of stage taken within 8” cutthroat flumes at the outlet of each catchment using standard equations for the flumes (American Society of Civil Engineers 1974). Stage was recorded every 10 min using Onset U20 level loggers (±2 mm) that were adjusted for barometric pressure using a second U30 logger installed at Main Met (Fig. 1). Instantaneous discharge was converted to average hourly discharge (Q; m3 s−1) so as to smooth out the hydrograph. In-situ water temperature (±1 °C), pH (±0.1 pH), and electrical conductivity (±2 % μS cm−1) were measured during stream sampling with an Extech Exstik II pH/conductivity meter. Shielded air temperature was recorded at Main Met 1.5 m above ground using an Onset Temperature/RH smart sensor (±0.21 °C temperature, ±2.5 % relative humidity) and precipitation was measured with an Onset industrial tipping bucket gauge (0.2 mm).
Hydrologic regimes
Seasonal hydrographs for PT and GS were divided into nival melt, baseflow, and stormflow seasons according to seasonal changes in discharge and the occurrence of rainfall. The nival melt period was determined to commence on June 5 and transitioned into baseflow on June 16, which lasted until the end of streamflow on June 24 in PT and July 1 in GS. The stormflow period encompasses two series of runoff-generating precipitation events that rejuvenated discharge in both streams from July 9–10 and from July 18–25 (Fig. 2b, d).
a Dissolved ion concentrations in Goose and daily cumulative rainfall. b \({\text{NO}}_{3}^{ - }\) concentrations and mean hourly discharge in Goose. c Dissolved ion concentrations in Ptarmigan and daily cumulative rainfall. d \({\text{NO}}_{3}^{ - }\) concentration and mean hourly discharge in Ptarmigan. Note the differences in scale for \({\text{NO}}_{3}^{ - }\) and major ion concentrations between Goose and Ptarmigan
Sample collection
Stream water sampling began on the first day of flow (June 5, 2012) and continued until the end of baseflow (June 24 in PT and July 1 in GS) and during stormflow (July 9–10; July 18–25). Samples were collected at stream outlets twice daily at approximately 10:00 and 18:00, near the times of minimum and maximum discharge, respectively, during the nival melt and stormflow periods, and once daily at approximately 18:00 during baseflow. Samples were collected using 1 L Nalgene bottles, which were tripled-rinsed with distilled water prior to deployment and with streamwater immediately before sampling. The bottles were completely filled and were kept cool and in the dark until filtration (typically within 30–120 min).
Precipitation samples were collected using passive rainfall samplers. These consisted of 20 L polycarbonate funnels (24 cm diameter) that were secured within a PVC frame so that the openings were elevated 50 cm above ground. The funnel drained into a removable 45 mL amber EPA vial by means of 10 cm long Tygon R-3603 tubing, which passed through the Teflon septum of the vial. The EPA vial was suspended within a removable 1 L Nalgene bottle that was secured to the collector and covered with black out material so as to protect the collection vial from UV light. In order to exclude dry deposition, the funnels were covered with plastic lids at all times except during precipitation events, when the covers were manually removed. Upon sample retrieval, the EPA vials were removed from the collectors, sealed with new caps, and replaced with a clean vial. All vials and caps were triple rinsed with distilled water before and after use.
All samples for dissolved ion analysis (including dissolved inorganic N (DIN)) were syringe filtered through 0.22 μm PVDF membrane filters. Syringes were triple-rinsed with distilled water before and after use, and new filters were used for each sample. All filters were rinsed with 10 mL of sample that was discarded before filling the vials. Samples were stored in HDPE vials or bottles that were pre-cleaned (triple soaked and rinsed with Milli-Q water at Queen’s University), rinsed with filtered sample, and filled with no headspace. Aliquots for dissolved ion analysis were refrigerated until analyzed at Queen’s University, while samples for stable isotope analyses (15N/14N and 18O/16O of \({\text{NO}}_{3}^{ - }\)) were frozen in the field and during shipment, and thawed immediately prior to analysis at Brown University.
Stream samples that were collected for TDN and dissolved organic carbon (DOC) analyses were filtered through pre-combusted 0.7 μm glass fiber filters (GF/F) with a glass filtration apparatus. The filtration apparatus was triple-rinsed with distilled water before use as well as between samples and soaked in 3 % hydrochloric acid every night. TDN and DOC samples were acidified to pH 2 and kept in 45 ml amber EPA vials with Teflon lined septa that were rinsed with filtered sample and filled to eliminate headspace. Samples were refrigerated and kept in the dark until analyzed at Queen’s University.
Analytical methods
Major ions and N species
Dissolved inorganic anions (Cl−, \({\text{SO}}_{4}^{2 - }\), \({\text{NO}}_{2}^{ - }\), \({\text{NO}}_{3}^{ - }\)) and cations (K+, Na+, Ca2+, and Mg2+) were quantified simultaneously by liquid ion chromatography with a Dionex ICS-3000. Anions were measured using KOH eluent with a gradient elution of 11–40 mM KOH flowing at 1.0 ml min−1 through an AS18 analytical column and an ASRS 300 suppressor. Cations were quantified isocratically using 16 mM methane sulphonic acid eluent flowing at 0.5 ml min−1 through a CS12A-5 analytical column and a CSRS 300 suppressor. Detection limits were calculated as three times the standard deviation of replicates of the low level standards, and were <0.005 ppm except for Mg2+ (0.016 ppm) and Ca2+ (0.039 ppm). Nonetheless, the concentrations of these species in the samples were at least 100 times higher than these background levels. The analytical uncertainty for most ion analyses, determined by the mean standard error of replicate samples, was better than 2 %. For \({\text{NO}}_{3}^{ - }\), the analytical uncertainty was 2 % and the detection limit was 0.003 ppm (or 0.7 ppb N). For \({\text{NO}}_{2}^{ - }\), the analytical error was 2.5 % and the detection limit was 0.01 ppb (or 0.03 ppb N); however \({\text{NO}}_{2}^{ - }\) in most samples was below detection limit.
Concentrations of \({\text{NH}}_{4}^{ + }\) were measured by colorimetry using an Astoria Pacific FASPac II Flow Analyser. The detection limit was <0.01 ppm N and the analytical error was 0.9 %. DIN was calculated as the sum of the N mass from \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\) and \({\text{NO}}_{2}^{ - }\) (when detectable).
Samples of DOC and TDN were analyzed simultaneously by high temperature combustion and NDIR and chemiluminescent detection using a Shimadzu TOC-VPCH/TNM equipped with a high sensitivity catalyst system. Analytical errors for DOC and TDN were 2.9 and 2.3 %, respectively based on replicate analyses of standards. Detection limits were 0.080 ppm for DOC and 0.015 ppm N for TDN. DON was determined as the difference between TDN and DIN.
