Abstract
During 2 years (2009, 2010), CO2 fluxes were measured in an oligotrophic fen located at the ecotonal limit of forest tundra and lichen woodland, in northeastern Canada. Peatlands in this region have registered a rise in surface moisture since Little Ice Age, allowing an increase in their pool size and density. In the studied peatland, 83 % of the surface is covered either with pools (42 %), hollows (28 %) and lawns (13 %) all of which present a mean water table higher than 7 cm. We evaluated how these conditions might influence the CO2 balance in these poor fens located in the northeastern section of the La Grande river watershed. During the two years measurements, results indicate a net CO2 source with 404 (±287) and 272 (±250) g CO2 m−2 year−1 emitted to the atmosphere throughout the growing seasons and winters. Modeled wintertime net ecosystem exchange resulted in a net loss of 150 (±36) and 150 (±37) g CO2 m−2 year−1 in 2009 and 2010 respectively, while 248 (±248) and 124 (±195) g CO2 m−2 was emitted during 2009 and 2010 growing seasons. This high CO2 source can be attributed to three factors: (1) the length of the cold season which represented 210–214 days of net CO2 loss (2) a high pool/vegetated surface ratio since pools are a net source of CO2 and presented 38–40 % of the annual spatially weighted CO2 budget and (3) hydroclimatic conditions during the growing season as dryer and warmer conditions in 2009 reduced photosynthesis and increased respiration rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In peatlands, carbon dioxide (CO2) is absorbed by vegetation via photosynthesis and released through both autotrophic and heterotrophic respiration. Net ecosystem exchange (NEE) represents the difference between gross primary productivity (GPP) and ecosystem respiration (R). As net primary production generally exceeds decomposition in peatlands, these ecosystems are considered as carbon sinks. In a recent paper, Yu et al. (2010) estimated that northern peatlands stock represents 473–621 Gt C while MacDonald et al. (2006) showed that peat initiation and development contributed to reduce atmospheric CO2 by 7 ppmv between 11 and 8 ka which corresponded to a net cooling of −0.2 to 0.5 W m−2 (Frolking and Roulet 2007).
However, peatlands can also behave as a source of carbon. Silvola et al. (1996) have assessed that 90 % of the absorbed carbon in peatlands is released to the atmosphere over a year period. The small difference between absorbed and released CO2 combined with peatlands sensitivity to hydro-climatic changes can lead to switch the peatlands’ CO2 budget from sink to source (Joiner et al. 1999; Cox et al. 2000; Griffis et al. 2000; Roulet et al. 2007). Moreover, the surface microtopography (hummock, lawn, hollow and pool) can influence the CO2 balance of a peatland. Pelletier et al. (2011) showed NEE response to water table fluctuations where higher water table led to increased NEE in hummocks but reduced NEE in hollows. Open water bodies also contribute to the CO2 budget (Waddington and Roulet 1996). In pools, Respiration in pools is mostly the result of organic matter decomposition (Kling et al. 1991; Hamilton et al. 1994; McEnroe et al. 2009). Moreover, CO2 uptake by photosynthesis is limited and generally offset by CO2 release to the atmosphere (Hamilton et al. 1994; Waddington and Roulet 1996; Macrae et al. 2004).
While several studies have demonstrated the response of peatlands to warmer and dryer conditions (Bubier et al. 2003a; Strack et al. 2006), the present research focuses in a peatland of northeastern Canada, where an overall rise of the regional water tables has been registered since the past 700 years (Arlen-Pouliot 2009; Van Bellen et al. 2013). This long-term disequilibrium of the hydrologic conditions generated gradual decomposition of strings and coalescence of pools. We refer to this phenomenon by the neologism ‘aqualysis’ (Dissanska et al. 2009; Proulx-McInnis et al. 2012; Trudeau et al. 2013). The main objective of this study was to evaluate the carbon balance of a peatland located at the ecotonal limit of the forest tundra and lichen woodland and to understand how a high general water table and pool coverage may play a role in this budget during two years. To understand the role of the hydrologic disequilibrium over carbon balance and as recent Canadian regional climate model (CRCM) outputs suggest future temperature and precipitation increase in this region of Canada (de Elia and Côté 2010), we analysed the influence of environmental variables such as water table on maximum rate of photosynthesis (PSNmax) and respiration (R) at microform scale and generated a two-year overall carbon budget using measured and modelled CO2 and CH4 flux data.
