Abstract
Temporal and spatial distributions of five volatile halogenated organic compounds (VHOC), including chloroform (CHCl3), trichloroethylene (C2HCl3), bromodichloromethane (CHBrCl2), chlorodibromomethane (CHBr2Cl), and bromoform (CHBr3), were determined in the southern Yellow Sea (SYS) during four cruises from July 2008 to May 2009. The five VHOC distributed in the study area were affected by river inputs, anthropogenic activities, circulation system, and biological processes. In this study, CHCl3, CHBrCl2, CHBr2Cl, and CHBr3 concentrations showed significant differences between summer 2008 and spring 2009. The sea-to-air fluxes calculated using the equation of Liss and Merlivat (In: Buat-Menard P (ed) The role of air-sea exchange in geochemical cycling, 1986) indicated that the SYS was a source of CHCl3 and C2HCl3 to the atmosphere during the investigation period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ocean is a major global near-surface reservoir of halogens including chlorine and bromine (Chuck et al. 2005). Volatile halogenated organic compounds (VHOC) include chlorocarbons such as chloroform (CHCl3) and trichloroethylene (C2HCl3), which are significant marine sources of chlorine to the atmosphere (McCulloch et al. 1999; McCulloch 2003). VHOC also include bromocarbons such as bromodichloromethane (CHBrCl2), chlorodibromomethane (CHBr2Cl), and bromoform (CHBr3) that are important marine sources of biogenic reactive organic bromine (Chuck et al. 2005). Marine emissions of trace gases have important implications for atmospheric composition and chemistry (Hughes et al. 2013). VHOC are involved in atmospheric chemical processes; as such, they are considered an important group of compounds. Once emitted to the atmosphere, the halogen-containing VHOC are broken down by photolysis or interaction with OH− or Cl− radicals to release inorganic chlorine and bromine. These halogens take part in reactions that decrease ozone levels of the atmosphere (McGivern et al. 2000; Nielsen and Douglass 2001; Laturnus et al. 2002; Wang et al. 2007) and have implications for the climate (Carpenter and Liss 2000; Cincinelli et al. 2012). Therefore, field investigation data from various marine ecosystems are required to understand VHOC production processes and the role of marine VHOC in global climate change.
The presence of CHCl3 and C2HCl3 in the marine environment is usually related to human activities such as the use of pesticide, anti-freezing agents, dry cleaning fluids, and industrial reagents (Class and Ballschmiter 1986; Wang et al. 1995; Blake et al. 1996; McCulloch et al. 1999). In addition, these two chlorinated compounds are also produced naturally. For example, marine algae are important global sources for CHCl3 and C2HCl3 (Abrahamsson et al. 1995; Nightingale et al. 1995; Scarratt and Moore 1999). Brominated volatile halocarbons (e.g., CHBrCl2, CHBr2Cl, and CHBr3) are generally believed to have mainly a biogenic origin, and are broadly produced by macroalgae and microalgae in the marine environment (Nightingale et al. 1995; Moore et al. 1996; Ekdahl et al. 1998; Quack and Wallace 2003; Colomb et al. 2008; Paul and Pohnert 2011). Successive chlorine substitution as a natural source of CHBrCl2 and CHBr2Cl mainly refers to open ocean and deeper waters, i.e., aged waters (Class and Ballschmiter 1988; Moore and Tokarczyk 1993; Fogelqvist and Tanhua 1995; Sturges et al. 1997). However, if chlorination occurs in coastal waters, the two compounds will also be produced. Additionally, the use of seawater as a cooling medium in coastal nuclear power plants and other plants can lead to the production of a wide range of byproducts, such as CHBrCl2, CHBr2Cl, and CHBr3, which are collectively called trihalomethanes (Fogelqvist and Krysell 1991; Allonier et al. 1999; Quack and Wallace 2003; Zhou et al. 2005; Liu et al. 2011).
Knowledge of seawater and atmospheric concentrations of these trace gases enables calculations of the direction and magnitude of their fluxes across the sea–air interface. Previous studies have reported that the open ocean acts as a source of CHCl3, C2HCl3, CHBrCl2, CHBr2Cl, and CHBr3 to the atmosphere (Abrahamsson et al. 2004b; Moore 2001; Chuck et al. 2005; Quack et al. 2004), as well as a sink for CHBrCl2, CHBr2Cl, and CHBr3 (Chuck et al. 2005; Liu et al. 2013). Moreover, coastal regions have gained increased attention because the concentrations of the compounds in these regions are higher. For instance, coastal Antarctic waters and the western Atlantic coastal waters of the United States of America were found to be important sources of atmospheric CHBr3 (Carpenter et al. 2007; Liu et al. 2011).
In the last decade, the distributions, sources, sea-to-air fluxes of global oceans, and production mechanism of many VHOC have been widely studied in different marine environments (Christof et al. 2002; Huybrechts et al. 2005; Zhou et al. 2005; Carpenter et al. 2007; Liu et al. 2011). However, the research of VHOC along China coastal waters is still at initial stages (Lu et al. 2010; He et al. 2013a, b). The primary objectives of the four cruises in summer 2008 and spring 2009 were to investigate the occurrences, distributions, and temporal variations of five VHOC—CHCl3, C2HCl3, CHBrCl2, CHBr2Cl, and CHBr3 in the southern Yellow Sea (hereafter referred to as SYS) and estimate the fluxes of CHCl3, C2HCl3, and CHBr3 to the atmosphere.
Materials and methods
Study area
The SYS, located in the northwestern Pacific Ocean, is an important marginal, semi-closed sea along China’s coast in the mid-latitude zones. The SYS has a total surface area of 30.9 × 104 km2 and an average depth of 46 m. The SYS opens east into the Pacific Ocean with the Shandong Peninsula to the north and two developed provinces to the west (Fig. 1). This sea is influenced by several rivers, including the Xinyi River, Huaihe River, and Sheyang River. The Xinyi River is a seasonal flood river in the Huaihe River Basin. The annual mean runoff of this river is 16.8 billion cubic meters (Peng et al. 2010). Treated wastewater from Xuzhou and Suqian cities is transferred to the Xinyi River via channelization project and then flows into the SYS to ensure the water quality of the South-to-North Water Diversion Project in Jiangsu Province (Ruan et al. 2008). The major pollution source of the river is industrial wastewater, followed by domestic sewage (Zhang et al. 2005). The volume of treated wastewater from cities discharged to the Xinyi River is 410,000 t d−1 and the pollutant load (pollutants bearing capacity) is 14,965 t a−1 (Ruan et al. 2008). The Huaihe River, one of the main rivers in eastern China, flows through Henan, Anhui, and Jiangsu provinces, and eventually enters the SYS in Yancheng, which is an important industrial city in the north of Jiangsu (Chen 2009). The Sheyang River, located in the city of Yancheng and surrounding industrial areas, is also a significant contributor to the contamination of the estuary (Peng et al. 2010). The SYS is heavily influenced by a circulation system consisting of Yellow Sea Cold Water Mass (YSCWM), Yellow Sea Warm Current (YSWC), and Yellow Sea Coastal Current (YSCC) on the western side (Feng et al. 1999; Yuan et al. 2008). The SYS is also an active zone with interactions among land, sea, and atmospheric processes. The SYS has a very complicated system because of its shallow depth, little deep-water exchange, relatively poor ability of self-purification, complex hydrological conditions, and strong human activities (Feng et al. 1999).
