Abstract
Recent identification of the widespread distribution of legacy sediments deposited in historic mill ponds has increased concern regarding their role in controlling land–water nutrient transfers in the mid-Atlantic region of the US. At Big Spring Run in Lancaster, Pennsylvania, legacy sediments now overlay a buried relict hydric soil (a former wetland soil). We compared C and N processing in legacy sediment to upland soils to identify soil zones that may be sources or sinks for N transported toward streams. We hypothesized that legacy sediments would have high nitrification rates (due to recent agricultural N inputs), while relict hydric soils buried beneath the legacy sediments would be N sinks revealed via negative net nitrification and/or positive denitrification (because the buried former wetland soils are C rich but low in O2). Potential net nitrification ranged from 9.2 to 77.9 g m−2 year−1 and potential C mineralization ranged from 223 to 1,737 g m−2 year−1, with the highest rates in surface soils for both legacy sediments and uplands. Potential denitrification ranged from 0.37 to 21.72 g m−2 year−1, with the buried relict hydric soils denitrifying an average of 6.2 g m−2 year−1. Contrary to our hypothesis, relict hydric layers did not have negative potential nitrification or high positive potential denitrification rates, in part because microbial activity was low relative to surface soils, as indicated by low nitrifier population activity, low substrate induced respiration, and low exoenzyme activity. Despite high soil C concentrations, buried relict hydric soils do not provide the ecological services expected from a wetland soil. Thus, legacy sediments may dampen N removal pathways in buried relict hydric soils, while also acting as substantial sources of NO3 − to waterways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While eutrophication is often attributed to nutrient pollution caused by contemporary land practices, growing evidence (Walter and Merritts 2008a; Brush 2008; Sharpley et al. 2013) suggests that past practices, like the ubiquitous construction of milldams, are also important. After European settlement of the mid-Atlantic region, upland soil erosion due to land clearing and plowing increased sedimentation rates throughout the Chesapeake Bay watershed (Jacobson and Coleman 1986; Brush 2008). Much of this sediment was captured behind milldams constructed in the seventeenth and eighteenth centuries. According to US manufacturing census data, there were tens of thousands of milldams in the mid-Atlantic region, and >65,000 water-powered mills in existence by 1840 in 872 counties across the eastern US (Walter and Merritts 2008a; Merritts et al. 2011). A large number of these milldams breached after abandonment in the nineteenth and twentieth centuries, leading to stream incision through the pond sediments. Stream incision lowers the water table, exposing the former mill pond sediments as a new valley bottom terrace commonly referred to as legacy sediment (Walter and Merritts 2008a).
Recent research suggests that legacy sediments have altered nutrient cycling at the land-stream water interface (i.e. the riparian zone) (Walter et al. 2007; Walter and Merritts 2008a; Merritts et al. 2011). While many studies have found that upland soil erosion can contribute to stream sediment flux (c.f. Toy et al. 2002; Montgomery 2007), it has recently been recognized that much of the fine sediment load carried by streams during storms in the mid-Atlantic region is likely from stream bank erosion (i.e., legacy sediments), rather than contemporary erosion from upland farms and urbanized sites (Walter et al. 2007; Walter and Merritts 2008a; Gellis et al. 2009). Though the pervasiveness of legacy sediments is still an area of active debate (Bain et al. 2008; Walter and Merritts 2008b; Wilcock 2008), many agree that the breaching of dams could represent a modern source of fine sediment to stream networks (Renwick et al. 2005; Schenk and Hupp 2009; Smith et al. 2011). Upland erosion rates have substantially declined over the past century (Trimble and Crosson 2000), while reservoir mill pond deposits can continue to be sources of fine-grained sediment for at least several decades following dam breaching (Merritts et al. 2013). Yet, most attention is still focused on upland sediment and nutrient sources (USEPA 2010) despite mounting evidence that stream bank legacy sediment erosion is a key contributor to sediment—and perhaps nutrient—loads in streams (c.f., Walter et al. 2007; Schenk and Hupp 2009; Mukundan et al. 2010; Gellis and Noe 2013). It is thus important to acknowledge that the use of milldams across the eastern US has greatly influenced fluvial and erosional processes of streams, and the activities associated with their use and demise represent significant sources of sediment to downstream environments.
Legacy sediments can affect eutrophication processes in two fundamental ways. Firstly, stream bank erosion is a significant non-point source of suspended sediment and nutrients entrained in the sediment (Trimble 1997; Walter and Merritts 2008a; Gellis et al. 2009; Gellis and Mukundan 2013), and can account for 50–100 % of the suspended sediment load in some places (Banks et al. 2010; Massoudieh et al. 2012; Gellis and Noe 2013). Secondly, channel formation in legacy sediments results in deep incised banks and de-watering of sediments as the new water level stabilizes (Doyle et al. 2003), affecting the contemporary transfer of nutrients from uplands to streams. Nutrient transport through legacy sediments with increased elevation and lower water levels is likely distinct from a floodplain that supports high nitrogen (N) retention in sediment of lateral water bodies (Forshay and Stanley 2005; Kaushal et al. 2008; Harrison et al. 2011), fringing stream plant communities (Forshay and Dodson 2011), and more frequent overbank deposition of nutrients (Junk et al. 1989; Roach et al. 2008). Yet, little is known about how legacy sediments influence the transfer of N from soils to streams. In this paper, we present a survey of potential N cycling rates in stream banks and upland soils impacted by legacy sediments. Because so little is known about the biogeochemistry of these landscapes, our goal was to identify zones of high or low potential microbial activity as benchmark observations for identifying controls on nitrate transport through legacy sediments.
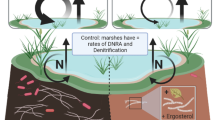
Legacy sediments found in the piedmont region of the eastern US (Walter and Merritts 2008a) typically include four principle stratigraphic units, which from top to bottom are: (1) recently formed A horizons that developed on the legacy sediment terraces as they became agricultural “bottom lands” for crops and/or grazing, (2) additional legacy sediment beneath the A horizon, (3) former (pre-settlement, Holocene) hydric soils that include abundant paleo-seeds of hydrophytes; and (4) Pleistocene periglacial gravels on bedrock. Recent studies of subsurface soils along upland-riparian-stream continuums indicate that buried horizons in the riparian zones are carbon-rich and can act as hot spots of microbial activity (Hill et al. 2004; Gurwick et al. 2008a, b). While none of these studies focused on sediments that were legacies of mill pond abandonment, the buried organic layers were analogous to the buried hydric layer beneath legacy sediments. This led us to hypothesize that relict hydric soils beneath legacy sediments would remain enriched in organic C and support high microbial activity and net NO3 − immobilization and/or denitrification, as found in other studies of buried organic-rich layers (c.f. Hill et al. 2004; Gurwick et al. 2008a, b; Hill et al. 2004; Kellogg et al. 2005).
While much is already known about the hydrologic interactions of uplands and near-stream riparian soils, and their control on N cycling and transport (Hynes 1975; Lowrance et al. 1985; Cirmo and McDonnell 1997; Mayer et al. 2007), it is unclear how the upland-riparian zone continuum is altered when the riparian zone consists predominately of legacy sediment. The lack of N cycling research within legacy sediments, and the former wetland soils that are buried beneath them, currently limits our ability to predict sources and sinks for N pollution from landscapes rich in legacy sediment (Walter and Merritts 2008a). To fill this gap, we quantified potential net N mineralization, nitrification, and denitrification, potential C mineralization, extracellular enzyme activity, and microbial C substrate use at two landscape positions (legacy sediment zone and upland soil zone) in three soil layers (surface, midlayer, and bottom). In the legacy sediment zone, surface, midlayer, and bottom strata corresponded to the A horizon recently formed within legacy sediment, colluvial legacy sediments beneath the A horizon, and the relict buried hydric soil, respectively (Fig. 1). We focused on three key questions: (1) Are net nitrification rates different in legacy sediment soils compared to upland soils? (2) Are there differences in net N mineralization, nitrification, denitrification, and carbon mineralization potentials among the distinct strata of riparian zones dominated by legacy sediment? (3) Is the buried relict hydric soil enriched in C, and if so does this promote microbial activity and sinks for subsurface NO3 − via immobilization or denitrification? We answered these questions in the Big Spring Run watershed of Lancaster County, Pennsylvania, which has become a national test case for research on legacy sediment and its impact on valley bottom ecosystems (USEPA 2009).
Materials and methods
Study site
Big Spring Run (39°59′N, 76°15′W) is a northward-flowing tributary of Mill Creek, in Lancaster County, Pennsylvania (drainage area ~4 km2). The site lies within the Conestoga River Watershed, which empties into the Susquehanna River, a river that ultimately provides over 50 % of the freshwater entering the Chesapeake Bay (Chang 2003; PA DEP 2011). The site has a typical humid temperate climate, with precipitation higher during summer months due to frequent convective storms. Soils along Big Spring Run are deep, silty loams derived from Conestoga limestone (Merritts et al. 2005), with the Newark Soils Series (Fluventic Endoaquepts) near the legacy sediment strewn stream, grading into the Pequea Soil Series (Typic Eutrudepts) in the uplands (Custer 1985). A typical Newark profile includes an A horizon (0–9 in.) underlain by B (9–32 in.) and C (32–60 in.) horizons, which can show signs of gleying. Pequea soil series typically occur on convex slopes of uplands and consist of A (0–10 in.), B (10–26 in.), and C (26–52 in.) horizons.