Materials and methods
Study area and site
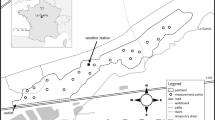
This study took place in the Northeastern region of the La Grande river watershed, James Bay, Québec (Fig. 1). Located at the ecotonal limit of forest tundra and lichen woodland, the region is sitting on Precambrian rocks, mostly granite and gneiss that were shaped by the last glaciation. Post glacial deposits such as till, esker, drumlins and moraines can be observed throughout the area (Dyke and Prest 1987). Characterised by acidic and poor soils, dominant vegetation of the region is represented by black spruce (Picea mariana (Mill) BSP) and lichens of the genus Cladina and Cladonia typical of the bioclimatic region of the lichen woodland (Payette et al. 2000). The area is under a subarctic climate with as mean annual temperature of −4.28 °C and mean annual precipitation of 738 mm between 1971 and 2003 (Hutchinson et al. 2009) (Fig. 3). These characteristics lead to low evapotranspiration which favours high water table in the region and a high peatland cover (15 %), most of which are represented by oligotrophic patterned fen (Tarnocai et al. 2002).
Study area and localisation of the peatland following Payette and Rochefort (2001) classification. The studied region (red circle) is located at the ecotonal limit between FO open boreal forest and TF forest toundra biomes
The study site, chosen in 2008 on the basis of air and ground regional survey and landscape characterisation, is a 3.5 ha oligotrophic patterned fen. Abeille peatland (54°06.9′N, 72°30.1′W) is formed by two distinct sections and one large pool downstream, close to the outlet (Fig. 2). It is characterised by elongated pools interspersed by strings perpendicular the general direction of the slope (Fig. 2). Pools cover 42 % of the total peatland area and their size vary between 4 and 99 m2 while peat depth ranges between 10 and 300 cm with an average of 108 cm (Proulx-Mc Innis 2010). The vegetation of the peatland is largely dominated by sedges (Carex exilis, C. Oligosperma and C. limosa) but seven terrestrial microforms characterised by different water table depth, peat temperature and vegetation assemblage were identified along with two types of pools (Tables 1, 2, 3). Evidences of aqualysis were observed in the Abeille peatland under the form of eroded hummocks, of a diminution of the area covered by trees such as Picea mariana and Larix laricina and by a degradation of strings thus allowing pool fusion by coalescence. Traces of former strings were also observed in the center of some pools.
CO2 flux measurements
CO2 terrestrial fluxes measurements
In June 2009, seven representative terrestrial microforms were identified in the peatland (Tables 1, 2) and two collars (25 cm diameter) were installed in each one of them. As some of them showed similar characteristics (water table depth, peat temperature, vegetation assemblage), we combined microforms into three subgroups at time of analysis; lawns (six collars grouping Cyperaceae strings, Cyperaceae lawns and Sphagnum lawns), hummocks (four collars grouping hummocks and the forest borders) and hollows (four collars grouping pool borders and hollows). Two pools respectively shallow (39.7 cm) and deep (78.5 cm) were also chosen for sampling (Table 3). Spatial microform coverage obtained with Geo Eye imagery classification (Dribault et al. 2012) show that the peatland is mainly represented by pools (42 %) and terrestrial microforms with water table close to peat surface such as hollows and lawns (41 %) while hummocks represent only a minor proportion of the peatland (17 %). To reduce the impacts of sampling, boardwalks were installed between the sites. NEE was measured during 6 field campaigns spread over two growing seasons. In 2009, three field campaigns were carried out in July, August and October while in 2010 three field campaigns were carried out in June, July and August respectively. Each campaign included 10 days of daily measurements.
During each campaign, CO2 fluxes were measured every day using a closed system composed of a climate-controlled static chamber connected to an infrared gas analyser (PPsystems EGM-4, Massachusetts, USA) placed on plastic collars. The 18L Plexiglas chamber transmitted about 92 % of the photosynthetically active radiation (PAR) and had a removable top in order to re-establish the ambient conditions between the measurements. It also had a climate-control system consisting in a fan and a pump immersed in cold water allowing water circulation in a radiator to maintain temperature inside the chamber close to ambient.
At the time of measurement, the chamber was placed on the collar with a water seal. We monitored the change in CO2 concentration in the chamber over a 2.5-min period, recording one data every 10 s for the first minute and one data every 30 s for the last 1.5 min. The CO2 flux was calculated from linear regression using the CO2 concentration change during the 2.5-min period. We also measured photosynthetically active radiation (PAR-1 sensor, PP Systems) during the fluxes sampling. Shrouds with different mesh size, partly blocking the incoming PAR, were used to create different light intensities. In each collar, 4 measurements were conducted, the first one at full light, then blocking 25 and 50 % of the light and using an opaque shroud to measure respiration.