Study area and sampling stations. Plus sign denotes July 2008 cruise, white circle denotes August 2008 cruise, white square denotes March 2009 cruise, and white up-pointing triangle denotes May 2009 cruise. Regional circulation models (YSCC Yellow Sea Coastal Current, YSWC Yellow Sea Warm Current) are indicated with arrows (cited from Bian et al. 2013), and the YSCWM area in summer is shadowed (cited from Wei et al. 2010). The location of the Tianwan nuclear power plant is indicated by star
Sampling stations
A total of four cruises were carried out aboard the R/V Dong Fang Hong 2 during summer 2008 and spring 2009 in the SYS. The study area and sampling stations are shown in Fig. 1. The concentrations of the five VHOC in the surface waters were measured at all stations during these four cruises. The first cruise was conducted at the end of the blooming season of Enteromorpha prolifera (Chlorophyta, Ulvophyceae) during 22–26 July 2008 within 120.5–122°E, 33.5–36°N. The blooming of green algae E. prolifera occurs in the SYS during summer season every year, but the affected area has been found to be largest in 2008. According to satellite data, E. prolifera originated from the coasts of Yancheng and Lianyunguang, Jiangsu Province in mid May 2008. In late May 2008, the green alga had grown rapidly, and green patches covered about 1,200 km2. Subsequently, the green alga moved into the middle of the Yellow Sea near to Qingdao, Shandong Province. The large green patches reached the coast of Qingdao in late June 2008 and formed a massive green tide covering 600 km2 along the coast of Qingdao (Liu et al. 2009). The green tide lasted for 2 weeks, and more than 10,000 persons were involved in the cleanup and removal of over 1 million tons of algae from the coast of Qingdao (China Ocean News 2008). The second cruise was performed during 10–14 August 2008 within 120.5–123°E, 34.5–36°N. The third and fourth cruises were carried out during 20–24 March 2009 and 9–16 May 2009 within 120.7–123°E, 34.5–36°N and 120.5–123°E, 33–36°N, respectively. Detailed information about the sampling stations and hydrographic and meteorological parameters in the four cruises is described in Table 1.
Analyses of VHOC and chlorophyll a in seawater
Surface seawater samples (average depth of 2 m) were collected by a Seabird 911 CTD Rosette (General Oceanics Co.) sampler. The concentrations of VHOC in the samples were analyzed immediately after collection using a cryogenic purge and trap system coupled to a gas chromatograph with an electron capture detector (GC-ECD). Seawater sample was drawn into a 100 ml glass syringe without ambient air. The sample was directly injected into a glass bubbling chamber at 40 °C and stripped with ultrapure nitrogen at a flow rate of 100 ml min−1 for 12 min. The extracted gases passed through two glass tubes containing anhydrous Mg(ClO4)2 and ascarite to remove water vapor and carbon dioxide, respectively. Subsequently, the extracted gases were trapped in an inert stainless steel tube immersed in liquid nitrogen. After extraction, the stainless steel tube was heated and the desorbed gases were introduced into a GC/ECD (Agilent 6890N) for analysis. Quantification of the target compounds was carried out based on retention times and peak areas of calibration standards. GC/ECD chromatograms of a working standard mixture of VHOC showed that their peaks were separated in the chromatograms (Fig. S1). The calibration standards were diluted twice with methanol to prepare working standard solutions. Microliter volumes of the working standards solutions were injected into the N2-purged seawater and subjected to the same procedure as seawater samples. The analytical precisions of the target compounds were generally in the range of 7–15 % in routine sample analysis. Their detection limits were 0.1–0.5 pmol L−1. The overall accuracy of the method for the target compounds, as determined by repeated analyses of low concentration standards (n = 7), was between 5 and 18 %. He et al. (2013a, b) further described the details of the method of analyses of VHOC.
Surface seawater temperature, salinity, and local water depth were obtained from the Seabird 911 CTD Rosette while ship-based in situ wind speeds were measured by a ship-borne weather instrument (RM Young, USA) at about 10 m above sea surface. A total of 200 ml seawater samples were filtered through glass fiber filters (Whatman GF/F, 47 mm diameter) for chlorophyll a (Chl-a) analysis. Chl-a on the filter was extracted with 10 ml 90 % acetone for 24 h in the dark at 4 °C. The fluorescence of the extract was measured with a fluorescence spectrophotometer (F-4500, Hitachi Co., Japan) after centrifugation according to Parsons et al. (1984).
Saturation anomaly and sea-to-air flux calculations
Saturation anomaly (SA; %) is defined as percent difference from the equilibrium between gas concentration in surface seawater and the atmosphere. A positive SA indicates a net flux from the ocean to the atmosphere (Hughes et al. 2009) calculated by the equation
where C w is the measured surface seawater concentration, C a is the atmosphere concentration, and H is the dimensionless temperature-dependent Henry’s law constant. The five VHOC had no in situ measured atmospheric concentrations, so we adopted the mean atmospheric concentrations of 28.34 pptv for CHCl3, 27.32 pptv for C2HCl3, and 5.8 pptv for CHBr3 measured by He et al. (2013a) in the SYS. However, no data on the atmospheric concentrations of CHBrCl2 and CHBr2Cl were available in the study area. Henry’s law constants for CHCl3, C2HCl3, and CHBr3 were calculated from the relationships described by Moore et al. (1995) and Moore (2000) using the measured surface seawater temperature at each station. The sea-to-air fluxes of CHCl3, C2HCl3, and CHBr3 were calculated by the following equation (Liss and Slater 1974):
where k w is the gas transfer coefficient (m day−1) calculated as a function of wind speed and Schmidt number. k w is calculated for CHCl3, C2HCl3, and CHBr3, as described by Liss and Merlivat (1986), respectively, using the following equations:
where u is the wind speed (m s−1) and Sc is the Schmidt number. The temperature-dependent Schmidt numbers for CHCl3, C2HCl3, and CHBr3 were calculated using the equations given by Khalil et al. (1999).