The soils at Big Spring Run impacted by legacy sediment deposition consist of four distinct stratigraphic layers (Fig. 1). Basal gravels are overlain by a 20–50 cm thick soil that formed in a fluvial wetland environment over the last 10,000 years (Merritts et al. 2005; Walter and Merritts 2008a; Merritts et al. 2011). The examination of hundreds of study sites across 20 mid-size watersheds throughout the mid-Atlantic Piedmont region, which combined stratigraphic evidence with geochemical and palynological analysis of pre-settlement material, has indicated that valley bottoms were once broad riparian wetlands, with a mosaic of small streams and low vegetated islands within the flood zone (Walter et al. 2007; Walter and Merritts 2008b). These small and shallow anabranching channels carried little sediment due to low, long-term erosion rates in pre-settlement times, and frequently flowed overbank onto a mix of wetlands (Walter et al. 2007; Walter and Merritts 2008b). The construction of numerous, small beaver dams during pre-settlement times in the mid-Atlantic region likely helped to create the anabranching stream networks and wetlands (Morgan 1867; Walter and Merritts 2008b; Brush 2008). While it is not possible to know whether these pre-settlement wetland soils would have been officially classified as hydric, they currently bear many hydric characteristics: they are dark gray to black in color (10 YR 2/1), fine-grained loamy in texture, and organic matter rich.
Following European settlement construction of milldams that spanned entire valley bottoms of dominantly 1st to 3rd order streams was extensive (Walter and Merritts 2008a). These dams created reservoirs that flooded valley bottoms and acted as efficient sediment retention ponds. Fine-grained legacy sediments (~80–100 cm thick at Big Spring Run) were deposited behind such low-head dams in slackwater environments (PA DEP 2006) on top of the hydric layer during the historic, post-settlement period. Currently, in the top 20 cm of the legacy sediment, an organic matter rich A horizon is developing. For the soil sampling described in the following sections, the A horizon, the thick layer of legacy sediment below the A horizon, and the relict hydric soil correspond to the surface layer, midlayer, and bottom layer, respectively.
Big Spring Run is a small, almost entirely agricultural watershed that is typical of the mid-Atlantic Piedmont region (Merritts et al. 2011). The majority of sedimentation at Big Spring Run is attributed to quiet-water deposition in slackwater mill ponds, and associated upstream backwater areas, created as a result of damming (Merritts et al. 2006). Behind the former milldam on Big Spring Run, a gradient of legacy sediment depth existed with sediments thickest near the location of the dam, and tapering off upstream away from the dam. Characterized by incised, high-banked channels, the stream has become disconnected from the floodplain, exposing the post-settlement legacy sediment, buried relict hydric soil, periglacial basal gravels, and underlying valley bedrock (Walter and Merritts 2008a; Merritts et al. 2011; Parola and Hansen 2011). Water table fluctuations can saturate the buried relict hydric soil or dry it out, impacting redox conditions. Few legacy sediment laden sites have been as extensively mapped as Big Spring Run. Being able to accurately identify the transition zone between legacy sediments and upland soils made Big Spring Run the ideal test site for our objectives. The area has also been recognized as a non-point source hot spot for N, phosphorus (P), and suspended sediment to the Chesapeake Bay (Hall et al. 1997; CBF 2004).
Soil sampling and analysis
Soils were sampled over three field campaigns as initial results informed more targeted analyses. Core sampling at the landscape scale revealed interesting patterns of N and C processing in soil layers (see results below), so we targeted the incised stream bank for further sampling, and integrated unpublished data from previous studies at the site to complete a more thorough analysis of microbial activity in legacy sediments. Sampling events, and the main analyses, are presented below according to decreasing scales—from the landscape scale to the narrower stream bank level—as opposed to chronological order.
Core sampling: potential N and C mineralization
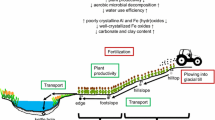
In April 2010, 29 soil cores (4.7 cm diameter, depth to refusal) were collected from Big Spring Run (Fig. 2) in two differing landscape positions: (1) an upland zone, not impacted by legacy sediment; and (2) the legacy sediment zone. The landscape zones were delineated based on Light Detection and Ranging (LiDAR) high-resolution topographic data (provided by the National Center for Airborne Laser Mapping to D. Merritts and R. Walter), field mapping, and trenching (Merritts et al. 2011). These mapping methods were integrated and used to determine the boundaries between non-buried upland soils and areas of valley-bottom legacy sediment deposition. A stratified random sampling design was used to account for spatial heterogeneity of soil properties within the two respective landscape zones. Eighteen cores were randomly collected from the upland (non-legacy), while eleven cores were randomly collected from the legacy sediment zone. Within each landscape zone the randomly collected cores were then treated as sample replicates to allow for comparison between the two landscape positions. The cores were collected using a Geoprobe model 6610DT (Geoprobe Systems, Salina, KS) direct push coring machine. Cores were sampled to the depth of refusal which corresponded to the Pleistocene gravels in the legacy zone, and to fractured bedrock in the upland zone. All cores were divided into 20 cm increments and several research teams subsampled these increments.
GIS map of Big Spring Run sampling sites taken in April 2010. Each dot represents a core location. The dashed lines around the stream depict the estimated area over which legacy sediments were deposited and were mapped from Light Detection and Ranging (LiDAR) high-resolution topographic data, field mapping, and trenching (Merritts et al. 2011). Any cores collected within the borders of the two dashed lines were classified as legacy zone soils. All cores taken outside the dashed lines, away from Big Spring Run, were classified as non-legacy upland soils (See Online Resource 1 for a GIS converted DEM color map of sampling sites)
Our main goal was to increase understanding of legacy sediment, so our subsampling of the soil cores prioritized sampling of the three key stratigraphic layers in the sediment: surface A horizons, legacy sediment beneath the A, and the buried relict hydric layer (Fig. 1). Texture and color were used to differentiate among these layers. The A horizon in the legacy sediments was sampled as the top 20 cm increment for each core, and hereafter referred to as the “surface” layer. A 20 cm increment from the midpoint (~50–70 cm depth) of the legacy sediment layer that extended below the A horizon was collected, and termed the “midlayer” sample (we could not homogenize all of the subsamples within the layer due to time constraints of obtaining fresh samples and because we wanted to preserve separate layers for potential future analyses). Beneath the legacy sediment, a 20 cm segment of “bottom” soil that overlapped with the midpoint (typical depths were 100–120 cm) of the relict hydric layer was sampled to minimize boundary effects and to ensure that we captured soil from only the former hydric soil.
The upland soils in this catchment also contained three key horizons, a surface A horizon, a B horizon, and a C horizon near the interface with bedrock (Fig. 1). Thus, we collected soil samples from these key layers using criteria similar to those described above for legacy sediment. For the “surface” layer of the upland soils, we collected the top 20 cm, which corresponded to the depth of the A horizon in the uplands as well as the legacy sediment. For the “bottom” layer (C horizon) in the uplands, we sampled the last complete 20 cm segment that did not include fractured bedrock (depth ranged from 100 to 240 cm). We chose this portion of the C horizon because the soil–bedrock interface is often a zone of preferential water flow (Mosley 1979; McDonnell 1990), which we expected to be analogous to the relict hydric layer that we sampled above basal gravels in the legacy sediment, but with differing long-term (mill pond vs. pedogenic) histories. The “midlayer” sample was taken from the midpoint (depth of ~40–80 cm) of the B horizon and is analogous to our midlayer sample from the legacy sediment in that it is unaffected by A horizon organic matter enrichment, or preferential flow that occurs at the bottom of soil profiles.
Core increments were weighed to determine bulk density, homogenized by hand, and subsampled for potential N and C mineralization within 2 h of collection. A 10 g subsample of fresh soil was sieved (2 mm) and oven dried (105 °C) to constant mass to determine the gravimetric water content. Another 10 g subsample of fresh soil was immediately extracted (100 mL of 2.0 M KCl). Lastly, a 10 g subsample of fresh soil was placed in a 120 mL glass Wheaton vial, sealed with a septum, and potential C mineralization and potential net N mineralization were estimated using 7-day laboratory incubations (Binkley and Hart 1989; Hart et al. 1994; Hart and Stark 1997) at 25 °C with soil moisture equal to the field conditions at time of sampling. Empty Wheaton vials were also sealed as controls to account for ambient CO2. After 1–2 days a syringe was used to mix and sample (1 mL) the headspace gas and the concentration of CO2 was determined (LI-7000, LI-COR Biosciences, Lincoln, NE). After sampling the headspace, vials were opened, fanned with ambient air to provide a uniform background CO2 concentration, and resealed with a new septum. This CO2 sampling protocol was repeated 2–3 times during the 7-day incubation. Potential C mineralization was calculated as total C released over the incubation period divided by the incubation duration (mg C kg soil−1 day−1).