CO2 pool fluxes measurements
Open water pools were sampled for CO2 and CH4 concentrations every two days during the field campaigns following Hamilton et al. (1994). Water samples were taken in glass bottles (Wheaton 125 mL) sealed with a rubber septum. The bottles were previously prepared in laboratory by (1) adding 8.9 g of KCl in order to inhibit water biological activity, (2) removing the air contained in the bottle with a vacuum pump and (3) adding 10 mL of UHP N2 to the bottle to create a headspace. In the field, as the air from the bottle was removed beforehand, samples were taken by submerging the bottle in water and by perforating the septum with a 18 gauge needle until filled. At sampling time, air and water temperature as well as wind speed were measured in order to be included in Eqs. 2 and 5.
At analysis time, bottles were shaken for 3 min to equilibrate water and N2. Using a 12 gauge needle, the headspace was sampled and analysed with a gas chromatograph (Shimadzu GC-14B) to determine CO2 partial pressure (Hamilton et al. 1994; Demarty et al. 2009). As a dissolved gas concentration in a solution is directly proportional to this gas partial pressure in the solution, Henry’s law was used to calculate CO2 concentration (Hamilton et al. 1994) using Eqs. 1 and 2:
where CO2wc is CO2 concentration in the water; CO2wp is CO2 partial pressure; KH(CO2) is CO2 solubility in water in mol L−1 atm−1 ; TK is water temperature in Kelvin
CO2 fluxes were calculated using the «thin boundary layer» as described by Demarty et al. (2009) (Eq. 3).
where:
where F is the gas flux at water/air interface; αCO2ac is the gas concentration in water exposed to the atmosphere calculated with Eq. 2 and global mean atmospheric partial pressure of 383 for carbon dioxide. k is the gas exchange coefficient.
To be applied to CO2 and ambient conditions, we adjusted k values with temperature, viscosity and CO2 diffusion coefficients with the Schmidt number (obtained by dividing water kinematic viscosity at temperature a’ by gas diffusion coefficient at temperature a’) following Cole and Caraco (1998) (Eq. 4):
where K600 is the exchange coefficient measured with sulphur hexafluoride (SF6), normalized to Schmidt number (Sc number) of 600, which is the Sc number for SF6 at 20 °C (Crusius and Wanninkhof 2003) and U10 represents wind speed at 10 m.
Finally, k was calculated with the following equations (Crusius and Wanninkhof 2003) (Eqs. 5 and 6): If wind speed <3 m/sec:
If wind speed ≥3 m/sec
where kCO2 is the gas coefficient exchange for CO2 and \( {\text{Sc}}_{{{\text{CO}}_{ 2} }} \) is Schmidt number for CO2
This method allowed us to measure CO2 fluxes from the pools to the atmosphere. Considering that there is low photosynthetic activity in the pools (Hamilton et al. 1994; Macrae et al. 2004), we did not measure CO2 invasion so the CO2 contribution may be overestimated.
CH4 measurements
CH4 fluxes were measured every two days over the same range of microforms than for CO2 sampling during 2009 and 2010 growing season (Trudeau et al. 2013). A 18L foil covered static chambers placed over collars sealed with water was used (Crill et al. 1988). CH4 samples (4) were taken every 6 min for a 24-min period. Air inside the chamber was mixed using a 60 mL syringe and a sample was taken and injected in a 10 mL pre-evacuated glass vial sealed with a rubber septum (butyl 20 mm septa, Supelco cie.) and a metal crimp (Ullah et al. 2009). Samples were kept at 4 °C until analysis with a gas chromatograph (Shimadzu GC-14B) equipped with a flame ionization detector for CH4. Fluxes were determined from a linear regression between the 4 measured CH4 concentrations during the 24-min sampling. CH4 fluxes from the pools were measured using the method described in the previous section, KH(CH4) being determined using the following equation (Eq. 7):
where CH4wc is CH4 concentration in the water; CH4wp is CH4 partial pressure; KH(CH4) is CH4 solubility in water in mol L−1 atm−1; TK is water temperature in Kelvin
Environmental variables
In each microform, water table depth and peat temperature were monitored continuously between June 13th 2009 and September 1st 2010. Water table depth was measured using a level logger (Odyssey Capacitance Water Level Logger) placed in PVC tube and inserted into peat or pool bottom. Peat and water temperatures were measured using HOBO probes (TMC6-HD Air/Water/Soil Temp Sensor) inserted at different depths (5–10–20–40 cm) on the terrestrial microforms. In the pools, water temperature was measured at the center and along the border both in surface and at the bottom. Aboveground biomass was measured by clipping vegetation inside collars at the end of 2010 growing season. Vascular plants stems and leaves were sorted and only capitula were kept for Sphagnum species. Vegetation was then dried in the oven at 80 °C and weighted to calculate biomass (Moore et al. 2002). Meteorological data were monitored from meteorological station located in a nearby peatland (<1 km) for 2009 and in Abeille peatland for 2010.