Knowledge of the oceanic and atmospheric concentrations of the trace gases enables calculations of their saturation anomalies in the water phase. Therefore, the direction and magnitude of their fluxes across the air–sea interface may also be calculated. The surface seawater concentrations, in situ water temperatures and wind speeds, and sea-to-air fluxes of the three compounds were estimated based on referenced atmospheric concentrations. This type of flux calculation is subject to several uncertainties, e.g., in atmospheric mixing ratios, which are probably inconsistent with the actual values because these gases are quite sensitive to seasonal change. Therefore, the results should be regarded as approximate estimates of the magnitude of fluxes.
Results and discussion
Surface distributions of VHOC in summer 2008 and spring 2009
According to the temperature–salinity (T/S) plot (Fig. 2), the two water masses in the study area during the investigation could be identified as (1) cold and low-salinity water in the coastal region and (2) warm and high-salinity water in open sea. Two perennial oceanic currents were noted in the study area: (1) the southward YSCC, characterized by low temperature and salinity, (2) the northwestward YSWC, a branch of the Kuroshio into the SYS, with protruding feature of high temperature and salinity (Figs. 3a, 4a, 5a, 6a, 3b, 4b, 5b, 6b). The observed hydrographic feature of the water masses was consistent with previous observations (Feng et al. 1999; Yuan et al. 2008). In addition, YSCWM (central position 123°E, 35°N, Fig. 1) in the bottom water forms during spring, matures in summer, and decays in autumn. The YSCWM is affected by thermocline, tidal mixing, the YSWC, and unique topography in the study area (Weng et al. 1989; Ren and Zhan 2005).
The mean (range) concentrations of CHCl3, C2HCl3, CHBrCl2, CHBr2Cl, and CHBr3 in the surface water of the SYS were 364.1 (87.7–802.2), 11.0 (1.2–23.6), 207.2 (106.5–366.8), 28.9 (1.8–114.0), and 11.5 (6.0–18.4) pmol L−1 in July 2008; 64.1 (32.2–116.8), 12.4 (6.8–19.3), 135.3 (67.9–248.6), 25.0 (11.4–62.9), and 6.1 (3.7–9.5) pmol L−1 in August 2008; 22.9 (13.9–86.1), 11.6 (3.7–33.2), 17.9 (10.6–26.2), 4.2 (2.2–11.6), and 13.5 (8.6–29.6) pmol L−1 in March 2009; and 37.2 (2.5–85.2), 12.3 (4.4–42.0), 21.5 (7.2–29.9), 4.6 (0.8–11.0), and 9.4 (6.8–12.1) pmol L−1 in May 2009, respectively. The mean concentrations of CHCl3 in the four cruises were higher than that of He et al. (2013a) (16.90 pmol L−1 in the East China Sea), but the averages of CHCl3 except for the first cruise were much lower than that of Roy (2010) (325.85 pmol L−1 in the coastal eastern Arabian Sea). The mean concentrations of C2HCl3 in the four cruises were slightly lower than that of He et al. (2013a) (16.27 pmol L−1 in the East China Sea), although evidently higher than that of Singh et al. (1983) (2.28 pmol L−1 in the eastern Pacific Ocean). The mean concentrations of CHBrCl2 in the present study were significantly higher than those of Class et al. (1986) (0.61 pmol L−1 in the South Atlantic Ocean) and Bravo-Linares et al. (2007) (0.98 pmol L−1 in the Irish Sea). The averages for CHBr2Cl were higher in the summer and lower in the spring than that of Bravo-Linares et al. (2007) (13.91 pmol L−1 in the Irish Sea). However, the concentration ranges of CHBr2Cl in the four cruises were in agreement with previously published results (Class et al. 1986; Carpenter et al. 2000). The mean concentrations of CHBr3 in this study were a little lower than that of He et al. (2013a) (17.11 pmol L−1 in the East China Sea) and also lower than those measured in other coastal waters (Zhou et al. 2005; Hughes et al. 2009; Liu et al. 2011) but higher than that of Bravo-Linares et al. (2007) (2.92 pmol L−1 in the Irish Sea). The discrepancy in the concentrations may be caused by the change in the source strengths of the different regions and seasons.
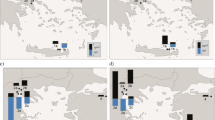
The elevated concentrations of CHCl3 were observed in the southernmost region and the northeast area in July and August 2008, respectively (Figs. 3d, 4d). Haloforms like CHCl3 are formed through water chlorination and sewage treatment (Bellar et al. 1974; Rook 1974). These processes possibly account for the elevated levels of CHCl3 in the southern study area of the first cruise, which resulted from the effluents from urban areas such as Yancheng and discharges from the Huaihe River and Sheyang River. Moreover, in the second cruise, elevated CHCl3 concentrations appeared in the northeast area near the YSCWM (Fig. 4d), suggesting the influence of water mass movement. In spring 2009, the highest CHCl3 concentrations in the two cruises were found in the southern coastal waters (Figs. 5d, 6d). This high concentration might be attributed to anthropogenic input from terrestrial pollutants (Blake et al. 1996; McCulloch et al. 1999).
The high concentrations of C2HCl3 occurred along the coast in summer 2008 and displayed a decreasing trend from inshore to offshore sites (Figs. 3e, 4e). In spring 2009, the elevated concentrations of C2HCl3 appeared in open sea in March (Fig. 5e) and in the central coastal waters in May (Fig. 6e). Chemical decomposition for C2HCl3 can be ruled out because the compound is very stable against hydrolysis in the aquatic environment, with half-lives of the order of hundred years (Mabey and Mill 1978; Fogelqvist 1984). Therefore, in summer 2008, its high concentrations along the coastal waters mirrored the impact of anthropogenic activities on the coastal environment (Class and Ballschmiter 1986; Wang et al. 1995; Blake et al. 1996). Moreover, decreased C2HCl3 concentrations with the distance from the coast should be ascribed to the influence of the YSCC and river runoff in the study area (Feng et al. 1999; Yuan et al. 2008). In March 2009, elevated C2HCl3 concentrations were observed in the open sea with higher temperature and salinity relative to the coastal waters (Fig. 5a, b), suggesting the influence of YSWC. In addition, previous studies have found relatively high abundance of phytoplankton populations in this region during the same season despite low levels of Chl-a (Huang et al. 2006). This phytoplankton abundance indicated that high C2HCl3 concentrations in the open sea were associated with phytoplankton production, in accordance with the previous observation that marine microalgae could produce C2HCl3 (Abrahamsson et al. 1995; Scarratt and Moore 1999). The elevated concentrations of C2HCl3 in the central coastal waters in May 2009 might be caused by the comprehensive action of YSCC, terrestrial inputs, and water mass movement.