Stable isotope ratios
The stable isotope ratio of oxygen in water (H2O; 18O/16O) was measured using a Los Gatos Research Liquid–Water Isotope Analyzer, which vaporizes injected sample and measures its absorbance relative to Vienna Standard Mean Ocean (VSMOW). All sample runs include replicate analyses of standards produced by Los Gatos (e.g., LGR1A, δ18O = −19.5 ‰ and δD = −154.3 ‰; LGR3A, δ18O = −13.1 ‰ and δD = −96.4 ‰), and samples are corrected to their values. Reproducibility (1σ) based on repeated measurements of standards is ±0.25 ‰ for δ18O. The standard deviation for replicate analyses of samples in this dataset was 0.05 ‰ for δ18O.
Stable isotope ratios of species of \({\text{NO}}_{3}^{ - }\) (15N/14N and18O/16O) were quantified using the denitrifier method at Brown University and standardized based on N2 in air and VSMOW, respectively. This technique employs denitrifying bacteria that lack N2O-reductase in order to transform as little as 5–10 nmol of \({\text{NO}}_{3}^{ - }\) in water samples to N2O, the latter of which is then analyzed for stable isotope composition by an isotope ratio mass spectrometer in continuous flow mode (Sigman et al. 2001; Casciotti et al. 2002). All sample runs include replicate analyses of \({\text{NO}}_{3}^{ - }\) standards USGS34, USGS35, and IAEA-N3, and sample values are corrected to the internationally recognized values for δ15N (USGS34 (−1.8 ‰); IAEA-N3 (+4.7 ‰)) and δ18O (USGS34 (−27.9 ‰), USGS35 (+57.5 ‰), IAEA-N3 (+25.6 ‰)) (Böhlke et al. 2003) based on the framework presented in Kaiser et al. (2007). Typical reproducibility (1σ) based on repeated measurements of isotopic standards is ±0.2 ‰ for δ15N and ±0.6 ‰ for δ18O. The paired standard deviation for replicate analyses of samples in this dataset was 0.1 and 0.5 ‰ for δ15N and δ18O, respectively. The majority of samples were run with 10 nmol of \({\text{NO}}_{3}^{ - }\), however, if sample volume or concentration was limited, analyses were completed with only 5 nmol of \({\text{NO}}_{3}^{ - }\).
Statistical analyses
Pearson’s correlation analyses were applied in order to determine relationships between \({\text{NO}}_{3}^{ - }\)and other dissolved organic and inorganic ions (e.g., DON, DOC, NH4 +, Cl−, \({\text{SO}}_{4}^{2 - }\), K+, Na+, Mg2+, and Ca2+) for the nival, baseflow, and stormflow periods in PT and GS. All analyses were performed in StatPlus using a significance level of α = 0.02.
Results
Hydrology
Seasonal discharge varied somewhat between catchments. Discharge commenced on June 5 in both streams, and peak snowmelt discharge occured on June 6 in GS and June 8 in PT (Fig. 2b, d). Streamflow decreased considerably after June 15 in both catchments and ceased in PT on June 24, while residual snow packs and wetter soils in GS sustained baseflow until July 1. Discharge was rejuvenated from July 9–10 in both streams due to a hydrologically significant rainfall event (Fig. 2b, d); however, dry antecedent moisture conditions muted the hydrograph responses. Due to wetter antecedent moisture conditions, subsequent smaller magnitude rainfall events regenerated and sustained discharge in both catchments from July 18 to July 25, and permitted the mean hourly discharge to reach 0.03 m3 s−1 in PT and 0.007 m3 s−1GS on July 24 (Fig. 2b, d).
Dissolved ion chemistry and N species composition
In GS, all dissolved ions except for \({\text{NO}}_{3}^{ - }\) followed similar trends and \({\text{NO}}_{3}^{ - }\) concentrations were generally low throughout the season (Fig. 2a, b). \({\text{NO}}_{3}^{ - }\) in GS was near detection limit at the onset of melt and peaked at 16.2 ppb N on June 7 concomitant with the second peak in snowmelt runoff (Fig. 2b). \({\text{NO}}_{3}^{ - }\) decreased to below detection limit (BDL) as discharge declined on June 9, where as major ions (Cl−, \({\text{SO}}_{4}^{2 - }\), Na+, Mg2+, and Ca2+) reached their peak concentrations of the nival melt period at this time. Hence, \({\text{NO}}_{3}^{ - }\) concentrations during the nival melt (June 5–15) in GS were not significantly correlated with any ion (Table 1). The mean \({\text{NO}}_{3}^{ - }\) concentration during nival melt in GS was 2.4 ± 4.0 ppb N and that during baseflow was 2.1 ± 2.2 ppb N (Table 2).
During stormflow, \({\text{NO}}_{3}^{ - }\) in GS runoff was mostly BDL even though rainfall \({\text{NO}}_{3}^{ - }\) concentrations were as high as 124 ppb N. All ion concentrations (except \({\text{NO}}_{3}^{ - }\)) increased dramatically with discharge on July 24 and reached their seasonal highs on July 25. No significant correlations existed between \({\text{NO}}_{3}^{ - }\) and other dissolved ions during stormflow (Table 1). The mean stormflow \({\text{NO}}_{3}^{ - }\) concentration (for all events from July 9 to 25) was 0.90 ± 1.5 ppb N (Table 2).
In PT, \({\text{NO}}_{3}^{ - }\) and major ion concentrations greatly exceeded those of GS and trends of \({\text{NO}}_{3}^{ - }\) closely followed those of major ion concentrations except during baseflow (June 16–24). \({\text{NO}}_{3}^{ - }\) concentrations during the nival melt were significantly correlated with DON, DOC, and all major ions (Table 1). The peak melt \({\text{NO}}_{3}^{ - }\) concentration in PT occurred on the second day of melt and measured 193 ppb N, thereby exceeding the peak \({\text{NO}}_{3}^{ - }\) concentration in GS by a factor of twelve. \({\text{NO}}_{3}^{ - }\) concentrations in PT decreased to 4.3 ppb N by June 9, but higher concentrations (15–51 ppb N) were sustained from June 10 to June 20 before gradually decreasing to near detection limit until the end of streamflow (June 24). During the nival melt, the mean \({\text{NO}}_{3}^{ - }\)concentration was 44 ± 42 ppb N. The mean \({\text{NO}}_{3}^{ - }\) concentration during baseflow was 18 ± 14 ppb N.
Stormflow runoff produced the highest measured solute concentrations in PT. On July 18, shortly after rainfall runoff recommenced, \({\text{NO}}_{3}^{ - }\) reached a seasonal high of 348 ppb N, thus exceeding the highest recorded rainfall \({\text{NO}}_{3}^{ - }\) concentration by 224 ppb N. Streamwater \({\text{NO}}_{3}^{ - }\) in PT measured above 128 ppb N until July 20. \({\text{NO}}_{3}^{ - }\) in PT during stormflow was significantly positively correlated with Na+ and \({\text{SO}}_{4}^{2}\) and had strong negative relationships with DON and DOC (Table 1). The dominant ion during stormflow was \({\text{SO}}_{4}^{2 - }\) (279 ± 87 ppm) and the mean stormflow \({\text{NO}}_{3}^{ - }\)concentration was 149 ± 121 ppb N.