Data analysis
The relationship between NEE and PAR was described by a rectangular hyperbola using a curve-fitting technique (Eq. 8) (Fig. 8) that was computed with JMP-IN statistical software (SAS institute 2007).
where: α is the initial slope of the rectangular hyperbola (apparent quantum yield)
PAR is photosynthetically active radiation
GPmax is the maximum gross photosynthesis in light saturation condition
R is the intercept of the curve or dark respiration value
CO2 uptake by the ecosystem is represented by positive signs and CO2 release to the atmosphere by negative values.
The length of the growing season was defined using peat temperature at 5 cm to determine date of spring thaw and autumn freeze. We assumed that there was no photosynthetic activity when peat was frozen. The 2009 growing season lasted from May 12th to October 9th (151 days) and April 28th to September 30th (155 days) in 2010.
Rectangular hyperbola curve (Eq. 8) was fitted in the data of each sampling site for every field campaign (July, August and October 2009; June, July and August 2010) and the parameters estimated were applied to an hourly model of NEE. Gaps were filled between field campaigns using a mobile mean to estimate the rectangular hyperbola parameters. Table 4 shows seasonal estimates of the rectangular hyperbola parameters for 2009 and 2010. As the rectangular hyperbola tend to underestimate respiration values, R was modeled separately using an exponential regression derived from relationship between daily dark respiration measurements for 2 years and peat temperature at 5 cm (Table 5, Fig. 6).
Peat temperature was modeled between January 1st 2009 and June 11th 2009 and between September 1st 2010 and December 31st 2010 using an exponential regression with air temperature. We did not model pool fluxes as no strong relationship between water temperature and CO2 emissions was detected. Thus, only the growing season budget includes pool emissions for which pool seasonal average fluxes (shallow and deep) were used. For the yearly model we assumed that the pools stopped emitting CO2 when an ice cover formed on the surface. However, no data are associated with the release following the ice melt in spring and therefore the annual pool contribution may be underestimated.
In order to identify the controlling factors of NEE, simple regressions (significance level = 0.05) were used. As NEE represents the difference between gross primary productivity (GPP) and respiration (R), we analysed the relationship between GPP and R and the environmental variables (water table depth, peat and water temperature and biomass) separately. As GPmax suggests an infinite limit of PAR, we used photosynthesis values under real light saturation condition (PAR > 1,000 μmol m−2 s−1) (PSNmax) (Bubier et al. 2003a) for a more realistic analysis of photosynthesis. NEEmax, which represents the difference between gross primary productivity and respiration under light saturation condition (PAR > 1,000 μmol m−2 s−1) was also used for analysis. One way ANOVA tests (significance level = 0.05) were conducted to compare means between 2009 and 2010. Shapiro–Wilk test was used to determine normality of the distributions (significance level = 0.05) and Cook’s distance was used to identify outliers. A total of 3,011 terrestrial fluxes were measured from which 2,994 were kept with 1,587 in 2009 and 1,407 in 2010. In the aquatic sites, 89 fluxes were kept out of 98 measured with 42 in 2009 and 47 in 2010.
The uncertainties associated with the data presented in this manuscript take into account the error associated with the derived relationships between fluxes and environmental variables (PAR, water table depth, temperature, biomass). However, numbers of errors that are hardly quantifiable are associated with the methods. The largest sources of probable error would derive from the measurement techniques and materials, from the assessment of an annual budget using non-continuous measurements (gap-filling) and from the scaling up of chamber measurements to the landscape level.
Results
Environmental conditions
Mean daily temperature between June 1st and September 1st was warmer than the 30 years average with 13.2 and 13 °C for 2009 and 2010 respectively (Fig. 3). For the same period, total precipitation was inferior to the 30 years mean (299 mm) in 2009 (269 mm) and exceeded the 30 years mean in 2010 (477 mm). In the hummocks, water table depth varied between −16.5 and −25.6 cm in the 2009 growing season and between −15.5 and −27.5 cm in 2010. In the lawns, water table depth ranged from −3.7 to −10.9 cm in 2009 and between −3.1 and −13.3 cm in 2010. In the hollows, water table depth varied between 0.2 and −6.2 cm in 2009 and between 0.9 and −9 cm in 2010. Lower precipitations during 2009 growing season did not lead to a large water table drawdown (Fig. 4) although the duration of the low precipitation period influenced Sphagnum surface desiccation on microforms with deeper water tables such as lawns and hummocks. This is consistent with Strack and Price (2009) observations which did not establish a constant direct correlation between water table depth and Sphagnum moisture content.