The concentrations of CHBrCl2 appeared to be obviously high in the southern coastal region and decreased gradually offshore in summer 2008 (Figs. 3f, 4f) as influenced by the river runoff and Yancheng and Lianyungang coastal terrestrial inputs. The concentration of CHBrCl2 was significantly correlated with CHCl3 (R = 0.66, p < 0.001) in July 2008 (Table 2). This result supported the theory that the same influence factors controlled the two compound distributions in the first cruise. In March 2009, relatively low sea surface temperature and salinity showed homogeneous mixing distribution patterns (Fig. 5a, b) because early spring maintained winter hydrologic characteristics (Tang et al. 1999). The high concentrations of CHBrCl2 were observed in the northeast study area (Fig. 5f). This observation might be related to the production of benthic algae or the emission of sediments that were carried to the surface seawater by vertical mixing of water masses (He et al. 2013a). In addition, high CHBrCl2 concentrations in the region corresponded well with high levels of Chl-a (Fig. 5c, f), which might be attributed to the influence of YSWC. YSWC could stimulate certain phytoplankton species to flourish and release more CHBrCl2 (Nightingale et al. 1995). CHBrCl2 revealed a decreasing trend from inshore to offshore sites in May 2009, particularly in the southern study area (Fig. 6f), contrary to the distribution pattern of sea surface salinity (Fig. 6b). This result suggested that CHBrCl2 distribution in this season was affected by the anthropogenic input from the low-salinity river water along the coast.
CHBr2Cl concentrations in the surface seawater during July 2008 showed distinct spatial variation with a declining trend from the coast to the central region (Fig. 3g). A linear relationship between CHBrCl2 and CHBr2Cl (R = 0.45, p < 0.05) in this cruise, as previously reported by Chuck et al. (2005), was also found in the present study (Table 2). This result indicated that the two compounds might have a common source. Elevated CHBr2Cl concentrations appeared in the southern study area in August 2008 with higher levels of Chl-a (Fig. 4c, g). Two possible explanations for these high values could be deduced. On one hand, this region was clearly affected by terrestrial input. On the other hand, high CHBr2Cl concentrations are caused by river discharges (e.g., Sheyang River, Huaihe River, and Xinyi River) with abundant nutrients that stimulate local phytoplankton growth and emission of CHBr2Cl (Chen 2009; Peng et al. 2010). In spring 2009, high values of CHBr2Cl were observed in the Lianyungang and Yancheng coastal waters, whereas low values were measured in the open sea (Figs. 5g, 6g). The elevated CHBr2Cl in the coastal waters might be attributed to the influence of anthropogenic inputs.
The elevated concentrations were measured in the open waters for CHBr3 in the first cruise (Fig. 3h). However, as shown in Fig. 4h, the concentrations of CHBr3 presented a decreasing trend from inshore to offshore sites in the second cruise. High concentrations of CHBr3 appeared in the northeast study area in the third cruise and in the Qingdao and Yancheng coastal waters in the fourth cruise (Figs. 5h, 6h). These results suggested that various impact factors controlled the CHBr3 distributions in the different cruises. The elevated CHBr3 concentrations in the open sea with relatively high levels of Chl-a (Fig. 3c) during July 2008 might be related to the in situ biogenic sources. Locally elevated concentrations of CHBr3 in the open waters have generally been caused by the planktonic production associated with upwelling and, most likely, diatom abundance (Atlas et al. 1993; Baker et al. 1999). The upwelling current was most significant during this period and gave rise to high abundance of diatoms in this region (Zhang et al. 2002). Nevertheless, CHBr3 concentrations in the second cruise took on a decreasing pattern from inshore to offshore sites. This pattern could be attributed to the influence of Tianwan nuclear power plant effluent and Xinyi River input. As an example, larger Xinyi River flow was observed during the investigation period, with the highest value reaching 4,800 m3/s (Sun et al. 2010). In March 2009, elevated CHBr3 concentrations observed in the northeast study area corresponded well with the elevated Chl-a levels (Fig. 5c, h). These concentrations might be attributed to in situ biological production because of high phytoplankton biomass. However, poor correlation was noted between CHBr3 and Chl-a in the present study (Table 2) because CHBr3 production was specific to certain species of phytoplankton (Moore et al. 1996). CHBr3 concentrations in the surface seawater during May 2009 displayed a declining trend from the Qingdao and Yancheng coasts to the central region (Fig. 6h). This distribution pattern might be caused by anthropogenic inputs. For instance, a great number of disinfection facilities were located in coastal urban areas such as Qingdao and Yancheng cities. A wide range of byproducts, such as trihalomethanes from the disinfection of seawater, could be discharged into coastal waters (Zhou et al. 2005; Liu et al. 2011).
Relationships between VHOC and oceanographic parameters
A simple statistical approach of linear regression was used for the analysis of our field data in the four cruises to investigate the relationships between VHOC concentrations and oceanographic parameters. Table 2 lists the results of the linear regression analyses among the five VHOC, temperature, salinity, and Chl-a in surface seawater, which were performed using SPSS. 19.0 (SPSS Inc., IBM, USA). As shown in Table 2, no obvious correlation appeared between the VHOC and Chl-a concentrations in the surface seawater, which was in agreement with previous studies (Abrahamsson et al. 2004a; Chuck et al. 2005; He et al. 2013b). These results suggested that Chl-a content, which is a general indicator of phytoplankton biomass, is not necessarily associated with VHOC production from biological activities in the SYS. A weak correlation was noted between the Chl-a contents and the concentrations of CHBrCl2 and CHBr2Cl in July 2008 (Table 2). This correlation might imply the biological production of these gases during this period. However, results show that CHBr3 is produced by algae at much higher rates than other bromomethanes (Nightingale et al. 1995; Ekdahl et al. 1998; Quack and Wallace 2003). Relatively low concentrations of CHBr3 and lack of correlation with other trihalomethanes were observed in this cruise, which illustrated an anthropogenic rather than biological source of CHBrCl2 and CHBr2Cl in the SYS. In addition, enhanced nutrient levels observed in the study area during the wet season because of riverine inputs (e.g., Sheyang River, Huaihe River and Xinyi River) could stimulate algal bloom in the SYS (Chen 2009; Peng et al. 2010). Consequently, Chl-a is a better marker of river water inflows than an indicator of biological production of the two bromochloromethane. In the physical parameters, sea surface salinity was negatively correlated with C2HCl3 in July 2008 and CHBrCl2 in both August 2008 and May 2009 (Table 2), implying a direct impact of the river runoff on the distributions of C2HCl3 and CHBrCl2 in the study area. A negative correlation between sea surface temperature and CHBrCl2 concentrations was found in August 2008. This correlation further proved that the terrestrial inputs had greater influence on CHBrCl2 concentration and distribution in summer 2008. No relationship could be found between other VHOC and sea surface temperature or salinity because of the complicated interactions of various water masses in the study area.