Following the final headspace gas sampling, inorganic N was extracted for each sample. Ammonium and nitrate + nitrite concentrations (μg N g soil−1) were determined on the initial and final KCl extracts using colorimetric analysis on a spectrophotometer microplate reader. Ammonium concentrations were measured via the salicylate method (Sims et al. 1995), while nitrate + nitrite concentrations were determined via the vanadium(III) chloride method (Doane and Horwath 2003). Nitrite concentrations were assumed to be negligible in this case, so the results are reported here as NO3 −–N concentrations. Potential net rates (μg N g soil−1 day−1) were determined by dividing the concentration change by the incubation period (7 days).
Incised bank sampling 1: denitrification
Soils along the incised stream bank (within the legacy sediment zone) at Big Spring Run were sampled three times over the course of a year—in November 2008, March 2009, and August 2009—at six different bank face locations. At each bank face location four horizontal cores were collected (within 1 m of each other) from each of the key stratigraphic layers (surface, legacy sediment, and relict hydric soil as described above) by inserting a 4.7 cm diameter soil core into the bank face. Each of the 6 bank face sites were cleared of debris and plant roots prior to sampling. Surface samples were collected in the top 10 cm of the incised bank, while legacy sediment and relict hydric samples were collected from the center (20–70 and 50–130 cm) of their respective layers to minimize boundary effects. Stream sediment samples were also collected near each of the six sampled incised faces, with coarse gravel >19 mm removed prior to incubation.
Denitrification bioassays were conducted on all samples using a modified acetylene block technique for sediments (Tiedje et al. 1989; Holmes et al. 1996; Groffman et al. 1999). 50 mL of fresh soil or sediment were transferred to 250 mL microcosms fitted with gas-tight lids and gas sampling-septa. To determine denitrification limitation by nitrate and/or organic carbon, soil samples were amended with 75 mL of stream water from their respective stream site plus 3,300 mg L−1 C as dextrose amendment (+C), 200 mg L−1 N as KNO3 (+N), or a combination of dextrose and nitrate (C + N). Control samples received only unamended site water. Each jar was degassed using helium and then injected with 10 % by volume acetylene. To ensure adequate acetylene distribution, as well as to block conversion to N2 gas, all microcosms were gently agitated. Initial gas samples were extracted from the headspace and injected into 5.9 mL gas-tight, evacuated Exetainer vials. Microcosms were incubated in the dark between 3 and 4 h within 5 °C of the sediment–water interface at the time of sample collection. N2O was measured using a gas chromatograph (Agilent Technologies, Palo Alto, CA) with a micro-electron capture detector. Total gas evolved includes the estimated dissolved N2O in water using the Bunsen’s coefficient (Young 1981) at incubation temperature, corrected for headspace and water volume. Once all soils were processed through the assay, the organic matter fraction of each sample was calculated as the change in mass after combusting overnight at 550 °C divided by the dry mass.
Incised bank sampling 2: assays of microbial activity
A second set of samples was collected from the stream bank at the Big Spring Run site in September 2010 to further investigate questions regarding buried relict hydric sediments (see question #3) that arose after interpreting results from the two previous sampling events. These samples were collected by inserting a hand-held core horizontally into the incised wall of the stream bank at depths corresponding to the middle of the three layers of interest; 0–20 cm for the surface, 55–75 cm for the middle layer, and 100–120 cm for the buried relict hydric layer. By taking samples in the middle of each of the bottom two layers boundary effects could again be minimized. Whole cores were not collected during this sampling event because the stream bank was more conducive to horizontal sampling. The horizontal sampling scheme was repeated along the bank faces of the stream at 5 different locations (all sites were different than those sampled for denitrification), with the bank face at each sampling site cleared of debris and plant roots prior to soil collection. The potential N and C mineralization protocol described above was carried out for all samples. Total soil C and N concentrations were determined by dry combustion elemental analysis. Organic matter was measured by loss on ignition (LOI) over 16 h at 450 °C (below the temperature where carbonate minerals can be lost) (Salonen 1979; Nelson and Sommers 1996; Santisteban et al. 2004).
An index of the nitrifier populations was quantified using the shaken soil-slurry method (Belser 1979; Belser and Mays 1980) adapted from Hart et al. (1994). Slurry conditions are optimized for high water, NH4 +, oxygen, and P availability, such that samples with the largest populations of nitrifiers will generate the largest increase in slurry nitrate concentration over time (Belser 1979). A 15 g subsample of fresh soil was sieved (2 mm) and placed into a 250 mL Erlenmeyer flask with 100 mL of solution containing 1.5 mM NH4 + and 1 mM PO4 3−. The flasks were agitated at 180 rpm for 28 h on an orbital shaker. From each flask, 10 mL of slurry was sampled at hours 2, 4, 26, and 28. Samples were centrifuged and the supernatant was analyzed colorimetrically for NO3 −. The rate of NO3 − production (mg N kg soil−1 day−1) was calculated by linear regression of the solution concentration versus time.
A community level physiological profile (CLPP) of each stream bank soil sample was developed using the MicroResp™ system (Macaulay Scientific Consulting Ltd., Aberdeen, Scotland) to describe the diversity of microbial substrate use (Campbell et al. 2003; Chapman et al. 2007). Catabolic response was determined from the short-term respiration responses of soils after the addition of 15 different organic C substrates: d-glucose, citric acid, ascorbic acid, urea, asparagine, l-cysteine, glycine, lignin, pepsin, N-acetyl glucosamine, α-ketobutyric acid, malic acid, oxalic acid, tannin, and humic acid.
Each carbon source was dissolved in deionized water at a concentration that delivered 30 mg of C per g of soil water with 25 μL of each C substrate (Campbell et al. 2003). The substrate solution was added directly to the soil samples after soils had been wetted and pre-incubated for ~5 days to reach an equilibrated water holding capacity of 60 %. Deep-well microplates with soil sample and substrate solutions were placed face to face with a second microplate containing CO2 detection gel composed of purified agar, cresol red, potassium chloride (KCl), and sodium bicarbonate (NaHCO3). The two microplates were sealed together and incubated in a dark cabinet at room temperature for four 6-h intervals. Immediately prior to sealing the two microplates to each other, and after each 6-h interval, the detection gel absorbance was measured at 570 nm using a Multiskan EX microplate spectrophotometer (Thermo Scientific, Waltham, MA). A calibration curve related absorbance to %CO2 (Macaulay Scientific 2010), and normalized absorbance (Ai) was calculated by dividing the absorbance at each time (Atx) by the absorbance at time zero (At0) and then multiplying by the mean absorbance at time zero (mean At0): Ai = (Atx/At0) × Mean (At0) (Macaulay Scientific 2010). Quantities of CO2 produced by each sample were calculated from the normalized absorbance readings (Ai) and reported as a CO2 rate (μg CO2–C g−1 h−1). Catabolic responses were also standardized for each substrate in order to determine the C sources microbes utilized most at different depths. Respiration data were converted to standardized catabolic response by dividing the respiration of each substrate by the mean respiration of all the substrates. A blank soil plus water sample served as a check to account for respiration of native soil C, and respiration from this water-only check was subtracted from all respiration rates, to isolate CO2 responses to the substrate additions.
Catabolic evenness (E) was calculated from the respiration response profiles, with p i representing the respiration response to individual substrates (r i ) as a proportion of total respiration activity induced by all substrates (\(\varSigma r_{i}\)) for each soil sample, i.e. \(p_{i} = r_{i} /\varSigma r_{i}\) (Degens et al. 2000). Since catabolic evenness is a measure of the relative variability in the catabolic functions of the soil, it is a dimension-less unit. Using 15 different substrates (excluding the no-substrate control of water) the maximum achievable evenness, where all substrates respond equally, was 15 (Degens et al. 2001). Richness, another component of diversity, was also determined, and defined as the number of substrates used by the microbes (Degens et al. 2001).