Stable isotopes of \({\text{NO}}_{3}^{ - }\)
Although samples for stable isotopes of \({\text{NO}}_{3}^{ - }\) were collected throughout the entire sampling campaign, only a subset of samples was selected for analysis based on notable changes in discharge and \({\text{NO}}_{3}^{ - }\) concentration. Note that \({\text{NO}}_{3}^{ - }\) concentrations in GS were often below the analytical requirement (5–10 nmol), hence there are fewer data points for GS relative to PT.
δ15N-\({\text{NO}}_{3}^{ - }\)
The seasonal δ15N values of streamwater \({\text{NO}}_{3}^{ - }\)were on average higher in PT (+4.1 ‰, n = 15) than in GS (+1.6 ‰, n = 11) and ranged from −7.1 to +15.8 ‰ in PT and from −7.9 to +13.6 ‰ in GS (Fig. 3a). Values of δ15N-\({\text{NO}}_{3}^{ - }\) were negative during the initial days of streamflow in both catchments (from −7.1 to −2.6 ‰ in PT and −7.3 to −4.1 ‰ in GS from June 5 to 7) and increased towards positive values after June 7. The δ15N-\({\text{NO}}_{3}^{ - }\) values in both streams were exceptionally high near the end of baseflow, peaking at +15.8 ‰ in PT on June 24 and reaching +12.3 ‰ on June 26 in GS (Fig. 3a). The seasonal peak δ15N-\({\text{NO}}_{3}^{ - }\)value in GS (+13.6 ‰) occurred at the onset of the second stormflow runoff event on July 18.
a Daily cumulative rainfall amount and values of δ15N-\({\text{NO}}_{3}^{ - }\) in rainfall and streamwater from Goose and Ptarmigan. b Values of δ18O-\({\text{NO}}_{3}^{ - }\) in rainfall and stream water from Goose and Ptarmigan. The black dashed box represents the expected range of δ18O-\({\text{NO}}_{3}^{ - }\) values of \({\text{NO}}_{3}^{ - }\) produced from nitrification (−10.6 to −2.2 ‰)
Rainwater δ15N-\({\text{NO}}_{3}^{ - }\) values generally remained between +0.1 and +2.0 ‰ (mean = +1.0 ‰ ± 1.0, n = 7), thus measuring several permil lower than stormflow runoff values. The highest rainfall δ15N value measured +2.7 ‰ and was collected on July 24 at 2:30 am (Fig. 3a).
δ18O-\({\text{NO}}_{3}^{ - }\)
Values of δ18O-\({\text{NO}}_{3}^{ - }\) were highly enriched during the beginning of streamflow (June 5–7) in both catchments compared to the rest of the season (Fig. 3b). During the first 3 days of sampling, δ18O-\({\text{NO}}_{3}^{ - }\) ranged from + 60.3 to + 71.3 ‰ (mean = 66.6 ‰ ± 5.7, n = 3) in PT and from +71.9 to +81.2 ‰ (mean = 77.4 ‰ ± 5.7, n = 4) in GS. The δ18O-\({\text{NO}}_{3}^{ - }\) values in PT dropped to +27.2 ‰ on June 8 and remained below +9.5 ‰ after June 13 except on the last day of baseflow (June 24) when δ18O reached +22.7 ‰. The δ18O-\({\text{NO}}_{3}^{ - }\) values in GS were typically higher than those of PT after June 12, but did fall to −9.7 ‰ on June 15 and −0.7 ‰ on June 20.
During stormflow, δ18O-\({\text{NO}}_{3}^{ - }\) values in PT plotted between −7.2 and +3.4 ‰, and those in GS measured +13.1 and +14.6 ‰. The δ18O-\({\text{NO}}_{3}^{ - }\) values of rainwater \({\text{NO}}_{3}^{ - }\) varied between +66.7 and +80.1 ‰ (mean = +71.4 ‰ ± 4.3).
Discussion
Constraining sources of \({\text{NO}}_{3}^{ - }\)
In unpolluted watersheds, \({\text{NO}}_{3}^{ - }\) in runoff predominantly originates from atmospheric (\({\text{NO}}_{3}^{ - }\) in snow or rain) or microbial (\({\text{NO}}_{3}^{ - }\) mineralized from soil or dissolved organic matter (SOM, DOM) or nitrified from \({\text{NH}}_{4}^{ + }\)) sources. Streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values can be used to determine the relative contributions of these two sources to \({\text{NO}}_{3}^{ - }\) in runoff because atmospheric and microbial processes impart δ18O-\({\text{NO}}_{3}^{ - }\) values that are readily distinguishable from one another (Kendall et al. 2007). Atmospheric \({\text{NO}}_{3}^{ - }\) is characterized by high δ18O values due to the signature imparted by ozone (O3) (~100 ‰) during formation of nitrogen oxides (NOx) in the atmosphere (Hastings et al. 2003). Hence, high streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values (>+60 ‰) indicate inputs primarily from atmospheric deposition (Kendall et al. 2007). Much lower streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values (−10 to +10 ‰ with δ18O-H2O values between −25 and +4 ‰) point towards microbial contributions because of the relatively low stable isotope values for oxygen in the water (H2O) and air (O2) involved in nitrification, a process whereby \({\text{NH}}_{4}^{ + }\) is oxidized to \({\text{NO}}_{3}^{ - }\) under aerobic conditions (e.g., \({\text{NH}}_{4}^{ + }\)→ \({\text{NO}}_{2}^{ - }\) → \({\text{NO}}_{3}^{ - }\)) (Kendall et al. 2007). Studies of nitrification have shown that atmospheric O2 and H2O typically provide 1/3 and 2/3, respectively, of the oxygen atoms in nitrified \({\text{NO}}_{3}^{ - }\) (Kendall 1998). Using this relationship, the range of potential streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values derived from microbial nitrification(δ18O-\({\text{NO}}_{{3\;{\text{nitrification}}}}^{ - }\)) can be calculated by employing the following equation:
where δ18O-O2 represents the stable isotope composition of atmospheric O2 (which is assumed to be +23.5 ‰, Kendall et al. 2007) and δ18O-H2O is the isotope composition of the streamwater collected. Using streamwater δ18O-H2O values from this study (−27.4 to −14.9 ‰, Table 3), Eq. 1 yields a range of δ18O-\({\text{NO}}_{{3\;{\text{nitrification}}}}^{ - }\) values between −10.6 and −2.2 ‰. Although more recent laboratory studies suggest that there may be a greater dependence on the 18O in H2O used during nitrification (Buchwald and Casciotti 2010; Snider et al. 2010), the data in this study are well captured by the range predicted by the 1/3 and 2/3 relationship. Note that δ18O-\({\text{NO}}_{{3\;{\text{nitrification}}}}^{ - }\) values can exceed this hypothetical range, as processes occurring within natural systems may violate the assumptions inherent to Eq. 1. Processes that could yield higher than expected values of δ18O-\({\text{NO}}_{{3\;{\text{nitrification}}}}^{ - }\) include: a higher fraction of O atoms derived from atmospheric O2 and H2O (Aravena et al. 1993); evaporative enrichment of the source water δ18O (Böhlke et al. 1997) and; incorporation of O2 which has become enriched relative to the assumed atmospheric value due to high rates of respiration in soils or sediments (Kendall 1998).