Monthly average temperature (symbols, °C) and precipitation (bars, mm) in 2009, 2010 and 1971–2003 (Hutchinson et al. 2009)
Spatial variability
One-way ANOVA test performed on individual CO2 fluxes measurements revealed that photosynthesis at PAR > 1,000 μmol m−2 s−1 (PSNmax), net ecosystem exchange at PAR > 1,000 μmol m−2 s−1 (NEEmax) and dark respiration (R) were significantly different between the microforms and during both years (p > 0.001).
The rectangular hyperbola curve parameters varied according to the microtopography sequence: hollow < lawn < hummock (Table 4). GPmax varied between 4.5 and 9.01 g CO2 m−2 d−1 in the hollows, between 15.75 and 18.75 g CO2 m−2 d−1 in the lawns and ranged from 31.98 to 32.2 g CO2 m−2 d−1 in the hummocks. Dark respiration varied from −1.29 to −1.6 g CO2 m−2 d−1 in the hollows, −4.33 and −4.89 g CO2 m−2 d−1 in the lawns and −11.22 and −11.76 in the hummocks (Table 4). In the pools, CO2 emissions ranged from −0.01 to −10 g m−2 d−1 with a mean of −2.3 (±0.3). Shallow pool was a stronger emitter than deep pool with respective mean seasonal flux of −5.38 (0.6) and −1.18 (±0.2) g CO2 m−2 d−1 in 2009 and −3.28 (±0.6) and 0.78 (±0.1) g CO2 m−2 d−1 in 2010.
Interannual variability
One-way ANOVA tests were performed to determine NEEmax, PSNmax and R statistical difference between 2009 and 2010 growing seasons in the terrestrial microforms (Fig. 5). For these tests, we used individual CO2 measurements collected during July and August. In the hollows, NEEmax, PSNmax and R were significantly higher in 2009 than in 2010 (p < 0.001). In the lawns, NEEmax was statistically similar during 2009 and 2010 (p = 0.101) while PSNmax and R were significantly higher in 2009 (p < 0.001). In the hummock, NEEmax and PSNmax were significantly higher in 2010 (p < 0.001) but R was higher in 2009 (p = 0.054). Pool fluxes were significantly greater in 2009 in shallow pool (p = 0.023) but were similar in deep pool (p = 0.089) of both years.
Environmental controls
In order to identify the controls on NEE, relationship between CO2 fluxes (PSNmax and R) and environmental variables (air temperature, peat temperature, water table depth and biomass) were established at seasonal and daily scale. Relationships were also determined at both ecosystem and microform scale (Table 6). R measurements were strongly correlated with water table depth, fluxes increasing with deeper water table. At the seasonal scale, water table depth explained 88 % (p < 0.001) of the respiration fluxes, a 25 cm decrease of the water table yielding fluxes about one order of magnitude higher. Fluctuations of the water table also showed a statistically significant relationship with respiration fluxes at the daily scale (p < 0.001; r2 = 0.55). However, the strength of the relationship varied among microforms. Water table depth explained 30 % of R variation in hollows (p < 0.001) and 52 % in lawns (p < 0.001) while it correlated very weakly in hummocks (r2 = 0.06; p < 0.001). Similar results for this relationship were obtained when 2009 and 2010 growing seasons were analysed separately. In pools, CO2 fluxes were also correlated with water levels (r2 = 0.39; p < 0.001) with higher fluxes measured at lower water levels.
The relationship between PSNmax and water table depth was also analysed. At the ecosystem level, PSNmax was strongly correlated with water table depth at seasonal scale (r2 = 0.83; p < 0,001) and also correlated at the daily scale (r2 = 0.58; p < 0,001) with more CO2 uptake with deeper water table. Similarly to respiration, a 25 cm decrease in the water table lead to fluxes about one order of magnitude higher. Daily measurements of CO2 at the microform scale showed that water table explained 34 % of PSNmax variation in the hollows (p < 0.001), 18 % in lawns (p < 0.001) while the relationship was very weak in hummocks (r2 = 0.02; p = 0.042).