The activities of seven extracellular enzymes were determined for the stream bank soil samples according to methods described in Allison and Vitousek (2004) and Sinsabaugh et al. (1993). We assayed the activity of the hydrolytic enzymes cellobiohydrolase (CBH), β-glucosidase (BG), β-N-acetyl glucosaminidase (NAG), leucine aminopeptidase (LA), and acid phosphatase (AP), and the oxidative enzymes polyphenol oxidase (PPO) and peroxidase (PerO). Soil enzyme activity was measured on ~2 g wet weight subsamples. Soil samples were frozen prior to analysis, as is common in other studies (Allison and Vitousek 2004; Keeler et al. 2009). Substrates were prepared as follows: AP: 5 mM pNP-phosphate; CBH: 2 mM pNP-cellobioside; BG: 5 mM pNP-β-glucopyranoside; NAG: 2 mM pNP-β-N-acetyl glucosaminide; LA: 5 mM leucine p-nitroanilide; PPO and PerO: 5 mM l-dihydroxy-phenylalanine; all in 50 mM acetate buffer. Samples were combined with 60 mL of 50 mM, pH 5, acetate buffer, and homogenized in a blender for 1 min. In a 2 mL Eppendorf tube 0.750 mL homogenate and 0.750 mL substrate were combined and shaken and incubated at 20 °C for one to 6 h. For every sample, three analytical replicates were prepared for each enzyme assay. Controls and blanks were included in order to account for any background absorbance of the homogenates or substrates. Following centrifugation, the supernatant of each sample was pipetted into a corresponding microplate well. 1.0 M NaOH was also added to each well of the hydrolytic enzymes to terminate the reaction and develop the color to be measured. The absorbances of the samples were quantified using a microplate spectrophotometer at 405 nm for the hydrolytic enzymes and 450 nm for the oxidative enzymes. Enzyme activity was measured as µmol of substrate converted per hour per gram soil organic matter. The ratios BG:AP, BG:(NAG + LA), (NAG + LA):AP, and BG:(PerO + PPO) were analyzed to provide information regarding enzymatic C:P, C:N, N:P, and labile C:recalcitrant C. Exoenzyme ratios (Sinsabaugh et al. 2009; Sinsabaugh and Follstad Shah 2011) can reveal shifts in resource allocation.
Mass balance calculation
To estimate the potential for formation and removal of NO3 − in near-stream soils affected by legacy sediments at Big Spring Run we constructed a mass balance using potential nitrification and denitrification rates from the stream bank. Due to the inherent variability in bank height we used a typical stream bank height of 1.4 m—we assumed the surface layer was 20 cm thick, the midlayer legacy sediment was 100 cm thick, and the buried relict hydric soil was 20 cm thick. The N cycling process rates in mg N kg soil−1 time−1 were multiplied by the bulk density of the soil (in kg m−3) and the thickness of the strata of interest (in m) to calculate potential N transformation rates in g N m−2 of near stream area covered by legacy sediment.
Statistical analysis and data treatment
PROC MIXED of the SAS 9.1 statistical software (SAS Institute Inc., Cary, NC) was used to examine whether soil properties differed among depths (surface, midlayer, bottom) and landscape positions (upland zone or legacy zone), and if there was an interaction between depth and landscape position. Depth, landscape position, and depth × landscape position were treated as fixed factors, while site replicates were treated as random factors. When interactions (depth x landscape position) were observed, data were further analyzed by a one-way ANOVA. If significance was found at the level of α = 0.05 a Fisher’s least-significance difference (LSD) multi-comparison test (with 95 % confidence limits) was used to compare specific depths or landscape positions. Microbial data from the stream bank samples were analyzed similarly, with depth as the only fixed factor and replicates as a random factor. All data were checked for normality and homoscedasticity, and rates log transformed when necessary. MINITAB (Minitab Inc., State College, PA) produced a correlation matrix among C and N response variables. Data were geographically managed and processed with the GIS software ArcGIS 10.0 (ESRI Inc., Redlands, CA).
SPSS 15.0 (SPSS, Inc., Chicago, IL) was used to determine whether differences in denitrification rates existed across stream bank strata (surface, legacy sediment, relict hydric soil), sampling date (November 2008, March 2009, August 2009), and nutrient amendment (control, +C, +N, C + N). Rates were natural log transformed (ln x + 1) to include zero values and to normalize increasing variance with increased measured values. Organic matter fraction measurements were transformed using arc sin square root to normalize variance of a fractional value. The differences among stream bank strata, sampling date, and nutrient amendment were compared using a three-way analysis of variance (ANOVA) followed by Tukey’s post hoc test with α = 0.05 on factors identified as significant in the ANOVA. Although significant two-way interactions were found, we also present the denitrification bioassays post hoc tests of the individual single subject effects to show the influence of the sediment substrata, season, and nutrient treatments to summarize the main effects.
Note that soils sampled along the stream bank were not sampled at the same time of year or in the same manner as the cores collected in the non-legacy uplands and legacy zone (e.g. stream bank legacy-zone only soils were collected by horizontal coring, while all other samples were extracted via vertical coring). For this reason, we draw inferences about landscape variability from the 29 deep cores, and use the stream bank legacy zone-only samples to resolve questions regarding denitrification and microbial activity in the relict hydric layer.
Results
Potential N and C mineralization in legacy sediments compared to non-legacy uplands
Surface soils from the non-legacy upland and legacy zone had significantly higher total soil C and N than in the midlayer and bottom soils (Table 1). The bottom soils of the legacy zone had significantly higher C and N than soils at the same depth in the non-legacy upland.
Concentrations of NH4 +–N in the soils of Big Spring Run were higher in the surface than in either the midlayer or bottom depths for both landscape positions (Table 1). Across landscape positions, surface soils in the legacy zone had significantly higher NH4 +–N concentrations than surface soils in the non-legacy upland. Legacy zone bottom soils (i.e. the buried relict hydric layer) had significantly higher NH4 +–N concentrations than the bottom soils of the non-legacy upland. In contrast to NH4 +–N, there were no significant differences in extractable NO3 −–N concentrations across depths. The bottom soils of the two landscape positions, however, had significantly different NO3 −–N levels, with those of the non-legacy uplands being twice as high as the legacy zone.
Potential net nitrification rates varied across both landscape position and depth from 9.2 to 77.9 g m−2 year−1. Surface soils had potential nitrification rates that were 352 and 743 % larger than those in the midlayer and bottom soils of the non-legacy uplands, respectively, and 312 and 284 % larger than those in the midlayer and bottom soils of the legacy zones, respectively (Table 1). Potential net ammonification rates were negative for all depths and landscape positions, ranging from −5.5 to −47.8 g m−2 year−1 (Table 1). Rates in surface soils were significantly more negative than deeper layers, regardless of landscape position. Only in the bottom soils did landscape position affect net ammonification rates. Soils from the non-legacy uplands had significantly higher (less negative) rates—63 % higher—than the legacy zone soils at depth.
Potential C mineralization ranged from 223 to 1,737 g m−2 year−1 across depths and landscape positions. Surface soils in both the non-legacy upland and legacy zone had significantly higher potential C mineralization rates than those found in the two lower depths (Table 1). While C mineralization rates were similar at the surface for the non-legacy upland and legacy zone, in the midlayer and bottom soils the legacy zone samples had higher potential C mineralization than the non-legacy uplands, 77 and 112 % higher, respectively.
Potential denitrification rates in incised stream banks
Nutrient treatment limitation is defined by the singular treatment that stimulates a significant increase in denitrification rate over the control (i.e. unamended sample). In the case of co-limitation +C, +N, and C + N had higher denitrification rates than the controls for all depths, but only the combination of C + N in the surface and legacy sediment midlayer were found to be significantly higher than their respective controls (Fig. 3). Nutrient co-limitation was not significant in the relict hydric soil. Overall potential denitrification rates were greatest in the surface soils, regardless of the nutrient amendment. Potential denitrification rates were not statistically different among the three sampling dates (P = 0.329), so co-limitation was determined by combining all subsets, allowing for a larger sample size (n = 18 vs. n = 6). Organic matter was not found to be a significant predictor of denitrification among the depths (P = 0.359).
Potential denitrification rates (g m−2 year−1) of 20 cm stream bank sample segments expressed as averages of all three sampling dates across three depths and four nutrient amendment treatments. Vertical bars denote one standard error of the mean (n = 18). For a given depth, bars with different lowercase letters represent statistically significant (P < 0.05) differences between nutrient amendments. For a given nutrient amendment, bars with different uppercase letters represent statistically significant (P < 0.05) differences with depth
Microbial activity in stream bank legacy sediments
The nitrifier population index was significantly greater in the surface soils compared to the mid-layer and relict hydric soils along the stream bank (Table 2). The catabolic response to added C (i.e. the substrate-induced respiration) was also greatest in the surface soils of the stream bank, regardless of substrate type (Fig. 4). Relict hydric soils had significantly lower responses to added substrates than surface soils, with the midlayer soils having an intermediate response. As a percentage of the total C respired (i.e. the standardized catabolic response), surface soils had significantly greater response to additions of labile carbon (d-glucose) and amino acids (glycine and asparagine) than to other types of C. In the relict hydric soil and the subsurface legacy sediments, the standardized catabolic response was greater for tannin and carboxylic acids (specifically α-ketobutyric acid, malic acid, and oxalic acid) than for other C substrates. There were no differences in richness or evenness (P = 0.7) of substrate use among the layers. Catabolic richness is not a very sensitive indicator of microbial functional diversity because most organic compounds can be used by soil microorganisms (Degens et al. 2000, 2001) as evidenced by this study in which all substrates were metabolized.