Since streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values typically lie between the values expected for atmospheric \({\text{NO}}_{3}^{ - }\) and microbially derived (nitrified) \({\text{NO}}_{3}^{ - }\), the relative contributions of atmospheric \({\text{NO}}_{3}^{ - }\) and nitrified-\({\text{NO}}_{3}^{ - }\) to streamwater \({\text{NO}}_{3}^{ - }\)were estimated by employing a two end-member mixing model (Kendall 1998; Barnes et al. 2008):
where δ18Ostream, δ18Oprecip, and δ18Onitrification represent the δ18O-\({\text{NO}}_{3}^{ - }\) values of the collected streamwater, rainfall, and the calculated minimum nitrification value (−10.6 ‰), respectively. Hence, Fprecip is the approximate fraction of streamwater \({\text{NO}}_{3}^{ - }\)derived purely from atmospheric \({\text{NO}}_{3}^{ - }\) (Fig. 4). Since rainfall δ18O-\({\text{NO}}_{3}^{ - }\) varied throughout the season, we estimated a range of Fprecip by using the mean and maximum δ18O-\({\text{NO}}_{3}^{ - }\) values found in the rain.
Results of mixing model showing the approximate range of the fraction of stream water \({\text{NO}}_{3}^{ - }\) derived from atmospheric deposition (Fprecip) and microbial \({\text{NO}}_{3}^{ - }\) in Goose and Ptarmigan using mean (71.4 ‰) and maximum (80.1 ‰) δ18O-\({\text{NO}}_{{3{\text{precip}}}}^{ - }\) values found in rainfall at Cape Bounty and the calculated minimum (−10.6 ‰) for the δ18Onitrification end-member value
While this model can help apportion \({\text{NO}}_{3}^{ - }\) between these two end member sources, the co-occurrence of other cycling processes such as assimilation or denitrification and their consequent fractionations on δ18O may confound the results (Kendall et al. 2007). For instance, the relative contribution of nitrified-\({\text{NO}}_{3}^{ - }\) may appear diminished while that of atmospheric \({\text{NO}}_{3}^{ - }\) may appear higher if denitrification and/or assimilation are occurring, as these processes both cause the δ18O value of the residual \({\text{NO}}_{3}^{ - }\) pool to increase (Kendall et al. 2007). The δ18O-\({\text{NO}}_{3}^{ - }\) values and mixing model results on June 24 in PT and June 26 in GS provide a good example of this (Figs. 3a, b, 4). The increases in δ18O-\({\text{NO}}_{3}^{ - }\) in PT on June 24 and in GS on June 26 relative to the previous samples suggest that the fraction of streamwater \({\text{NO}}_{3}^{ - }\) derived from atmospheric deposition increased by approximately 27 % in PT and 21 % in GS compared to the previous samples. However, since there was no rainfall during that interval, it is unlikely that there was an increase in the \({\text{NO}}_{3}^{ - }\) contributed from atmospheric deposition. Instead, what appears to be a greater contribution from atmospheric sources on June 24 in PT and June 26 in GS is actually more likely the result of consumptive processes (denitrification and assimilation) generating higher δ18O-\({\text{NO}}_{3}^{ - }\) and δ18N values and relatively lower \({\text{NO}}_{3}^{ - }\) concentrations (Fig. 5). Hence, the results of this mixing model must be interpreted with the potential impact of these processes in mind.
Rainwater \({\text{NO}}_{3}^{ - }\) and sources of stream water \({\text{NO}}_{3}^{ - }\) in Goose and Ptarmigan for select samples collected during the nival melt, baseflow, and stormflow periods. The larger black rectangle denotes δ18O-\({\text{NO}}_{3}^{ - }\) values characteristic of atmospheric \({\text{NO}}_{3}^{ - }\); the dashed rectangle delineates the expected range of nitrified δ18O-\({\text{NO}}_{3}^{ - }\) values (−10.6 to −2.2 ‰); the gray dashed lines encompass mixing of atmospheric and microbially derived \({\text{NO}}_{3}^{ - }\); and the two black arrows depict hypothetical denitrification vectors at slopes of 0.5 and 1 (δ18O/δ15N) (Kendall et al. 2007). The date of collection (mm-dd) is indicated on select samples. (Color figure online)
The substrate from which the N in \({\text{NO}}_{3}^{ - }\) originated (DOM, SOM, \({\text{NH}}_{4}^{ + }\) in organic matter or minerals) and the expected range of δ15N-\({\text{NO}}_{3}^{ - }\) values produced from nitrification (δ15Nnitrification) can be estimated by examining the δ18N of the product and reactants. The only such data from our study area includes a soil sample collected from the surface organic layer at the base of an ALD and one from the scar zone of the same ALD in the PT catchment, which were analyzed for the δ15N of organic N in SOM and had values of +0.2 and +1.6 ‰, respectively (Lafrenière, unpublished data). Given the limited dataset from our study area, we follow the methodology of Ansari et al. (2013) and use δ15N-\({\text{NO}}_{3}^{ - }\) values obtained from other studies (Tye and Heaton 2007; Wynn et al. 2007) with similar potential N substrates to supplement our data and to delineate our range of δ18Nnitrification values (between −5.8 and +7.7 ‰) (Fig. 5; Table 4). These values have been attributed to \({\text{NO}}_{3}^{ - }\) nitrified from substrates including snowpack \({\text{NH}}_{4}^{ + }\), SOM, and \({\text{NH}}_{4}^{ + }\) in clay and/or rock in European High Arctic streams (Tye and Heaton 2007; Wynn et al. 2007; Ansari et al. 2013) (Fig. 5; Table 4). Note that the δ15N values of the SOM in PT are within the range reported for δ15N of \({\text{NH}}_{4}^{ + }\) in SOM by Tye and Heaton (2007). \({\text{NH}}_{4}^{ + }\) released from N-rich sedimentary bedrock has also been shown to contribute \({\text{NO}}_{3}^{ - }\) in some geologic settings (Holloway et al. 1998, 2001; Holloway and Dahlgren 2002), thus \({\text{NH}}_{4}^{ + }\) present in mineral soils cannot be ignored as a possibles ource of \({\text{NO}}_{3}^{ - }\) at this site. It should be emphasized that the δ15N values in Table 4 only provide a means of constraining the most plausible, but not exact, source from which \({\text{NO}}_{3}^{ - }\) was derived in these watersheds.