In summary, the surface distribution patterns of the five VHOC were complicated and controlled by riverine runoffs (e.g., Sheyang River, Huaihe River, and Xinyi River), various current systems (e.g., the YSCC, YSWC, and YSCWM), anthropogenic input from terrestrial pollutants (e.g., water chlorination and sewage discharge), and biological processes such as phytoplankton production. Moreover, oceanographic environmental factors such as sea surface temperature and salinity also potentially have an important function in controlling these VHOC distributions.
Seasonal variations in the concentrations of VHOC in the study area
The average concentrations of the five VHOC and Chl-a in the SYS during the four cruises are shown in Fig. 7. The average concentration of CHCl3 was (364.1 ± 176.0) pmol L−1 in July 2008, but reduced to (64.1 ± 23.8) pmol L−1 in less than a month. In March and May 2009, the concentration of CHCl3 changed slightly, but was 50 % lower than that in August 2008. No obvious variation was observed in the concentrations of C2HCl3, unlike CHCl3, in the four cruises. The overall variations of concentrations of CHBrCl2 and CHBr2Cl were similar to CHCl3. On the average, CHBrCl2 and CHBr2Cl concentrations in summer were 8.7 and 6.1 times higher than those in spring, respectively (Fig. 7). The highest average concentration of CHBr3 was found in March 2009, which is nearly two times higher than the lowest mean concentration observed in August 2008 (Fig. 7). Consequently, the VHOC (except C2HCl3) displayed significant seasonal variations. In the present study, an obvious seasonal variation was also observed for Chl-a. The mean concentration of Chl-a in summer was two times higher than that in spring (Fig. 7). River runoff, which brought massive terrestrial contaminants into the SYS, was obviously higher in summer than in spring (Chen 2009). In addition, enhanced input of nutrients by terrestrial runoff during the summer could stimulate phytoplankton growth (Wang et al. 2001), which might produce more VHOC. The VHOC seasonal variations may partially be explained by differences in river runoff, various anthropogenic contaminant inputs, and biological processes in different seasons.
Our investigation results in March and May 2009 were compared with published data of VHOC concentrations in spring 2011 from the SYS and the East China Sea to better understand the temporal variations of VHOC concentrations in the SYS (He et al. 2013b). Result showed that the average concentrations of CHCl3, C2HCl3, CHBr2Cl, and CHBr3 in spring 2009 were clearly lower than those in spring 2011. This discrepancy could be attributed to regional and source differences. As an example, four measured VHOC concentrations in the SYS and the East China Sea during spring 2011 were greatly influenced by the Yangtze River effluent (He et al. 2013b). In addition, the concentration difference of these VHOC in the four cruises was possibly caused by regional differences because the four cruises did not cover exactly the same area (Fig. 1). However, data from coastal and shelf regions covered by the four cruises shared the trend toward higher VHOC concentrations in summer. For example, the average concentrations of CHCl3, CHBrCl2, and CHBr2Cl at stations A1 and B1 were 98.7, 145.8, and 26.7 pmol L−1 in summer, whereas their spring values were 11.9, 17.5, and 4.9 pmol L−1, respectively. These results clearly indicated that the significant differences in these VHOC concentrations between summer and spring in the present study were caused mainly by temporal change rather than regional difference. In summer rainy season, a large amount of terrestrial pollutants and sewage flowed into the SYS by flood (Li et al. 2009; Sun et al. 2010). The surface seawater concentrations of VHOC except C2HCl3 revealed obvious seasonal variations, indicating that the indirect effects of significantly high river runoff and terrestrial inputs dominated the VHOC concentrations. Moreover, a large part of the coastal water and YSWC also brought abundant nutrients, stimulating local phytoplankton growth and emission of these VHOC. The nutrients even altered phytoplankton species composition and community structure (Li et al. 2009).
Saturation anomalies and sea-to-air fluxes of three VHOC during the four cruises
According to the above-described equation, the saturation anomalies of VHOC were calculated in the study area. The saturation anomalies of CHCl3, C2HCl3, and CHBr3 were 5,798.2 (1,383.4–13,017.6), 378.8 (−45.8 to 1,033.7), and 30.8 (−28.8 to 126.7) % in July 2008; 1,007.3 (478.4–1,861.7), 498.5 (219.4–796.6), and −32.5 (−61.3 to 24.0) % in August 2008; 50.1 (−5.9 to 444.3), 108.2 (−27.9 to 555.5), and −51.3 (−68.0 to 0.6) % in March 2009; and 259.3 (−75.8 to 764.4), 220.3 (19.0–964.6), and −47.1 (−66.2 to 28.4) % in May 2009, respectively. Results from the four cruises showed that CHCl3 and C2HCl3 were generally supersaturated in the SYS, with the exception of only a few locations (e.g., stations A19, C5, and D2) where CHCl3 and C2HCl3 were undersaturated in the surface seawater. By contrast, CHBr3 was undersaturated at most locations of the SYS except for the first cruise. In the present study, the saturation anomalies of CHCl3 and C2HCl3 were higher in summer 2008 than in spring 2009. The results were consistent with the seasonal variation in the sea surface temperature, which were 24.9 (19.2–26.8) and 26.8 (23.1–30.8) °C in summer 2008, 8.2 (5.8–10.4) and 15.1 (13.3–16.2) °C in spring 2009 during the four cruises, respectively (Table 3). The highest saturation anomalies of CHCl3 and C2HCl3 appeared in July and August 2008, which corresponded to the greatest level of CHCl3 (Fig. 6) and the highest temperature in the same cruise, respectively. Moreover, the maximum negative saturation anomaly of CHBr3 was observed in March 2009, in agreement with the highest concentration of CHBr3 during the same period (Fig. 6). Therefore, the large temporal discrepancies in the saturation anomalies of CHCl3, C2HCl3, and CHBr3 were mainly dependent on both sea surface temperature and VHOC concentration gradient between water and air. The observed positive saturation anomalies indicated that the SYS was a source of CHCl3 and C2HCl3 to the atmosphere during the investigation period. However, CHBr3 was generally undersaturated in surface seawater at most locations. The negative saturation anomalies of CHBr3 in the current study were consistent with the result of Chuck et al. (2005), who reported that CHBr3 was usually undersaturated in a broad area of the temperate Atlantic Ocean. Moreover, negative saturation anomalies and fluxes of CHBr3 were observed in the Florida Straits and along the east coast of Florida (Liu et al. 2011).