A correlation between R fluxes and air temperature was observed at the daily scale for both years (r2 = 0.37; p < 0.001 in 2009 and r2 = 0.19; p < 0.001 in 2010), warmer temperature leading to higher fluxes. Within the microforms, the strongest relationship between individual R fluxes and air temperature was measured in lawns (r2 = 0.65; p < 0.001), followed by hummocks (r2 = 0.54; p < 0.001) and hollows (r2 = 0.38; p < 0.001). A weak but significant relationship was also measured in pools (r2 = 0.10; p = 0.002). R fluxes also varied according to peat temperature at 5 cm (closely correlated to air temperature) (Fig. 6). An exponential curve was fitted between R fluxes at the daily scale and peat temperature and explained 10 % of R variation within the ecosystem (p < 0.001). When considered at the microform scale, peat temperature explains 35 % of the variation of R fluxes in the hollows (p < 0.001), 44 % in the lawns (p < 0.001) and 42 % in the hummocks (p < 0.001) (Table 5). In pools (shallow and deep), water temperature at the border bottom explained 11 % of the variations (p = 0.004). The relationship was stronger in deep pool (r2 = 0.35; p = 0,004) than in the shallow pool (r2 = 0.13; p = 0.014). For each microform, the relationship between R fluxes and temperature (air and peat) was stronger during the dryer and warmer 2009 growing season.
PSNmax was also correlated to air temperature at the daily scale, warmer temperature leading to increasing uptake. However, the relationship is weaker than the one for R. Within the microforms, air temperature explained 24 % of PSNmax variation in the hollows (p < 0.001), 15 % in the lawns (p < 0.001) and the relationship was not significant in the hummocks (p = 0.06). Peat temperature also correlated with PSNmax. However, the relationship was variable between the two growing seasons. In 2009, PSNmax decreased with warmer temperature while it increased with rising temperature in 2010. At the microform scale, hollows peat temperature explained 30 and 42 % of PSNmax variation during 2009 and 2010 respectively (p < 0.001). In the lawns and hummocks, the relationship was non-significant in 2009 (p = 0.286 and p = 0.68 respectively) but in 2010 explained 30 % of variation in the lawns (p < 0.001) and 32 % in the hummocks (p < 0.001). Q10 values associated with PSNmax were higher in 2010 in lawns and hummocks and were similar during both years in hollows which suggests that microforms with water table >7 cm had a photosynthesis process more responsive to temperature during the 2010 season. At last, the relationship between mean CO2 fluxes and living biomass at the end of the growing season was also analysed. Both mean seasonal respiration and PSNmax strongly correlated with green biomass (respectively r2 = 0.89; p = 0.001 and r2 = 0.87; p = 0.002).
Annual CO2 budget
Yearly budgets represented a net loss of CO2 to the atmosphere with greater emissions in 2009 [404 (±287) g CO2 m−2 year−1 compared to 272 (±250) g CO2 m−2 year−1 in 2010] (Table 7) (Fig. 7). Both the wintertime and growing season periods represented a net loss of CO2 towards the atmosphere. During 2009 growing season, the peatland has lost 248 (±248) g CO2 m−2 (151 days), while 124 (±195) g CO2 m−2 were lost in 2010 (155 days). Mean modeled NEE in winter was similar during both years with a net loss of −150 (±36) g m−2 year−1 in 2009 (214 days) and −150 (±37) g m−2 year−1 in 2010 (210 day) representing 37 and 55 % of the respective annual CO2 budget, suggesting that the interannual variation of the CO2 balance is caused by variation of photosynthesis or respiration during the growing season.
The CO2 balance also varied according to microforms (Table 7). Hollows remained CO2 sinks both in 2009 and 2010 although they absorbed less in 2010 with an average NEE of 6 (±175) g m−2 year−1 compared to 60 (±246) g m−2 year−1 in 2009. In this microform, GPP and R decreased in 2010. Lawns represented a CO2 source during both years with a NEE of −219 (±483) g CO2 m−2 year−1 in 2009 and −191 (±404) in 2010. Both GPP and R decreased in 2010. Hummocks were also a source of CO2 with NEE of −870 (±589) and −546 (±568) g m−2 year−1 in 2009 and 2010 respectively. However, during the 2010 growing season, hummocks were a small CO2 sink with 5 (±419) g CO2 m−2 absorbed between May and October. In contrast with other microforms, GPP in hummocks increased in 2010 while R decreased. Pool fluxes were larger in 2009 and contributed to 40 % of the annual spatially weighted CO2 budget compared to 38 % in 2010.
Annual carbon balance
In order to obtain an average annual carbon balance, we modelled the Abeille peatland C balance by combining spatially weighted annual daily CO2 fluxes measured and modelled with spatially weighted annual daily CH4 fluxes measured in Abeille peatland during the same period. CH4 fluxes and budget from 2009 and 2010 were described and analysed in a previous publication (Trudeau et al. 2013).