Catabolic response (µg substrate per g of soil) and standardized catabolic response (percent) as averages for stream bank samples across three depths. Abbreviations for substrates are: d-glucose (GLUC), citric acid (CITR), ascorbic acid (ASCO), urea (UREA), asparagine (ASPA), l-cysteine (CYST), glycine (GLYC), lignin (LIGN), pepsin (PEP), N-acetyl glucosamine (NAG), α-ketobutyric acid (KETO), malic acid (MALI), oxalic acid (OXAL), tannin (TANN), and humic acid (HUMI). Vertical bars denote one standard error of the mean (n = 5). For a given substrate, bars with different lowercase letters represent statistically significant (P < 0.05) differences between depths
Enzymatic activity (µmol h−1 gOM−1) was significantly higher in the surface soils along the stream bank than in the samples collected in the midlayer and bottom soils (Fig. 5). The bottom (relict hydric) layer had a significantly higher ratio of BG:(PerO + PPO) than the two upper soil layers.
Enzyme activity (µmol of substrate utilized per hour per gram of soil organic matter) expressed as averages for stream bank samples across three depths. Abbreviations for extracellular enzymes are: acid phosphatase (AP), leucine aminopeptidase (LA), cellobiohydrolase (CBH), β-glucosidase (BG), β-N-acetyl glucosaminidase (NAG), polyphenol oxidase (PPO), and peroxidase (PerO). Vertical bars denote one standard error of the mean (n = 5). For a given enzyme, bars with different lowercase letters represent statistically significant (P < 0.05) differences between depths. For AP depth was not statistically significant (P = 0.077), but a post hoc test was still performed. The results of the Fisher’s least-significance difference (LSD) test for the AP enzyme are noted by the asterisks next to the letters
Mass balance
After accounting for the bulk density and depth of the different legacy sediment-affected layers, we found that potential denitrification rates were not significantly different than potential nitrification rates across a typical cross-section of stream bank at Big Spring Run (Table 3). Differences in potential formation versus removal rates of NO3 − did not exist within any of the three depths of interest, either. Depth comparisons did show, however, that rates for both N removal and formation processes were highest in the surface legacy sediment layer, which differed significantly from the buried relict hydric soils. Potential denitrification rates in the buried relict hydric soil were also significantly higher than those in the midlayer legacy sediment, but such differences were not found for potential nitrification rates.
Discussion
Legacy sediments compared to upland soils
Given the divergent histories of the upland and legacy zone soils, we expected to observe large differences across the landscape in soil C and N storage and in net N cycling rates. Surprisingly, we found that legacy zone surface soils, developing for <300 years, have C and N pools and net N and C mineralization rates that are comparable to adjacent upland soils. Strong depth gradients were expected for upland soils, as previous studies have shown that organic matter, C, N, and mineralization rates decline dramatically with depth (Holden and Fierer 2005). It takes time for soil horizons to develop, and for the depth distribution of nutrients to become well defined. Thus, we predicted that depth gradients would be weaker in the younger (less developed) legacy zone soils. However, the changes in pools and fluxes between surface and midlayer soils are similar in uplands and legacy soils.
We do not know exactly how the depth distribution of nutrients in legacy sediments arises over time, but here we critique two possible scenarios: (1) the depth distribution of C and N could be due to differential soil deposition into the former millpond, or (2) the nutrient depth profile could be due to soil development after the dam breached. For the first scenario to be true a high nutrient soil could have been lain down at the end of the depositional period within the millpond. This is unlikely, as upland erosion from land clearing and agriculture would have initially included surface soils with high C and N, followed by deeper soils with lower nutrient status. Alternatively, if the retention pond remained undisturbed, it could have become increasingly eutrophic over time, creating a layer of organic matter overtop the sediment due to accumulation of dead phytoplankton. Water flushing events from storms, and mixing of groundwater within the retention pond, however, likely caused enough disturbance to prevent eutrophic conditions from developing. We infer that it is unlikely that a nutrient-rich soil was deposited as a cap on the millpond sediment, and that the nutrient depth distribution in legacy sediments is probably not due to depositional processes. A more probable scenario would be that the C and N content of eroded upland material was constant over time, leading to a uniform distribution of nutrients across all depths within the millpond. Once the dam breached the soil profile that we observed likely developed from two processes. Soils at depth probably lost C and N over time as mineralization outpaced inputs. Surface soils, however, would be exposed to new inputs or processes that would replenish nutrient losses, ultimately resulting in a higher nutrient status as development continued. Differences in soil horizon development are likely responsible for the current nutrient depth distribution found in legacy sediment profiles.
In contrast to surface and midlayer soils, which were relatively uniform across the landscape (at least in N and C contents), we observed significant landscape variation in C and N in deeper soil layers. Indeed, a key contribution of our research is documenting how buried relict hydric layers affect the depth distribution of C. Unlike upland soils, legacy zone soils have higher C and N at the bottom of the profile, which supports the geomorphological, geochemical, and paleobotanical evidence that legacy sediment from millpond dams buried widespread valley bottom Holocene wetlands (Walter and Merritts 2008a; Voli et al. 2009; Merritts et al. 2011). Loamy hydric soils tend to have organic C contents from 8 to 18 % (USDA NRCS 2010), which suggests that the relict hydric soils at Big Spring Run have lost some C over time, however. Such losses likely occurred following dam breaching and subsequent incision into the legacy sediment, which would have allowed more oxygen to diffuse into the deeper layers, leading to losses via C oxidation. Thus these buried layers are considered relicts of the past hydric soils once present at Big Spring Run.
Nitrification versus denitrification in legacy sediments
Our results show that potential nitrification and denitrification adjacent to stream systems with legacy sediments is high in surface sediments, with substantially lower microbial N cycling activity in the subsurface, including the C-rich buried relict hydric soils. Mass balance calculations suggest that potential net nitrate production in a given layer could be fully removed via denitrification if the available NO3 − does not move between strata. However, it is unlikely that this balance between the two N processes will be realized in nature because conditions for high nitrification do not coincide temporally with conditions that are optimal for denitrification. Even though both potential nitrification and denitrification are high in the surface soils, and the use of the modified acetylene block technique can greatly underestimate potential denitrification rates (Seitzinger et al. 1993; Groffman et al. 2006), the environmental conditions that predominate in the surface layer are expected to favor nitrification—abundant O2 would restrict denitrification.
While hot spots and hot moments of denitrification are possible in the organic-rich surface layers of riparian zones (Ambus and Lowrance 1991; Groffman et al. 1992; Burt et al. 2002), at Big Spring Run, where the stream is hydrologically disconnected from the floodplain, reduced denitrification rates are expected. The high cut banks at Big Spring Run can cause incoming NO3 − from groundwater to bypass active sites of riparian denitrification in the surface (Groffman et al. 2003; Böhlke et al. 2007), and, should surface runoff from uplands occur, the residence time of water in the surface riparian area is too low to promote high denitrification rates (Kasahara and Hill 2006; Kaushal et al. 2008). This leads us to hypothesize that the actual (rather than potential) mass balance would include high nitrification in the surface. Further investigation would be needed to test this hypothesis, and requires comparing potential rates to actual N processing rates measured in situ, which would also entail the use of different analytical techniques.
In contrast, in the buried relict hydric soil where environmental conditions favor denitrification over nitrification, potential denitrification rates were found to be low, which suggests the sink potential is low. Well-connected floodplains are thought to enhance N retaining processes through denitrification or plant uptake. At Big Spring Run, however, legacy sediments cause a physical separation of biogeochemically active zones (surface soils) from subsurface hydrologic flowpaths. Surface soils are sites of net positive NO3 − production, which if transported to depth will not be balanced by N removal via denitrification. Overall, legacy sediments expand the area over which high nitrification rates are expected, which is not matched by a similar expansion in high denitrification potential. Thus, with the pervasive distribution of legacy sediments (Walter and Merritts 2008a) it appears that legacy sediments are a likely contributor to NO3 − pollution in watersheds with historic millponds.
Our results were surprising because groundwater NO3 − concentrations were found to be lower (<1 mg L−1) in the stream’s hyporheic area along the legacy zone, compared to that in upland wells in the surrounding landscape (5–20 mg L−1) in the years prior to this study (RC Walter, pers. comm.). Likewise, bottom layer NO3 − concentrations are lower in the legacy zone than in the non-legacy zone (Table 1). We expected that net NO3 − immobilization and/or denitrification in the buried relict hydric layer would account for some of the decline between upland wells and the hyporheic zone since previous studies have shown the potential for considerable denitrification activity in buried, organic-rich layers (Hill and Cardaci 2004; Hill et al. 2004). The fact that we did not observe net NO3 − immobilization or high denitrification in our samples suggests that immobilization and/or denitrification may be occurring slightly below our sampling depth, or subsurface hydrologic residence time may be sufficiently long to induce a change with very low metabolic rates. The water table at the site usually fluctuates near the boundary between the buried relict hydric soil and the basal gravels. Hot spots and hot moments of denitrification, which can frequently account for a high percentage of the denitrification that occurs in an ecosystem, tend to occur at these oxic-anoxic interfaces, where hydrological flowpaths intersect (McClain et al. 2003; Vidon et al. 2010). Our samples, however, were taken from the middle of the buried hydric soil, not at the base, where hyporheic exchange at the interface with the basal gravels may play an important role. Future research should include high spatial resolution sampling at the contact zone between the buried relict hydric soil and basal gravels to test this hypothesis.