The sea-to-air fluxes of CHCl3, C2HCl3, and CHBr3 across the air–sea interface in the SYS were calculated according to the previously described equations. As shown in Table 3, the average sea-to-air fluxes of the three compounds were within the previously reported range, except CHCl3 in the first cruise with a considerably high flux associated with the highest mean concentration (364.1 pmol L−1). The wind speeds were 5.5 (3.0–8.0), 8.5 (3.8–11.6), 7.7 (3.0–11.6), and 7.4 (1.4–17.6) m s−1 during the four cruises from July 2008 to May 2009, respectively. The fluxes of CHCl3 in the four cruises showed an obvious seasonal variation, with the summer values largely exceeding the spring measurements. The mean flux of CHCl3 in summer was higher by a factor of 8.6 than in spring, whereas the mean concentration in summer was 7.1 times that of spring observations. This result indicated that the higher concentrations of dissolved CHCl3 in summer were responsible for the larger fluxes. The sea-to-air fluxes of C2HCl3 appeared to be the highest in August 2008, although the concentrations of C2HCl3 were not the highest, which was attributed to the maximum wind speed and sea surface temperature in this cruise. Furthermore, the sea-to-air fluxes of CHBr3 appeared to be most negative in March 2009, which corresponded to the minimum sea surface temperature and maximum concentration of CHBr3 during the same period. Consequently, the large temporal variabilities in the sea-to-air fluxes of VHOC were controlled not only by their surface concentrations, but also by wind speed and sea surface temperature.
Based on our data (Table 3), the average flux differences for CHCl3, C2HCl3, and CHBr3 between the two cruises of 2008 were –266.7, 19.0, and –10.3 nmol m2 day−1, respectively. The data above show that C2HCl3 fluxes increased, whereas CHCl3 and CHBr3 fluxes decreased in less than 1 month after the bloom of the green alga. This difference indicated that during the post-bloom period, more C2HCl3 was released into the atmosphere. The mean wind speed and seawater temperature in the second cruise were 3.0 m s−1 and 1.9 °C higher than those in the first cruise, respectively (Table 3). Although the surface concentrations of C2HCl3 decreased in the second cruise, increasing wind speed and temperature led to the higher fluxes.
The major uncertainty in the assessment of sea-to-air fluxes is in the calculation of gas transfer velocities, which depends on measured wind speed and sea surface temperature. In this work, the instantaneous wind speeds were used to calculate the gas transfer coefficient for the sea-to-air fluxes at each station. Therefore, the preliminary calculation fluxes of the three compounds should be regarded as instantaneous and approximate assessment. In addition, the introduction of these atmospheric mixing ratios also led to larger uncertainties in the sea-to-air flux estimates. Further study, including simultaneous measurement of concentrations of these VHOC in both aqueous and gaseous phases, is necessary to accurately estimate the sea-to air fluxes of these VHOC in the study area.
Conclusions
This study provided preliminary understanding of the spatio-temporal variations of five VHOC and their sea-to-air fluxes in the SYS. The distributions of these VHOC in the study area were complicated and influenced by river runoff, anthropogenic inputs, various current systems, and biological processes. Environmental factors (e.g., Chl-a, sea surface temperature, and salinity) potentially had a significant function in controlling VHOC distributions. Salinity appeared to be negatively correlated with C2HCl3 in July 2008 and CHBrCl2 in August 2008 and May 2009, whereas temperature was found to be negatively correlated with CHBrCl2 concentrations in August 2008. Fully understanding the influence of these oceanographic parameters on VHOC distributions is necessary in further studies. All VHOC except C2HCl3 revealed apparent seasonal variations in the study area, which was caused mainly by temporal change rather than regional difference. The calculation of saturation anomalies showed that CHCl3 and C2HCl3 were supersaturated, whereas CHBr3 was generally undersaturated in surface seawater at most locations during the study period. The calculation of sea-to-air fluxes indicated that the SYS was a significant source of CHCl3 and C2HCl3 to the atmosphere.
References
Abrahamsson K, Ekdahl A, Collén J, Pedersen M (1995) Marine algae: a source of trichloroethylene and perchloroethylene. Limnol Oceanogr 40:1321–1326
Abrahamsson K, Bertilsson S, Chierici M, Fransson A, Froneman PW, Lorén A, Pakhomov EA (2004a) Variations of biochemical parameters along a transect in the Southern Ocean, with special emphasis on volatile halogenated organic compounds. Deep-Sea Res II 51:2745–2756
Abrahamsson K, Lorén A, Wulff A, Wängberg SÅ (2004b) Air–sea exchange of halocarbons: the influence of diurnal and regional variations and distribution of pigments. Deep-Sea Res II 51:2789–2805
Allonier AS, Khalanski M, Camel V, Bermond A (1999) Characterization of chlorination by-products in cooling effluents of coastal nuclear power stations. Mar Pollut Bull 38:1232–1241
Atlas E, Pollock W, Greenberg J, Heidt L, Thompson AM (1993) Alkyl nitrates, nonmethanehydrocarbons, and halocarbon gases over the equatorial Pacific Ocean during SAGA 3. J Geophys Res 98:16933–16947
Baker JM, Reeves CE, Nightingale PD, Penkett SA, Gibb SW, Hatton AD (1999) Biological production of methyl bromide in the coastal waters of the North Sea and open ocean of the northeast Atlantic. Mar Chem 64:267–285
Bellar TA, Lichtenberg JJ, Kroner RC (1974) The occurrence of organohalides in chlorinated drinking waters. J Am Water Works Assoc 66:703–706
Bian CW, Jiang WS, Richard JG, Ding H (2013) The suspended sediment concentration distribution in the Bohai Sea, Yellow Sea and East China Sea. J Ocean Univ China 12:345–354
Blake DR, Blake NJ, Smith TW, Wingenter OW, Rowland FS (1996) Nonmethane hydrocarbon and halocarbon distributions during Atlantic stratocumulus transition experiment/marine aerosol and gas exchange, June 1992. J Geophys Res 101:4501–4514
Bravo-Linares CM, Mudge SM, Loyola-Sepulveda RH (2007) Occurrence of volatile organic compounds (VOCs) in Liverpool Bay, Irish Sea. Mar Pollut Bull 54:1742–1753