In 2009, the average spatially weighted carbon flux was −101.2 (±175) g C m−2 year−1 for which 92 % is accounted for CO2–C (93.3 (±171) g m−2 year−1) and 11 % for CH4–C (7.9 (±4) g m−2 year−1). In 2010, mean spatially weighted C flux was −71.3 (±136) g m−2 year−1 for which 90 % is accounted for CO2–C (64.5(±132) g m−2 year−1) and 10 % for CH4–C (6.8 (±4) g m−2 year−1) .
Discussion
We measured CO2 fluxes along a microtopographical gradient in a peatland influenced by rising water table since the Little Ice Age, slowly allowing an increased pool cover and development of wet microforms (Dissanska et al. 2009; Tardif et al. 2009). 83 % of the aqualysed Abeille peatland is covered either with pools (42 %), hollows (28 %) or lawns (13 %) all of which present a mean water table higher than 7 cm. We evaluated with two years of data how these hydrologic conditions influenced the CO2 balance at the microtopographic and ecosystem scale on this two years basis.
We observed an important variability in the fluxes at spatial and temporal scales. However, due to large measured uncertainties, it is crucial to mention that spatial and temporal variabilities discussed in this section can’t be affirmed with certitude. Annual and seasonal CO2 budgets differed between the two years, 2009 represented a large net loss to the atmosphere while 2010 was a smaller source (Fig. 7). In the terrestrial microforms, the 2009 growing season represented a net loss to the atmosphere while 2010 was a small sink (Table 7). Several studies have mentioned a switch from sink to source in the annual CO2 budget (Shurpali et al. 1995; Joiner et al. 1999; Griffis et al. 2000; Roulet et al. 2007) and attributed the change to the hydroclimatic variability during the growing season. In the Laforge region, the 2009 growing season was dry and warm when compared to the 30-years normal (1971–2003) while 2010 was also warmer but much wetter (Fig. 3). We also observed that fluxes responded to variations in the water table and temperatures as stated by Moore and Dalva (1993), Bubier et al. (1998), Waddington et al. (1998), Updegraff et al. (2001) Strack et al. (2009). The correlation between environmental variables and fluxes varied annually and according to microtopography.
An important portion of the spatial variability of the fluxes has been linked to microtopography. GPP and R varied along a surface moisture gradient (hollow < lawn < hummock) (Figs. 8, 5, 6), which is consistent with the observed correlations between living biomass, water table and CO2 fluxes. Nonetheless, inter-annual and intra-annual variability of NEE among the microforms indicates that they are controlled by different variables. During the 2009 and 2010 growing seasons, hollows were a CO2 sink (Table 7). Lawns were a source both in 2009 and 2010 while hummocks were a large source in 2009 and a small sink in 2010 (Table 7). These results infer that seasonal hydro-climatic variations do influence GPP and R and vary according to microtopography. Intrinsic characteristic of the microforms such as dominant vegetation, biomass, water table depth and peat temperature play an important role in determining the fluxes.
For all the microforms of the peatland, important correlations were observed between R fluxes and peat and air temperature with warmer temperature leading to higher R fluxes (Fig. 7). Similar results were reported by Bubier et al. (2003a, 2003b) and attributed to increased decomposition. Relationship between GPP and temperature is more complex. In lawns and hummocks, warmer peat temperature did not lead to increasing rates of photosynthesis during dry periods. In June and July 2009, warmer temperature increased R rate but did not affect photosynthesis which lead to a negative NEE (Table 7). Under wetter conditions, photosynthesis was more responsive to peat temperature than R, which lead to positive NEE in August 2009 and during the 2010 growing season (Table 7). In the hollows, photosynthesis was not reduced under dry conditions. In this microform, water table was close to peat surface during both 2009 and 2010 (3.6 cm in 2009 and 2.4 cm in 2010) and did not represent a constraint for the vegetation. Respiration and photosynthesis in the hollows showed greater response to peat and air temperature than to variation of the water table. This could explain why hollows were smaller sinks during the slightly colder 2010 while CO2 uptake was increased in the other microforms (Table 7). It is also possible that wetter conditions of 2010 limited the capacity of vegetation to photosynthesise as it was flooded during a part of the growing season. As for pools, fluxes were reduced in 2010 in response to colder temperature and higher water table (Table 7).