However, even if high denitrification or NO3 − immobilization rates do occur at depth, leading to reduced NO3 − levels in groundwater entering the stream, the existence of many other entry pathways suggest that high NO3 − pollution is still possible. Two such pathways—erosional processes, like stream bank slumping, and runoff events, like surface overland flow—can serve as conduits for high NO3 − in surface soils to directly enter the waterway, bypassing the hyporheic zone completely. Given their prevalence in Pennsylvania, there is a critical need for future work to focus on understanding how different hydrological flowpaths may impact contemporary N flow from soils to streams in legacy sediment-strewn reaches.
The microbial activity of the relict hydric layer
Originally, we hypothesized that the long-buried relict hydric soil would support high levels of microbial activity due to higher levels of organic C and more favorable moisture conditions (Groffman et al. 1992). After our initial results revealed low net N immobilization and low denitrification in the relict hydric layer, we collected another set of samples (from the stream bank with obvious relict hydric sediment stratigraphy) to increase our mechanistic understanding of controls on microbial activity in this layer.
When stream bank samples were exposed to a range of labile and recalcitrant C substrates, relict hydric soils responded weakly relative to the surface and midlayer soils of the stream bank. Thus, the low microbial activity in the long-buried relict hydric soils of the stream bank was not due to a lack of a specific C substrate. The low nitrifier population index for the subsurface horizons suggests that the NH4 + oxidizer community is largely absent, and that low net nitrification potentials at depth are not due to a short-term lack of NH4 + (Table 2). Koval (2012) also found low activity in the buried relict hydric soils of Big Spring Run—denitrification potentials were several orders of magnitude lower than in the surface, even though denitrifier populations (identified through nosZ T-RFLP analysis) were present. A dialysis transplant experiment, however, suggests that microbial communities currently existing in the relict hydric soil of Big Spring Run may show increased denitrification potentials if wetland hydrology is restored (Koval 2012)—these rates, though, would still be much lower than those typical of a fully functioning wetland.
Exoenzyme activity was also lower in the buried relict hydric layer than in soils higher in the profile. Each gram of C in the buried relict hydric layer sustains a much lower level of microbial enzyme activity than a gram of C in the surface (Fig. 5). The relative demand for different nutrients can be assessed with the stoichiometric activity ratios of enzymes (Sinsabaugh et al. 2009). The only shift in enzymatic ratios was found in BG:(PerO + PPO), which increased in the relict hydric soil. This suggests that compared to surface soils, microbes in the relict hydric layer allocated more of their C acquiring enzyme activity to labile C forms than recalcitrant forms. However, this finding was not consistent with some of our substrate additions; for example, relict hydric soils had a larger percentage of total respired C from tannins than surface soils, likely owing to the age of the buried strata and the likely respiration of more labile forms over time.
Taken together, the C substrate additions, nitrifier population assays, and exoenzyme analyses suggest that microbial activity is low in the buried relict hydric layer and that no single resource was isolated as the primary constraint. The microbial community in the relict hydric layer appears to be inefficient at utilizing new C inputs, and in using existing C to generate exoenzyme activity. This is in contrast to recent studies of organic-rich buried horizons, which have been found to contain microbially available C supporting ecologically significant element cycling rates (Hill and Cardaci 2004; Gurwick et al. 2008a, b). The buried organic deposits studied by Hill and Cardaci (2004) post-dated European settlement, while those included in the work by Gurwick et al. (2008a, b)—which more closely resembled the buried relict hydric soil of this study—were thousands of years old. In light of the similarities in C mineralization rates measured in both these studies, Gurwick et al. (2008a, b) posited that the availability of C in these buried horizons is due to the variation in the quality and quantity of organic matter at the time of horizon formation or burial, as opposed to the duration since burial. If Gurwick’s hypothesis is correct, then the hydric soil at BSR may have had low levels of microbially available C prior to burial by legacy sediments, perhaps due to poor litter quality.
One caveat to our work is that we did not mimic the oscillating redox conditions that the relict hydric layer likely experiences. The majority of Big Spring Run is in a former backwater environment that developed upstream of a millpond. Now that the stream has incised into the sediment, the water table is often close to the bedrock layer (Parola and Hansen 2011) and much of the time, the surface, midlayer, and bottom layers along the stream banks are exposed to the air. Our laboratory assays, conducted under oxic conditions, are most analogous to this low-water state. However, when the water table fluctuates in response to precipitation events the buried relict hydric soil may become saturated, producing sub-oxic or anoxic conditions in the sediments. Future work would benefit by mimicking field conditions that account for the varying redox conditions in the buried relict hydric soil (Mayer et al. 2010).
Conclusions and implications
This is the first study of N and C mineralization in legacy sediments and of the impact of nitrate processing within these newly recognized, near-stream sediments as a source of nitrate to streams. Our research identified clear patterns of N cycling in legacy sediments of Big Spring Run, with the three key discoveries being: (1) potential net nitrification and denitrification are greatest in the surface soils, (2) buried relict hydric soils exhibit low net NO3 − immobilization and denitrification potential, and (3) low potential NO3 − immobilization and denitrification result in part from low microbial activity in the buried layer. These results have important implications for water quality research and stream bank restoration where legacy sediments exist.
Legacy sediments are pervasive throughout the mid-Atlantic of the US, but their origin and widespread impacts were recognized only recently (Walter and Merritts 2008a). Our data suggest that surface soils on legacy sediment terraces possess relatively high potential net nitrification rates and can be a source of NO3 − that may be transported through buried relict hydric layers that are not effective NO3 − sinks either via net immobilization (Table 1) or via denitrification (Fig. 3). Of course, an increase in steam NO3 − due to the influence of legacy sediment must be considered a working hypothesis until future research explores how hydrological flowpaths impact NO3 − formation and transport at not only Big Spring Run, but also within the whole of the mid-Atlantic Piedmont region.
A second working hypothesis that can be drawn from our research is that millpond construction and abandonment has increased net nitrification rates by increasing the total area of aerobic surface soils near the stream. Historically, during the Holocene when valley bottom wetland ecosystems expanded, surface soils at Big Spring Run were anoxic more often and organic matter rich (Walter and Merritts 2008a). Riparian wetlands filter NO3 − via net immobilization and denitrification (Groffman et al. 1992; Simmons et al. 1992; Kellogg et al. 2005). The deposition of legacy sediment may have had an impact on stream water NO3 − concentrations by increasing the land area with high net nitrification (surface soils have higher rates of net nitrification than extant hydric soils, c.f., Groffman et al. 1992; Duncan and Groffman 1994; Clement et al. 2002) and by decreasing N immobilization and denitrification in the buried relict hydric layer (our values for the buried relict hydric layer are low compared to active wetlands; Hanson et al. 1994; Casey et al. 2001; Gold et al. 2001). Thus, in systems with historic millpond deposits, near stream net NO3 − production is likely higher and NO3 − immobilization and denitrification are likely lower than in the same locations prior to legacy sediment accumulation. In the future, this model can be tested by comparing nitrification and immobilization/denitrification processes in streams impacted by legacy sediment to actively functioning natural wetlands or to restored wetlands.
The identification of Best Management Practices (BMPs) to mitigate the impacts of legacy sediments on streams and wetlands is an important goal for resource managers in the Mid-Atlantic region (USEPA 2009). In September of 2011, legacy sediments were removed throughout a portion of the Big Spring Run watershed to evaluate a new BMP specifically targeted to streams in the east that were impacted by damming. This restoration effort, which involves hydrologically reconnecting the stream to its floodplain via legacy sediment removal, represents a unique opportunity to assess the effects of watershed restoration on ecological function. Our baseline, pre-restoration data suggest that buried relict hydric layers may initially be a weak sink for NO3 − in the riparian zone, but the removal of highly nitrifying surface sediment may eliminate a source of NO3 − to the system. It will be important to investigate changes in microbial community activity and N retention over time following restoration to determine whether unburied hydric soils eventually provide the same ecosystem services as wetlands unaffected by milldam sediment.