Seasonality of streamwater \({\text{NO}}_{3}^{ - }\) sources
The trend in δ18O-\({\text{NO}}_{3}^{ - }\)values in PT and GS shows that atmospheric \({\text{NO}}_{3}^{ - }\) was only a dominant source of streamwater \({\text{NO}}_{3}^{ - }\) during early melt (June 5–7; δ18O-\({\text{NO}}_{3}^{ - }\)values from +60.3 to +81.5 ‰) (Figs. 3b, 4, 5). The δ15N-\({\text{NO}}_{3}^{ - }\)values in runoff during the first few days of melt (−7.9 to −2.6 ‰) also sit within the range of values expected from atmospheric deposition (Fig. 3a) (Hastings et al. 2003, 2004; Heaton et al. 2004; Kendall et al. 2007). This demonstrates that in this continuous permafrost setting, the bulk ofstreamwater \({\text{NO}}_{3}^{ - }\) during initial melt largely results from the elution of atmospheric \({\text{NO}}_{3}^{ - }\) from the snowpack, as is observed in other snow-dominated catchments (Williams et al. 1995; Brooks and Williams 1999). However, lower δ18O-\({\text{NO}}_{3}^{ - }\) values in PT from June 5 to 7 (mean = 66.6 ‰ ± 5.7) relative to mean atmospheric δ18O-\({\text{NO}}_{3}^{ - }\) (71.4 ‰ ± 4.3, n = 7) and significant relationships of \({\text{NO}}_{3}^{ - }\) with DON and DOC in PT suggest that a proportion of \({\text{NO}}_{3}^{ - }\) in PT originated from nitrification or the mineralization of organic matter within the snowpack or soil surface, which is not uncommon in other permafrost regions (Wynn et al. 2007; McNamara et al. 2008; Ansari et al. 2013).
The more depleted δ18O-\({\text{NO}}_{3}^{ - }\) values observed after initial discharge (−9.7 to +27.2 ‰) in both catchments indicate that the supply of \({\text{NO}}_{3}^{ - }\) from the snowpack was largely exhausted and that microbial mineralization and/or nitrification provided the majority of streamwater \({\text{NO}}_{3}^{ - }\) throughout the remainder of the season (Figs. 3b, 4, 5). Despite this change in source, concentrations of \({\text{NO}}_{3}^{ - }\) in GS remained near or below detection limit, signifying that consumptive processes or storage of \({\text{NO}}_{3}^{ - }\) in the expanding active layer outweighed \({\text{NO}}_{3}^{ - }\) production and continued to minimize \({\text{NO}}_{3}^{ - }\) export (Fig. 2b). This is not unexpected in GS given that nutrient limitation in these systems drives strong inorganic N retention (Shaver et al. 1992; Yano et al. 2010).
During stormflow, both catchments received large inputs of \({\text{NO}}_{3}^{ - }\) from rainfall (Louiseize and Lafrenière 2013), but the relatively low δ18O-\({\text{NO}}_{3}^{ - }\) values in GS (13.1 to +14.6 ‰) and PT (−7.2 to −2.0 ‰) reveal that microbial \({\text{NO}}_{3}^{ - }\) composed upwards of 70 and 80 % of the \({\text{NO}}_{3}^{ - }\) in runoff in GS and PT, respectively (Fig. 4). In GS, δ15N-\({\text{NO}}_{3}^{ - }\) values peaked and streamwater \({\text{NO}}_{3}^{ - }\) concentrations remained near or below detection limit despite rainfall \({\text{NO}}_{3}^{ - }\) concentrations between 33 and 124 ppb N (Figs. 2b, 3a); hence, much of the atmospheric \({\text{NO}}_{3}^{ - }\) was denitrified, assimilated by plants and/or microbes, and/or hydrologically stored within the catchment, and a small amount processed \({\text{NO}}_{3}^{ - }\) was flushed out of the active layer via piston flow (Figs. 4, 5). In PT, the drop in solute concentrations that occurred concomitantly with a rapid increase in discharge during the end of stormflow indicates that rainwater flowed through the system and reached the stream outlet (Fig. 2b); however, streamwater δ18O-\({\text{NO}}_{3}^{ - }\) was at its lowest value on July 25, indicating that almost all of the \({\text{NO}}_{3}^{ - }\) from precipitation had been retained or recycled in the disturbed catchment as well (Figs. 3b, 4) (Burns and Kendall 2002; Campbell et al. 2002). To our knowledge, this is the first study that uses isotopic analyses to confirm nitrified or mineralized \({\text{NO}}_{3}^{ - }\) as the dominant source of streamwater \({\text{NO}}_{3}^{ - }\) following significant rainfall events in High Arctic catchments.
Impact of ALDs on \({\text{NO}}_{3}^{ - }\) cycling and sources
The ALDs in PT, two of which compose a long stretch of the well-carved stream channel, are potential cause for high \({\text{NO}}_{3}^{ - }\) export. These disturbances lack organic-rich soils, where nitrification is usually limited by strong competition/biological demand for N and where \({\text{NO}}_{3}^{ - }\) export is further curbed by denitrification (Giblin et al. 1991; Nordin et al. 2004; Booth et al. 2005; Yano et al. 2010; Harms and Jones 2012). The clay-rich scar zones of the ALDs limit vertical water movement (Woo and Young 2006) and channelize flow downslope (Kokelj and Lewkowicz 1998) through surficial mineral soils, which are typically characterized by larger pools of inorganic N and higher nitrification rates compared to organic soils (Nadelhoffer et al. 1991; Hobbie and Gough 2002; Harms et al. 2014). Hence, not only do the ALDs in PT limit the potential for denitrification or uptake/immobilization of \({\text{NO}}_{3}^{ - }\) by displacing vegetation and the organic horizon, they may also represent a source of nitrified-\({\text{NO}}_{3}^{ - }\).