Carpenter LJ, Liss PS (2000) On temperate sources of bromoform and other reactive organic bromine gases. J Geophys Res 105:20539–20547
Carpenter LJ, Malin G, Liss PS, Küpper FC (2000) Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal east Atlantic. Glob Biogeochem Cycles 14:1191–1204
Carpenter LJ, Wevill DJ, Palmer CJ, Michels J (2007) Depth profiles of volatile iodine and bromine-containing halocarbons in coastal Antarctic waters. Mar Chem 103:227–236
Chen YH (2009) The system of flood & drought management in the Huaihe River. Chin Flood Drought Manag 1:144–152 (in Chinese)
China Ocean News (2008) http://epaper.oceanol.com/zghyb/20080822/index.htm
Christof O, Seifert R, Michaelis W (2002) Volatile halogenated organic compounds in European estuaries. Biogeochemistry 59:143–160
Chuck AL, Turner SM, Liss PS (2005) Oceanic distributions and air–sea fluxes of biogenic halocarbons in the open ocean. J Geophys Res 110:C10022. doi:10.1029/2004JC002741
Cincinelli A, Pieri F, Zhang Y, Seed M, Jones KC (2012) Compound Specific Isotope Analysis (CSIA) for chlorine and bromine: a review of techniques and applications to elucidate environmental sources and processes. Environ Pollut 169:112–127
Class T, Ballschmiter K (1986) Chemistry of organic traces in air VI: distribution of chlorinated C1–C4 hydrocarbons in air over the northern and southern Atlantic Ocean. Chemosphere 15:413–427
Class T, Ballschmiter K (1988) Chemistry of organic traces in air, VIII: sources and distribution of bromo- and bromochloromethanes in marine air and surface water of the Atlantic Ocean. J Atmos Chem 6:35–46
Class T, Kohnle R, Ballschmiter K (1986) Chemistry of organic traces in air VII: bromo- and bromochloromethanes in air over the Atlantic Ocean. Chemosphere 15:429–436
Colomb A, Yassaa N, Williams J, Peeken I, Lochte K (2008) Screening volatile organic compounds (VOCs) emissions from five marine phytoplankton species by head space gas chromatography/mass spectrometry (HS-GC/MS). J Environ Monit 10:325–330
Ekdahl A, Pedersén M, Abrahamsson K (1998) A study of the diurnal variation of biogenic volatile halocarbons. Mar Chem 63:1–8
Feng SZ, Li FQ, Li SQ (1999) Marine science introduction. Higher Education Press, Beijing, pp 434–498 (in Chinese)
Fogelqvist E (1984) Low molecular weight chlorinated and brominated hydrocarbons in seawater. Ph. D., Thesis, Department of Analytical and Marine Chemistry, CTH/GU, S-412 96 Gothenburg, Sweden
Fogelqvist E, Krysell M (1991) Naturally and anthropogenically produced bromoform in the Kattegatt, a semi-enclosed oceanic basin. J Atmos Chem 13:315–324
Fogelqvist E, Tanhua T (1995) Iodinated C1–C4-hydrocarbons released from ice algae in Antarctica. Naturally-produced organohalogens. Kluwer Acad, Norwell, pp 295–306
He Z, Yang GP, Lu XL (2013a) Distributions and sea-to-air fluxes of volatile halocarbons in the East China Sea in early winter. Chemosphere 90:747–757
He Z, Yang GP, Lu XL, Zhang HH (2013b) Distributions and sea-to-air fluxes of chloroform, trichloroethylene, tetrachloroethylene, chlorodibromomethane and bromoform in the Yellow Sea and the East China Sea during spring. Environ Pollut 177:28–37
Huang BQ, Liu Y, Chen JX, Wang DZ, Hong HS, Lü RH, Huang LF, Lin YA, Wei H (2006) Temporal and spatial distribution of size-fractionated phytoplankton biomass in the East China and Huanghai Sea. Acta Oceanol Sin 28:157–164 (in Chinese)
Hughes C, Chuck AL, Rossetti H, Mann PJ, Turner SM, Clarke A, Chance R, Liss PS (2009) Seasonal cycle of seawater bromoform and dibromomethane concentrations in the coastal bay on the western Antarctic Peninsula. Global Biogeochem Cycles 23:GB2024. doi:10.1029/2008GB003268
Hughes C, Johnson M, Utting R, Turner S, Malin G, Clarke A, Liss PS (2013) Microbial control of bromocarbon concentrations in coastal waters of the western Antarctic Peninsula. Mar Chem 151:35–46
Huybrechts T, Dewulf J, Langenhove HV (2005) Priority volatile organic compounds in surface waters of the southern North Sea. Environ Pollut 133:255–264
Khalil MAK, Moore RM, Happer DB, Lobert JM, Erickson DJ, Koropalov V, Sturges WT, Keene WC (1999) Natural emission of chlorine-containing gases: reactive chlorine emission inventory. J Geophys Res 104:8333–8346
Laturnus F, Haselmann KF, Borch T, Grøn C (2002) Terrestrial natural sources of trichloromethane (chloroform, CHCl3)—an overview. Biogeochemistry 60:121–139
Li DP, Yang YQ, Dong HY, Guo LN, Liu XZ, Ma Y (2009) Cause analysis and synoptic characteristics for outbreaks of Enteromorpha around Qingdao in 2008. Period Ocean Univ Chin (Nat Sci) 39:1165–1170 (in Chinese)
Liss PS, Merlivat L (1986) Air–sea gas exchange rates: introduction and synthesis. In: Buat-Menard P (ed) The role of air–sea exchange in geochemical cycling. Reidel, Dordrecht, pp 113–127
Liss PS, Slater PG (1974) Flux of gases across the air–sea interface. Nature 247:181–184
Liu D, Keesing JK, Xing Q, Shi P (2009) World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull 58:888–895
Liu Y, Yvon-Lewis SA, Hu L, Salisbury JE, O’Hern JE (2011) CHBr 3, CH2Br2, and CHClBr2 in the U.S. coastal waters during the Gulf of Mexico and East Coast Carbon cruise. J Geophys Res 116:C1004. doi:10.1029/2010JC1006729
Liu Y, Yvon-Lewis SA, Thornton DC, Butler JH, Bianchi TS, Campbell L, Hu L, Smith RW (2013) Spatial and temporal distributions of bromoform and dibromomethane in the Atlantic Ocean and their relationship with photosynthetic biomass. J Geophys Res 118:3950–3965
Lu XL, Yang GP, Song GS, Zhang L (2010) Distributions and fluxes of methyl chloride and methyl bromide in the East China Sea and the Southern Yellow Sea in autumn. Mar Chem 118:75–84
Mabey W, Mill T (1978) Critical review of hydrolysis of organic compounds in water under environmental conditions. J Phys Chem Ref Data 7:383–415
McCulloch A (2003) Chloroform in the environment: occurrence, sources, sinks and effects. Chemosphere 50:1291–1308
McCulloch A, Aucott ML, Graedel TE, Kleiman G, Midgley PM, Li YF (1999) Industrial emissions of trichloroethene, tetrachloroethene, and dichloromethane: Reactive Chlorine Emissions Inventory. J Geophys Res 104:8417–8427
McGivern WS, Sorkhabi O, Deresckei-Kovacs A, North SW (2000) Primary and secondary processes in the photodissociation of CHBr 3. J Phys Chem 104:10085–10091