Abbreviations
- CLPP:
-
Community level physiological profile
- CBH:
-
Cellobiohydrolase
- BG:
-
β-Glucosidase
- NAG:
-
β-N-Acetyl glucosaminidase
- LA:
-
Leucine aminopeptidase
- AP:
-
Acid phosphatase
- PPO:
-
Polyphenol oxidase
- PerO:
-
Peroxidase
References
Allison SD, Vitousek PM (2004) Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36:285–296
Ambus P, Lowrance R (1991) Comparison of denitrification in two riparian soils. Soil Sci Soc Am J 55:994–997
Bain DJ, Smith SMC, Nagle GN (2008) Reservations about dam findings (comment and reply), Bain et al. and Walter/Merritts. Science 321:910–912
Banks WSL, Gellis AC, Noe G (2010) Sources of fine-grained suspended sediment in Mill Stream Branch Watershed, Corsica River Basin, a tributary to the Chesapeake Bay, Maryland, 2009. In: Proceedings, 2nd Joint Federal Interagency Conference, Las Vegas, NV, June 27 July 1, 2010 CD-ROM ISBN 978-0-0779007-3-2, 6B, pp 1–12
Belser LW (1979) Population ecology of nitrifying bacteria. Annu Rev Microbiol 33:309–333
Belser LW, Mays EL (1980) Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol 39:505–510
Binkley D, Hart SC (1989) The components of nitrogen availability assessments in forest soils. Adv Soil Sci 10:57–112
Böhlke JK, O’Connell ME, Prestegaard KL (2007) Ground water stratification and delivery of nitrate to an incised stream under varying flow conditions. J Environ Qual 36:664–680
Brush GS (2008) Historical land use, nitrogen, and coastal eutrophication: a paleoecological perspective. Estuaries Coasts. doi:10.1007/s12237-008-9106-z
Burt TP, Pinay G, Matheson FE, Haycock NE, Butturini A, Clement JC, Danielescu S, Dowrick DJ, Hefting MM, Hillbricht-Ilkowska A, Maitre V (2002) Water table fluctuations in the riparian zone: comparative results from a pan-European experiment. J Hydrol 265:129–148
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Casey RE, Taylor MD, Klaine SJ (2001) Mechanisms of nutrient attenuation in a subsurface flow riparian wetland. J Environ Qual 30:1732–1737
CBF (Chesapeake Bay Foundation) (2004) Manure’s impact on rivers, streams and the Chesapeake Bay: keeping manure out of the water. Report by the Chesapeake Bay Foundation 1–26
Chang H (2003) Basin hydrologic response to changes in climate and land use: the Conestoga River Basin, Pennsylvania. Phys Geogr 24:222–247
Chapman SJ, Campbell CD, Artz RRE (2007) Assessing CLPPs using MicroRespTM, a comparison with Biolog and multi-SIR. J Soils Sedim 7:406–410
Cirmo CP, McDonnell JJ (1997) Linking the hydrologic and biogeochemical controls of nitrogen transport in near-stream zones of temperate-forested catchments: a review. J Hydrol 199:88–120
Clement J, Pinay G, Marmonier P (2002) Seasonal dynamics of denitrification along topohydrosequences in three different riparian wetlands. J Environ Qual 31:1025–1037
Custer BH (1985) Soil Survey of Lancaster County, Pennsylvania. United States Department of Agriculture Soil Conservation Service in cooperation with Pennsylvania State University, College of Agriculture, and Pennsylvania Department of Environmental Resources, State Conservation Commission. USDA
Degens BP, Schipper LA, Sparling GP, Vojvodic-Vukovic M (2000) Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol Biochem 32:189–196
Degens BP, Schipper LA, Sparling GP, Duncan LC (2001) Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol Biochem 33:1143–1153
Doane TA, Horwath WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722
Doyle MW, Stanley EH, Harbor JM (2003) Channel adjustments following two dam removals in Wisconsin. Water Resour Res 39:1011–1026
Duncan CP, Groffman PM (1994) Comparing microbial parameters in natural and constructed wetlands. J Environ Qual 23:298–305
Forshay KJ, Dodson SI (2011) Macrophyte presence is an indicator of enhanced denitrification and nitrification in sediments of a temperate restored agricultural stream. Hydrobiologia 668:21–34
Forshay KJ, Stanley EH (2005) Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry 75:43–64
Gellis AC, Mukundan R (2013) Watershed sediment source identification: tools, approaches, and case studies. J Soils Sedim 13:1655–1657
Gellis AC, Noe GB (2013) Sediment source analysis in Linganore Creek watershed, Maryland, USA, using the sediment fingerprinting approach: 2008 to 2010. J Soils Sediments 13:1735–1753
Gellis AC, Hupp CR, Pavich MJ, Landwehr JM, Banks WSL, Hubbard BE, Langland MJ, Ritchie JC, Reuter JM (2009) Sources, transport, and storage of sediment at selected sites in the Chesapeake Bay watershed. Reston, VA: US Department of the Interior, US Geological Survey Scientific Investigations Report 2008–5186:1–95
Gold AJ, Groffman PM, Addy D, Kellogg DQ, Stolt M, Rosenblatt AD (2001) Landscape attributes as controls on ground water nitrate removal capacity of riparian zones. J Am Water Resour Assoc 37:1457–1464
Groffman PM, Gold AJ, Simmons RC (1992) Nitrate dynamics in riparian forests: microbial studies. J Environ Qual 21:666–671
Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X (1999) Denitrification. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 272–288
Groffman PM, Bain DJ, Band LE, Belt KT, Brush GS, Grove JM, Pouyat RV, Yesilonis IC, Zipperer WC (2003) Down by the riverside: urban riparian ecology. Front Ecol Environ 6:315–321
Groffman PM, Altabet MA, Bohlke JK, Butterback-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Gurwick NP, Groffman PM, Yavitt JB, Gold AJ, Blazejewski G, Stolot M (2008a) Microbially available carbon in buried riparian soils in a glaciated landscape. Soil Biol Biochem 40:85–96
Gurwick NP, McCorkle DM, Groffman PM, Gold AJ, Kellogg DQ, Seitz-Rundlett P (2008) Mineralization of ancient carbon in the subsurface of riparian forests. J Geophys Res 113. doi:10.1029/2007JG000482
Hall DW, Lietman PL, Koerkle EJ (1997) Evaluation of agricultural best-management practices in the Conestoga River Headwaters, Pennsylvania: effects of nutrient management on quality of surface runoff and groundwater at a small carbonate-rock site near Ephrata, Pennsylvania, 1984–1990. Lemoyne, PA: Pennsylvania Department of Environmental Protection, US Geological Survey Water-Resources Investigations Report 95-4143:1–59
Hanson GC, Groffman PM, Gold AJ (1994) Denitrification in riparian wetlands receiving high and low groundwater nitrate inputs. J Environ Qual 23:917–922
Harrison LR, Legleiter CJ, Wydzga MA, Dunne T (2011) Channel dynamics and habitat development in a meandering gravel bed river. Water Resour Res 47:W04513. doi:10.1029/2009WR008926
Hart SC, Stark JM (1997) Nitrogen limitation of the microbial biomass in old-growth forest soil. Ecoscience 4:91–98
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabi A, Wollum A (eds) Methods of soil analysis, part 2—microbiological and biochemical properties. SSSA Book Series, No 5, Madison, pp 985–1018
Hill AR, Cardaci M (2004) Denitrification and organic carbon availability in riparian wetland soils and subsurface sediments. Soil Sci Soc Am J 68:320–325
Hill AR, Vidon PGF, Langat J (2004) Denitrification potential in relation to lithology in five headwater riparian zones. J Environ Qual 33:911–919
Holden PA, Fierer N (2005) Microbial processes in the vadose zone. Vadose Zone J 4:1–21
Holmes RM, Jones JB, Fisher SG, Grimm NB (1996) Denitrification in a nitrogen-limited stream ecosystem. Biogeochemistry 33:125–146
Hynes HBN (1975) The stream and its valley. Verhandlungen des Internationalen Verein Limnologie 19:1–15
Jacobson RB, Coleman DJ (1986) Stratigraphy and recent evolution of Maryland Piedmont floodplains. Am J Sci 286:617–637
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the international large river symposium. Canadian Special Publication of Fisheries and Aquatic Sciences 106:110–127
Kasahara T, Hill AR (2006) Effects of riffle-step restoration on hyporheic zone chemistry in N-rich lowland streams. Can J Fish Aquat Sci 63:120–133
Kaushal S, Groffman P, Mayer P, Striz E, Gold A (2008) Effects of stream restoration on denitrification in an urbanizing watershed. Ecol Appl 18:789–804
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kellogg DQ, Gold AJ, Groffman PM, Addy K, Stolt MH, Blazejewski G (2005) In situ ground water denitrification in stratified, permeable soils underlying riparian wetlands. J Environ Qual 34:524–533
Koval J (2012) Assessing restoration potential in relict wetland soils: investigating the effect of wetland hydrology on soil microbial community composition and denitrification potential. M.S. thesis, University of Illinois, Urbana-Champaign
Lowrance R, Leonard R, Sheridan J (1985) Managing riparian ecosystems to control nonpoint pollution. J Soil Water Conserv 40:87–91
Massoudieh A, Gellis A, Banks WS, Wieczorek ME (2012) Suspended sediment source apportionment in Chesapeake Bay watershed using Bayesian chemical mass balance receptor modeling. Hydrol Process. doi:10.1002/hyp.9429
Mayer PM, Reynolds SK, McCutchen MD, Canfield TJ (2007) Meta-analysis of nitrogen removal in riparian buffers. J Environ Qual 36:1172–1180