The effects of ALDs on \({\text{NO}}_{3}^{ - }\) concentrations in PT were evident since initial melt, when the peak \({\text{NO}}_{3}^{ - }\) concentration was twelve times higher in PT than in GS. Temporal asynchronies between \({\text{NO}}_{3}^{ - }\) supply and demand can fuel large \({\text{NO}}_{3}^{ - }\) fluxes during initial melt, and export of atmospheric \({\text{NO}}_{3}^{ - }\) during this time confirms that biological demand for \({\text{NO}}_{3}^{ - }\) was limited compared to supply in both catchments (Perakis 2002; Barnes et al. 2008; Yano et al. 2010). However in GS, low \({\text{NO}}_{3}^{ - }\) concentrations and enriched streamwater δ18O-\({\text{NO}}_{3}^{ - }\) values from June 5 to 7 (mean = 77.4 ‰ ± 5.7, n = 4) relative to the mean atmospheric value (71.4 ‰ ± 4.3, n = 7) indicate that some snowpack \({\text{NO}}_{3}^{ - }\) was likely denitrified since the onset of melt, which is not surprising given that saturated shallow organic soils encourage denitrification and provide an effective reservoir of \({\text{NO}}_{3}^{ - }\) during early melt (Sickman et al. 2003; Brooks et al. 1996; Hodson et al. 2005; Harms and Jones 2012). This early biological demand for \({\text{NO}}_{3}^{ - }\) in GS suggests that the 12-fold higher peak \({\text{NO}}_{3}^{ - }\) concentration in PT likely did not simply result from a temporal disparity between \({\text{NO}}_{3}^{ - }\) supply and demand. Rather higher \({\text{NO}}_{3}^{ - }\) concentrations in PT during this time were likely enabled by the ALDs, which created a spatial discrepancy by displacing organic soils and reducing the potential for denitrification and retention via uptake or hydrological storage.
The impacts of ALDs in PT were most pronounced during stormflow. Despite higher N demand during the growing season (Lipson and Monson 1998), which is typically between late June and mid August at CBAWO, concentrations of \({\text{NO}}_{3}^{ - }\) in PT peaked at 348 ppb N and were almost twice as high as rainfall concentrations due to flushing of nitrified or mineralized \({\text{NO}}_{3}^{ - }\) from the watershed (Figs. 2d, 3, 4). High \({\text{NO}}_{3}^{ - }\) export during times of high N demand in these nutrient limited systems only usually occurs when sources and sinks of \({\text{NO}}_{3}^{ - }\) are spatially separated from one another (Perakis 2002; Lafrenière and Lamoureux 2008). Hence, the nitrified or mineralized \({\text{NO}}_{3}^{ - }\) likely bypassed \({\text{NO}}_{3}^{ - }\) sink areas during stormflow runoff, and this led to high streamwater \({\text{NO}}_{3}^{ - }\) concentrations in PT. During stormflow in PT, strong negative relationships of \({\text{NO}}_{3}^{ - }\) with organic constituents and significant correlations of \({\text{NO}}_{3}^{ - }\)with Na+ and \({\text{SO}}_{4}^{2 - }\), which cannot be attributed to precipitation because of their low concentrations in rain but high concentration in streamwater, suggest that microbial \({\text{NO}}_{3}^{ - }\)was derived from mineral soils that are enriched in major ions (Table 1, 2). The ALDs are the most probable cause for the exceptionally high concentrations of microbial \({\text{NO}}_{3}^{ - }\) during stormflow in PT, as they provide an area where nitrified or mineralized \({\text{NO}}_{3}^{ - }\) can emanate from mineral soils and bypass zones of higher N demand (e.g., vegetated, organic soils) during hydrologic transport. These results are in line with those of Nowak and Hodson (2014) who reported that \({\text{NO}}_{3}^{ - }\) supplied from nitrification, which likely occurred in reactive sediments that were exposed due to glacier retreat, was accompanied by sulphide oxidation and therefore high concentrations of \({\text{SO}}_{4}^{2 - }\). Our findings are also similar to those of Harms et al. (2014), who found that mineral soils provided a source of inorganic N in thermokarst gullies. The thermokarst gullies in that study also experienced less denitrification and higher rates of nitrification compared to adjacent undisturbed soils (Harms et al. 2014).
Nitrification of ammonium bound to clay particles has been shown as a source of \({\text{NO}}_{3}^{ - }\) in glaciated settings (Amoroso et al. 2010; Ansari et al. 2013) and provides an explanation that is consistent with enhanced export of microbially produced \({\text{NO}}_{3}^{ - }\) from mineral-rich scar zones in the disturbed PT catchment during stormflow. Based on the proportions of the mixing model and the mean atmospheric δ15N-\({\text{NO}}_{3}^{ - }\) value (+1.0 ‰ ± 1.0), we would expect δ15N values of the substrate to range between approximately +4.4 and +8.1 ‰ unless denitrification or uptake of \({\text{NO}}_{3}^{ - }\) was prominent (Wynn et al. 2007). Although we cannot affirm the substrate used during nitrification, the δ15N values of nitrified-\({\text{NO}}_{3}^{ - }\) in PT (+4.7 to +7.3 ‰) tended to be several permil higher than the δ15N of SOM from the organic and mineral soils in PT (+0.2 and +1.6 ‰) as well as those expected for \({\text{NO}}_{3}^{ - }\) produced from surface organic sources in other glaciated regions (e.g., +1.2 to +2.7 ‰; Table 4), which have low δ15N values because of inputs from litter depleted in δ15N (Högberg 1997; Tye and Heaton 2007). Values of δ15N for soil N and DIN generally tend to be higher in mineral soils (Högberg 1997; Nadelhoffer et al. 1996; Kendall et al. 2007); hence it is plausible that the nitrified \({\text{NO}}_{3}^{ - }\) from PT originated from mineral soils in ALD scar zones.
Conclusions
This study is the first to report on and compare the seasonal dynamics and sources of \({\text{NO}}_{3}^{ - }\) in runoff from an undisturbed catchment (GS) and one that has been subject to a series of ALDs (PT) in a High Arctic region. Isotopic evidence indicates that \({\text{NO}}_{3}^{ - }\) from atmospheric deposition was only a dominant source of streamwater \({\text{NO}}_{3}^{ - }\) in both catchments during initial snowmelt (June 5–7) and that streamwater \({\text{NO}}_{3}^{ - }\) predominantly originated from microbial sources afterwards. Although both catchments shared this trend, the relative importance of \({\text{NO}}_{3}^{ - }\) sources and sinks differed between catchments since the beginning of melt. Low concentrations of \({\text{NO}}_{3}^{ - }\) and high δ18O-\({\text{NO}}_{3}^{ - }\) values in the undisturbed catchment indicate that hydrologic storage and biological sink mechanisms in the vegetated stream channel and organic soils effectively retained or removed \({\text{NO}}_{3}^{ - }\) since the onset of melt. In the disturbed catchment, high \({\text{NO}}_{3}^{ - }\) concentrations and relatively low δ18O-\({\text{NO}}_{3}^{ - }\) values in runoff indicate that large areas of exposed mineral soils in ALDs facilitated the export of \({\text{NO}}_{3}^{ - }\) from microbial sources and reduced \({\text{NO}}_{3}^{ - }\) retention and/or denitrification despite evidence of \({\text{NO}}_{3}^{ - }\) demand or storage within the catchment. δ15N-\({\text{NO}}_{3}^{ - }\) values several permil higher than what is expected of \({\text{NO}}_{3}^{ - }\) derived from organic substrates and relationships between \({\text{NO}}_{3}^{ - }\) and major ions during times of high \({\text{NO}}_{3}^{ - }\) concentration suggest that mineral soils were a likely source of the nitrified-\({\text{NO}}_{3}^{ - }\) in the disturbed catchment.