Moore RM (2000) The solubility of a suite of low molecular weight organochlorine compounds in seawater and implications for estimating the marine source of methyl chloride to the atmosphere. Chemosphere-Global Change Sci 2:95–99
Moore RM (2001) Trichloroethylene and tetrachloroethylene in Atlantic waters. J Geophys Res 106:27135–27143
Moore RM, Tokarczyk R (1993) Volatile biogenic halocarbons in the northwest Atlantic. Global Biogeochem Cycles 7:195–210
Moore RM, Geen CE, Tait VK (1995) Determination of Henry′s law constants for a suite of naturally occurring halogenated methanes in seawater. Chemosphere 30:1183–1191
Moore RM, Webb M, Tokarczyk R, Wever R (1996) Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures. J Geophys Res 101:20899–20908
Nielsen JE, Douglass AR (2001) A simulation of bromoform’s contribution to stratospheric bromine. J Geophys Res 106:8089–8100
Nightingale PD, Malin G, Liss PS (1995) Production of chloroform and other low molecular halocarbons by some species of macroalgae. Limnol Oceanogr 40:680–689
Nightingale PD, Malin G, Law CS, Watson AJ, Liss PS, Liddicoat MI, Boutin J, Upstill-Goddard RC (2000) In situ evaluation of air–sea gas exchange parameterizations using novel conservative and volatile tracers. Global Biogeochem Cycles 14:373–387
Parsons TR, Maita Y, Lalli CM (1984) A manual for chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Paul C, Pohnert G (2011) Production and role of volatile halogenated compounds from marine algae. Nat Prod Rep 28:186–195
Peng YQ, Wei MJ, Xu DN, Cai MT, Liu C, Liu ZW (2010) Research on the distribution characteristics and the formation causes of sporo-pollen of the west of Southern Yellow Sea. J Cap Norm Univ (Nat Sci) 31:24–30 (in Chinese)
Quack B, Wallace DWR (2003) Air–sea flux of bromoform: controls, rates, and implications. Global Biogeochem Cycles 17:1023–1050
Quack B, Atlas E, Petrick G, Stroud V, Schauffler S, Wallace DW (2004) Oceanic bromoform sources for the tropical atmosphere. Geophys Res Lett 31:L23S05. doi:10.1029/2004GL020597
Ren HJ, Zhan JM (2005) A numerical study on the seasonal variability of the Yellow Sea cold water mass and related dynamics. J Hydrodyn 20:887–896 (in Chinese)
Rook JJ (1974) Formation of haloforms during chlorination of natural waters. J. Wat. Treat. & Exam 23:234–243
Roy R (2010) Short-term variability in halocarbons in relations to phytoplankton pigments in coastal waters of the central eastern Arabian Sea. Estuar Coast Shelf S 88:311–321
Ruan XH, Ni LX, Wan QH, Zhao ZH (2008) Integrated treatment of channelized tail water of the eastern route of the South-to-North Water Transfer Project. South-to-North Water Trans Water Sci Techol 6:81–84 (in Chinese)
Scarratt MG, Moore RM (1999) Production of chlorinated hydrocarbons and methyl iodide by the red microalga Porphyridium purpureum. Limnol Oceanogr 44:703–707
Singh HB, Salas LJ, Stiles RE (1983) Methyl halides in and over the eastern Pacific (40°N–32°S). J Geophys Res 88:3684–3690
Sturges WT, Cota GF, Buckley PT (1997) Vertical profiles of bromoform in snow, sea ice, and seawater in the Canadian Arctic. J Geophys Res 102:25073–25083
Sun YY, Zhang YT, Chen JD (2010) Test and analysis of flood capacity in the Xinyi River. Zhi Huai 2:11–13 (in Chinese)
Sweeney C, Gloor E, Jacobson AR, Key RM, McKinley G, Sarmiento JL, Wanninkhof R (2007) Constraining global air–sea gas exchange for CO2 with recent bomb 14C measurements. Global Biogeochem Cycles 21:GB2015. doi:10.1029/2006GB002784
Tang YX, Zou EM, Li XZ, Li ZX (1999) Analysis of hydrographic features and circulation situation in the southern Huanghai Sea in early spring. Acta Oceanol Sin 21:1–11 (in Chinese)
Wang CJL, Blake DR, Rowland FS (1995) Seasonal variations in the atmospheric distribution of a reactive chlorine compound, tetrachloroethene (CCl2=CCl2). Geophys Res Lett 22:1097–1100
Wang BD, Liu F, Zhan R (2001) A review of the biogeochemical study on the biogenic elements in the Yellow Sea. J Oceanogr Huanghai Bohai Seas 19:99–106 (in Chinese)
Wang JX, Qin P, Sun SC (2007) The flux of chloroform and tetrachloromethane along an elevational gradient of a coastal salt marsh, East China. Environ Pollut 148:10–20
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. J Geophys Res 97:7373–7382
Wei QS, Fu MZ, Ge RF, Zang JY (2010) Chemicohydrographic characteristics and the seasonal variations of nutrients at 35°N transect in the Cold Water Mass of the Southern Yellow Sea. Environ Sci 31:2063–2074 (in Chinese)
Weng XC, Zhang YK, Wang CM, Zhang QL (1989) The variational characteristics of the Huanghai Sea (Yellow Sea) Cold Water Mass. J Ocean Univ Qingdao (Nat Sci) 19:119–131 (in Chinese)
Yuan DL, Li JR, Hu DX (2008) Cross-shelf circulation in the Yellow and East China Seas indicated by MODIS satellite observations. J Mar Syst 70:134–149
Zhang WS, Xia CS, Yuan YL (2002) Effect of the upwelling current on the vertical chlorophyll distribution in the Yellow Sea Cold Water Mass. Adv Mar Sci 20:9–14 (in Chinese)
Zhang XD, Ruan XH, Sun M (2005) Experimental study on treatment of wastewater from Xinyi River by overland flow system. J Hohai Univ (Nat Sci) 33:273–276 (in Chinese)
Zhou Y, Varner RK, Russo RS (2005) Coastal water source of short-lived halocarbons in New England. J Geophys Res 110:D21302. doi:10.1029/2004JD005603
Acknowledgments
The authors are grateful to the captain and crew of the R/V Dong Fang Hong 2 for help and cooperation during the study. We are thankful to colleagues of our laboratory for their assistance in sample collection and Chl-a analysis. We also wish to thank the two anonymous reviewers for constructive comments and suggestions which greatly improved the manuscript. We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 41320104008 and 40776039), the Natural Science Foundation of Shandong Province (Z2005E01), the Changjiang Scholars Program, Ministry of Education of China, and the Taishan Scholars Program of Shandong Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, GP., Yang, B., Lu, XL. et al. Spatio-temporal variations of sea surface halocarbon concentrations and fluxes from southern Yellow Sea. Biogeochemistry 121, 369–388 (2014). https://doi.org/10.1007/s10533-014-0007-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0007-x