Mayer PM, Groffman PM, Striz EA, Kaushal SS (2010) Nitrogen dynamics at the groundwater–surface water interface of a degraded urban stream. J Environ Qual 39:810–823
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
McDonnell J (1990) A rationale for old water discharge through macropores in a steep, humid catchment. Water Resour Res 26:2821–2832
Merritts DJ, Walter RC, deWet A (2005) Sediment and soil site investigation, Big Spring Run, West Lampeter Township. For LandStudies Inc, Lancaster County
Merritts D, Walter R, Rahnis M, Heister K, Fraley L, Miller A, Oberholtzer W (2006) Buried Holocene streams and legacy sediment: late Pleistocene to historical changes in stream form and process and implications for stream restoration, mid-Atlantic Piedmont region. Annual Meeting Geological Society of America Field Trip, pp 1–48
Merritts D, Walter R, Rahnis M, Hartranft J, Cox S, Gellis A, Potter N, Hilgartner W, Langland M, Manion L, Lippincott C, Siddiqui S, Rehman Z, Scheid C, Kratz L, Shilling A, Jenschke M, Datin K, Cranmer E, Reed A, Matuszewski D, Voli M, Ohlson E, Neugebauer A, Ahamed A, Neal C, Winter A, Becker S (2011) Anthropocene streams and base-level controls from historic dams in the unglaciated mid-Atlantic region, USA. Philos Trans R Soc A 369:976–1009
Merritts D, Walter R, Rahnis M, Cox S, Hartranft J, Scheid C, Potter N, Jenschke M, Reed A, Matuszewski D, Kratz L, Manion L, Shilling A, Datin K (2013) The rise and fall of mid-Atlantic streams: millpond sedimentation, milldam breaching, channel incision, and stream bank erosion. In: De Graff JV, Evans JE (eds) The challenges of dam removal and river restoration, 21st edn. Geological Society of America Reviews in Engineering Geology, Boulder, pp 183–203
Montgomery DR (2007) Soil erosion and agricultural sustainability. Proc Nat Acad Sci USA 104:13268–13272
Morgan LH (1867) The American beaver and his works. Burt Franklin, New York (reprinted 1970)
Mosley P (1979) Streamflow generation in a forested watershed, New Zealand. Water Resour Res 15:795–806
Mukundan R, Radcliffe DE, Ritchie JC, Risse LM, McKinley RA (2010) Sediment fingerprinting to determine the source of suspended sediment in a southern piedmont stream. J Environ Qual 39:1328–1337
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL et al (eds) Methods of soil analysis, part 3—chemical methods, 2nd edn. SSSA Book Series, Madison, pp 1001–1005
Parola AC, Hansen C (2011) Reestablishing groundwater and surface water connections in stream restoration. Sustain Mag 24:2–7
Pennsylvania Department of Environmental Protection (PA DEP) (2006) Legacy Sediment Definitions. Legacy Sediment Workgroup (Chaired by Hartranft J, Merritts D, Walter R). http://www.portal.state.pa.us/portal/server.pt/community/chesapeake_bay_program/10513/workgroup_proceedings/553510
Pennsylvania Department of Environmental Protection (PA DEP) (2011) Lower Susquehanna region geology and groundwater. White Paper. Pennsylvania Department of Environmental Protection, Harrisburg
Renwick WH, Smith SV, Bartley JD, Buddemeier RW (2005) The role of impoundments in the sediment budget of the conterminous United States. Geomorphology 71:99–111
Roach WJ, Heffernan JB, Grimm NB, Arrowsmith JR, Eisinger C, Rychener T (2008) Unintended consequences of urbanization for aquatic ecosystems: a case study from the Arizona desert. Bioscience 58:715–727
Salonen K (1979) The selection of temperature for high temperature combustion of carbon. Acta Hydrochim Hydrobiol 7:591–597
Santisteban JI, Mediavilla R, Lopez-Pamo E, Dabrio CJ, Zapata MBR, Garcia MJG, Castano S, Martinez-Alfaro PE (2004) Loss on ignition: a qualitative or quantitative method for organic matter and carbonate mineral content in sediments? J Paleolimnol 32:287–299
Schenk ER, Hupp CR (2009) Legacy effects of colonial millponds on floodplain sedimentation, bank erosion, and channel morphology, mid-Atlantic, USA. J Am Water Resour Assoc 45:597–606
Scientific Macaulay (2010) MicroResp™ technical manual. Macaulay Scientific Consulting Ltd, Scotland, pp 1–22
Seitzinger SP, Nielsen LP, Caffrey J, Christensen PB (1993) Denitrification measurements in aquatic sediments: a comparison of three methods. Biogeochemistry 23:147–167
Sharpley A, Jarvie HP, Buda A, May L, Spears B, Kleinman P (2013) Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J Environ Qual 42:1308–1326
Simmons RC, Gold AJ, Groffman PM (1992) Nitrate dynamics in riparian forests: groundwater studies. J Environ Qual 21:659–665
Sims GK, Ellsworth TR, Mulvaney RL (1995) Microscale determination of inorganic nitrogen in water and soil extracts. Commun Soil Sci Plant Anal 26:303–316
Sinsabaugh RL, Follstad Shah JJ (2011) Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102:31–43
Sinsabaugh RL, Antibus RK, Linkins AE, McClaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Smith SMC, Belmont P, Wilcock PR (2011) Closing the gap between watershed modeling, sediment budgeting, and stream restoration. In: Simon A, Bennett SJ, Castro JM (eds) Stream restoration in dynamic fluvial systems: scientific approaches, analyses, and tools. Geophysical Monograph Series, vol. 194, Washington, pp 293–317
Tiedje JM, Simkins S, Groffman PM (1989) Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 115:261–284
Toy TJ, Renard KG, Foster GR (2002) Soil erosion: processes, prediction, measurement, and control. Wiley, New York
Trimble SW (1997) Contribution of stream channel erosion to sediment yield from an urbanizing watershed. Science 278:1442–1444
Trimble SW, Crosson P (2000) US soil erosion rates—myth and reality. Science 289:248–250
USDA NRCS (US Department of Agriculture, Natural Resources Conservation Service) (2010) Field indicators of hydric soils in the United States, version 7.0. In: Vasilas LM, Hurt GW, Noble CV (eds) USDA, NRCS, in cooperation with the National Technical Committee for Hydric Soils
USEPA (US Environmental Protection Agency) (2009) Effects of watershed restoration on nitrogen in a stream impacted by legacy sediments: Big Spring Run stream restoration project as a case study. White Paper. US Environmental Protection Agency, National Risk Management Research Laboratory Ground Water and Ecosystems Restoration Research, Washington
USEPA (US Environmental Protection Agency) (2010) Guidance for federal land management in the Chesapeake Bay Watershed. EPA841-R-10-002. US Environmental Protection Agency, Nonpoint Source Pollution, Office of Wetlands, Oceans, and Watersheds, Washington
Vidon P, Allan C, Burns D, Duval TP, Gurwick N, Inamdar S, Lowrance R, Okay J, Scott D, Sebestyen S (2010) Hot spots and hot moments in riparian zones: potential for improved water quality management. J Am Water Resour Assoc 46:278–298
Voli M, Merritts D, Walter R, Ohlson E, Datin K, Rahnis M, Kratz L, Deng W, Hilgartner W, Hartranft J (2009) Preliminary reconstruction of a pre-European settlement valley bottom wetland, southeastern Pennsylvania. Water Resour Impact 11:11–13
Walter RC, Merritts DJ (2008a) Natural streams and the legacy of water-powered mills. Science 319:299–304
Walter RC, Merritts DJ (2008b) What to do about those dammed streams (Comment and Reply), Peter Wilcock and Walter/Merritts. Science 321:910–912
Walter R, Merritts D, Rahnis M (2007) Estimating volume, nutrient content, and rates of stream bank erosion of legacy sediment in the Piedmont and Valley and Ridge physiographic provinces of Southeastern and Central PA. A Report to the Pennsylvania Department of Environmental Protection, pp 1–38
Wilcock P (2008) What to do about those dammed streams (Comment and Reply), Peter Wilcock and Walter/Merritts. Science 321:910–912
Young CL (1981) Oxidies of nitrogen. Pergamom Press, Oxford
Acknowledgments
We thank Dorothy Merritts, Stacey Sosenko, and Michael Rahnis from Franklin and Marshall College, Jeff Hartranft of PA DEP, and Angela Kent from the University of Illinois, who provided assistance in the development of the experimental design and sample collection. We would also like to thank the EPA ORD drilling crew members Ken Jewell and Russell Neil. LiDAR data was provided to the Project by the National Center for Airborne Laser Mapping to Merritts and Walter. This material is based upon work supported by the National Science Foundation under Grant No. DEB 0816668. Notice: The US Environmental Protection Agency through its Office of Research and Development partially funded and collaborated in the research described herein. It has been subject to an administrative review but does not necessarily reflect the views of the Agency. No official endorsement should be inferred. Mention of trade names, products, or services does not convey, and should not be interpreted as conveying, official EPA approval, endorsement, or recommendation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weitzman, J.N., Forshay, K.J., Kaye, J.P. et al. Potential nitrogen and carbon processing in a landscape rich in milldam legacy sediments. Biogeochemistry 120, 337–357 (2014). https://doi.org/10.1007/s10533-014-0003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0003-1