ALDs impacted \({\text{NO}}_{3}^{ - }\) export most evidently during the onset of snowmelt and especially throughout stormflow. The isotopic evidence supports that elevated export of microbial \({\text{NO}}_{3}^{ - }\) in the disturbed catchment was largely the result of spatial segregation between \({\text{NO}}_{3}^{ - }\) sources (e.g., snowmelt, mineral soils) and sinks (e.g., organic soils) created by the ALDs, which resulted in hydrological flow paths through mineral soils where N demand was likely low and nitrification was likely high relative to undisturbed soils. ALDs that are physically coupled to waterways have the potential to impact seasonal N transport by perpetuating \({\text{NO}}_{3}^{ - }\) export through increasing \({\text{NO}}_{3}^{ - }\) production and limiting \({\text{NO}}_{3}^{ - }\) retention.
References
American Society of Civil Engineers (1974) Generalized discharge relations for cutthroat flumes. J Irrig Drain Div-ASCE 98(IR4):569–583
Amoroso A, Domine F, Esposito G et al (2010) Microorganisms in dry polar snow are involved in the exchanges of reactive nitrogen species with the atmosphere. Environ Sci Technol 44:714–719
Ansari AH, Hodson AJ, Heaton THE et al (2013) Stable isotopic evidence for nitrification and denitrification in a High Arctic glacial ecosystem. Biogeochemistry 113:341–357. doi:10.1007/s10533-012-9761-9
Aravena R, Evans ML, Cherry JA (1993) Stable isotopes of oxygen and nitrogen in sources identification of nitrate from septic systems. Ground Water 31(2):180–186. doi:10.1111/j.1745-6584.1993.tb01809.x
Atkinson DM, Treitz PM (2012) Arctic Ecological classifications derived from vegetation community and satellite spectral data. Remote Sens 4:3948–3971. doi:10.3390/rs4123948
Barnes RT, Raymond PA, Casciotti KL (2008) Dual isotope analyses indicate efficient processing of atmospheric nitrate by forested watersheds in northeastern U.S. Biogeochemistry 90(1):15–27. doi:10.1007/s10533-008-9227-2
Böhlke JK, Eriksen GE, Revesz K (1997) Stable isotope evidence for an atmospheric origin of desert nitrate deposits in northern Chile and southern California, U.S.A. Chem Geol 136:135–152
Böhlke JK, Mroczkowski SJ, Coplen TB (2003) Oxygen isotopes in nitrate: new reference materials for 18O:17O:16O measurements and observations on nitrate-water equilibration. Rapid Commun Mass Spectrom 17:1835–1846
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Bowden WB, Gooseff MN, Balser A et al (2008) Sediment and nutrient delivery from thermokarst features in the foothills of the North Slope, Alaska: potential impacts on headwater stream ecosystems. J Geophys Res 113:G02026. doi:10.1029/2007JG000470
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol Process 13:2177–2190
Brooks PD, Williams MW, Schmidt SK (1996) Microbial activity under snowpacks, Niwot Ridge, Colorado. Biogeochemistry 32(2):93–113
Buchwald C, Casciotti KL (2010) Oxygen isotopic fractionation and exchange during bacterial nitrite oxidation. Limnol Oceanogr 55:1064–1074
Burns DA, Kendall C (2002) Analyses of 15N and 18O to differentiate NO3 − sources in runoff at two watersheds in the Catskills Mountain of New York. Water Resour Res 38(5):1051. doi:10.1029/2001WR000292
Campbell DH, Kendall C, Chang CCY, Silva SR (2002) Pathways for nitrate release from an alpine watershed: determination using 15N and 18O. Water Resour Res 38(5):1052. doi:10.1029/2001WR000294
Casciotti KL, Sigman DM, Hastings MG et al (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74(19):4905–49012. doi:10.1021/ac020113w
Cooper R, Thoss V, Watson H (2007) Factors influencing the release of dissolved organic carbon and dissolved forms of organic nitrogen from a small upland headwater during autumn runoff events. Hydrol Process 21:622–633
Favaro EA, Lamoureux SF (submitted) Antecedent Controls on Rainfall Runoff Response and Sediment Transport in a High Arctic Catchment, Geografiska Annaler, Series A: Physical Geography, GAA1405-043, submitted May 23, 2014.
Frey KE, McClelland JW (2009) Impacts of permafrost degradation on arctic river biogeochemistry. Hydrol Process 23:169–182. doi:10.1002/hyp.7196
Giblin AE, Nadelhoffer KJ, Shaver GR et al (1991) Biogeochemical diversity along a riverside toposequence. Arctic Alaska Ecol Monogr 61:415–435
Harms TK, Jones JB (2012) Thaw depth determine reaction and transport of inorganic nitrogen in valley bottom permafrost soils. Glob Change Biol 18:2958–2968. doi:10.1111/j.1365-2486.2012.02731.x
Harms TK, Abbott BW, Jones JB (2014) Thermo-erosion gullies increase nitrogen available for hydrologic export. Biogeochemistry 117:299–311. doi:10.1007/s10533-013-9862-0
Hastings MG, Sigman DM, Lipschultz F (2003) Isotopic evidence for source changes of nitrate in rain at Bermuda. J Geophys Res 108(D24):4790. doi:10.1029/2003JD003789
Hastings MG, Steig EJ, Sigman DM (2004) Seasonal variations in N and O isotopes of nitrate in snow at Summit, Grennland: implications for the study of nitrate in snow and ice cores. J Geophys Res 109:D20306. doi:10.1029/2004JD004991
Heaton THE, Wynn P, Tye AM (2004) Low 15N/14N ratios for nitrate in snow in the high arctic (79°N). Atmos Environ 38:5611–5621
Hobbie SE, Gough L (2002) Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in northern Alaska. Oecologia 131:453–462
Hodson AJ, Mumford PN, Kohler J, Wynn PM (2005) Arctic glacial ecosystems: new insights from nutrient budgets. Biogeochemistry 72:233–256
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Holloway JM, Dahlgren RA (2002) Nitrogen in rock: occurrences and biogeochemical implications. Global Biogeochem Cycles 16(4):1118
Holloway JM, Dahlgren RA, Casey WH (2001) Nitrogen release from rock and soil under simulated field conditions. Chem Geol 174:403–414
Holloway JM, Dahlgren RA, Hansen B, Casey WH (1998) Contribution of bedrock nitrogen to high nitrate concentrations in stream water. Nature 395:785–788
Jones JB, Petrone KC, Finlay JC et al (2005) Nitrogen loss from watersheds of interior Alaska underlain with discontinuous permafrost. Geophys Res Lett 32(L02401):2005. doi:10.1029/2004GL021734
Kaiser J, Hastings MG, Houlton BZ, Rockmann T, Sigman DM (2007) Triple oxygen isotope analysis of nitrate using the denitrifier method and thermal decomposition of N2O. Anal Chem 79(2):599–607. doi:10.1021/ac061022s
Kendall C (1998) Tracing nitrogen sources and cycling in catchments, Chapter 16. In: Kendall C, Mcdonnell JJ (eds) Isotope tracers in catchment hydrology. Elsevier Science B.V, Amsterdam, pp 519–576
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems, chapter 12, In: Michener RH and Lajtha K (eds.) Stable isotopes in ecology and environmental science, 2nd edn, Blackwell Publishing, New York, pp. 375–449
Keuper F, van Bodegom PM, Dorrepaal E et al (2012) A frozen feast: thawing permafrost increases plant-available nitrogenin subarctic peatlands. Glob Change Biol 18:1998–2007
Kokelj SV, Lewkowicz AG (1998) Long-term influence of active layer detachment sliding on permafrost slope hydrology, Hot Weather Creek, Ellesmere Island, Canada. In: Proceedings, seventh international conference on permafrost, yellowknife, June 2–27. Québec City Nordicana, Centre d’études nordiques, pp. 583–589.
Lafrenière MJ, Lamoureux SF (2008) Seasonal dynamics of dissolved nitrogen exports from two High Arctic watersheds, Melville Island, Canada. Hydrol Res 39(4):323–335. doi:10.2166/nh.2008.008
Lafrenière MJ, Lamoureux SF (2013) Thermal perturbation and rainfall runoff have greater impact on seasonal solute loads than physical disturbance of the active layer. Permafrost Periglac 24(3):241–251
Lamoureux SF, Lafrenière MJ (2009) Fluvial impacts of extensive active layer detachments, Cape Bounty, Melville Island, Canada. Arct Antarct Alp Res 41(1):59–68
Levine MA, Whalen SC (2001) Nutrient limitation of phytoplankton production in Alaskan foothill lakes. Hydrobiologia 455:189–201
Lewkowicz AG, Harris C (2005) Frequency and magnitude of active-layer detachment failures in discontinuous and continuous permafrost, northern Canada. Permafrost Periglac 16:115–130
Lipson DA, Monson RK (1998) Plant-microbe competition for soil amino acids in the alpine tundra: effects of freeze-thaw and dry-rewet events. Oecologia 113:406–414
Louiseize NL, Lafrenière MJ (submitted) Active layer slope disturbances affect magnitude and composition of dissolved nitrogen export from High Arctic headwater catchments. J Hydrol HYDROL17010
McClelland JW, Stieglitz M, Pan F et al (2007) Recent changes in nitrate and dissolved organic carbon export from the upper Kuparuk River, North Slope, Alaska. J Geophys Res 112:G04S60. doi:10.1029/2006JG000371
McNamara JP, Kane DL, Hobbie JE, Kling GW (2008) Hydrologic and biogeochemical controls on the spatial and temporal patterns of nitrogen and phosphorus in the Kuparuk River, arctic Alaska. Hydrol Process 22:3294–3309. doi:10.1002/hyp.6920
Nadelhoffer KJ, Giblin AE, Shaver GR, Laundre JA (1991) Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecology 72(1):242–253
Nadelhoffer KJ, Shaver GR, Fry B et al (1996) 15 N natural abundances and N use by tundra plants. Oecologia 107:386–394
Nordin A, Schmidt KS, Shaver GR (2004) Nitrogen uptake by soil microbes and plants in relation to soil nitrogen supply. Ecology 85(4):955–962
Nowak A, Hodson A (2014) On the biogeochemical response of a glacierized High Arctic watershed to climate change: revealing patterns, processes and heterogeneity among micro-catchments. Hydrol Process. doi:10.1002/hyp.10263
Paulter BG, Simpson AJ, McNally DJ et al (2010) Arctic permafrost active layer detachments stimulate microbial activity and degradation of soil organic matter. Environ Sci Technol 44:4076–4082
Perakis SS (2002) Nutrient limitation, hydrology and watershed nitrogen loss. Hydrol Process 16:3507–3511
Petrone KC, Jones JB, Hinzman LD, Boone RD (2006) Seasonal export of carbon, nitrogen, and major solutes from Alaskan catchments with discontinuous permafrost. J Geophys Res 111:G02020. doi:10.1029/2005JG000055
Shaver GR, Billings WD, Chapin FS et al (1992) Change and the carbon balance of arctic ecosystems. Bioscience 42:433–441
Sickman JO, Leydecker AL, Chang CCY et al (2003) Mechanisms underlying export of N from high-elevation catchments during seasonal transition. Biogeochemistry 64:1–24
Sigman DM, Casciotti KL, Andreani M et al (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in marine and fresh waters. Anal Chem 73:4145–4153
Snider DM, Spoelstra J, Schiff SL, Venkiteswaran JJ (2010) Stable oxygen isotope ratios of nitrate produced from nitrification: 18O-labeled water incubations of agricultural and temperate forest soils. Environ Sci Technol 44:5358–5364
Tye AM, Heaton THE (2007) Chemical and isotopic characteristics of weathering and nitrogen release in non-glacial drainage waters on arctic tundra. Geochim Cosmochim Acta 71:4188–4205
Vincent WF, Lemay M, Allard M (2013) Adapting to permafrost change: a science framework. EOS 94(42):373–375
Williams MW, Bales RC, Brown AD, Melack JM (1995) Fluxes and transformations of nitrogen in a high-elevation catchment, Sierra Nevada. Biogeochemistry 28:1–31
Woo MK, Young KL (2006) High Arctic wetlands: their occurrence, hydrological characteristics and sustainability. J Hydrol 320:432–450
Woods GC, Simpson MR, Paulter BG et al (2011) Evidence for the enhanced lability of dissolved organic matter following permafrost slope disturbance in the Canadian High Arctic. Geochim Cosmochim Acta 25:7226–7241
Wynn PM, Hodson AJ, Heaton THE, Chenery SR (2007) Nitrate production beneath a High Arctic glacier, Svalbard. Chem Geol 244:88–102. doi:10.1016/j.chemgeo.2007.06.008
Yano Y, Shaver GR, Giblin AE et al (2010) Nitrogen dynamics in a small arctic watershed: retention and downhill movement of 15N. Ecol Monogr 80(2):331–351
Acknowledgments
This research was funded by the Arctic Net Network of Centres of Excellence of Canada. We also extend our gratitude to the Polar Continental Shelf Program (PCSP), for exceptional logistical support. Valuable field, laboratory, and GIS assistance was provided by K. Rutherford, G. Montross, S. Montross, D Lamhonwah and A. Rudy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rights and permissions
About this article
Cite this article
Louiseize, N.L., Lafrenière, M.J. & Hastings, M.G. Stable isotopic evidence of enhanced export of microbially derived \({\text{NO}}_{3}^{ - }\) following active layer slope disturbance in the Canadian High Arctic. Biogeochemistry 121, 565–580 (2014). https://doi.org/10.1007/s10533-014-0023-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0023-x