Several studies have previously concluded that the CO2 balance of peatlands is determined by temperature and surface-moisture conditions (Shurpali et al. 1995; Alm et al. 1999; Joiner et al. 1999; Griffis et al. 2000; Lafleur et al. 2001; Frolking et al. 2002; Bubier et al. 2003a; Aurela et al. 2009). In a subarctic fen near Churchill (Manitoba), Griffis et al. (2000) measured a net source of CO2 during a hot and dry growing season and sink during warm and wet growing season. According to that study, photosynthetic activity and fitness of the vegetation is influenced by the moisture conditions during early growing season and determine the magnitude of the source/sink. Lafleur et al. (2001) also measured smaller sinks during dry growing seasons in a subarctic fen and concluded that NEE is highly sensitive to dry conditions. In a northern Finland fen, Aurela et al. (2009) measured lower CO2 uptake during a dry and warm year which was attributed to reduced photosynthetic capacity of the vegetation.
Other authors have reported that the carbon balance response to changing climatic conditions also vary with microtopography (Alm et al. 1999; Heikkinen et al. 2002; Bubier et al. 2003a; Strack et al. 2006; Pelletier et al. 2011). Bubier et al. (2003a) suggested that the influence of the changing hydrologic conditions will vary with vegetation composition. In hummocks mainly dominated by ericaceous shrubs, Strack et al. (2006), Bubier et al. (2003a) and Alm et al. (1999) reported lower rates of gross photosynthesis and greater respiration under dry conditions. Heikkinen et al. (2002) measured greater NEE in microsites with water table closer to peat surface while Alm et al. (1999) found that during a dry year, hollows dominated by Sphagnum balticum had better water retention and increased its photosynthetic capacity. In the Eastmain region (mid-boreal Québec), Pelletier et al. (2011) observed that rising water table in hummocks led to greater NEE in response to increased productivity which is consistent with our results. Moreover, this study also mentions lower photosynthesis in hollows during a wetter year which was also observed our study. This was attributed to the vegetation flooding during the 2008 wet boreal summer.
Pools also played an important role in the carbon dioxide budget. Although pool fluxes were similar to respiration in the hollows (Fig. 5c), flux direction was uniquely towards the atmosphere which generated high emission rates, similar to Hamilton et al. (1994) in a peatland complex of the Hudson Bay Lowlands. During the 2009 growing season, pools emitted −990 (±122) g CO2 m−2 (non-area-weighted) to the atmosphere while the vegetated surface emitted −247 (±1120) g CO2 m−2. In 2010, the vegetated surface represented a net sink of 11 (±868) g CO2 m−2 during the growing season which was offset by pool emissions of −631 (±99) g CO2 m−2. Thus, being a constant large source of CO2 to the atmosphere that will offset vegetated surface sink, pools are a major contributor to the overall carbon budget, especially in the context where they represent a dominant microform of a system.
Modeled winter fluxes for Abeille peatland suggest their important contribution in the annual CO2 balance. Mean modeled fluxes for wintertime [−0.71 (±0.2) and −0.72 (±0.2) g m−2 d−1 in 2009 and 2010 respectively] (Table 7) were consistent with those of −0.69 g m−2 d−1 measured by Aurela et al. (2009) in a northern boreal fen. Mean daily winter fluxes were quite small but as the study region is characterised by long winter period (210–214 days), they represent an important part of the annual CO2 balance particularly in hummocks, where growing season fluxes could not offset winter fluxes.
Tarnocai et al. (2002) estimated that 15 % of northeastern Canada is covered by peatlands which are mostly represented by oligotrophic patterned fen. Pools are a predominant component of this type of peatland. Patterned fen are also observed in northern Europe (e.g. aapa mires) (Charman 2002) and peatlands of the Hudson Bay Lowlands also have large pools (Hamilton et al. 1994). Our study showed that pools contribution is non-negligible in the C balance of the peatlands. In the context where climate projections predict increased precipitations for the next decade over northeastern Canada (de Elia and Côté 2010) one can assume that these peatlands will generate important impacts in the C budget as they represent a net C source to the atmosphere.
Conclusion
Northeastern Canadian peatlands distributed at the ecotonal limit of the forest tundra and lichen woodland, showed a net carbon loss towards the atmosphere during two years of measurements. This carbon source can be explained by three main variables: meteorological conditions during the growing season, pool/vegetated surface ratio and length of the winter season.
Results show that environmental conditions during the growing season controlled C fluxes. During a dry year (2009), decreased photosynthetic activity was accompanied by increased respiration and peatland represented a net source of CO2 both during the growing season and annually. However, this phenomenon varied according to microtopography. Microforms with water table close to surface such as hollows were less strongly influenced by drought and showed increased photosynthetic activity in 2009 in response to warmer temperature. On the other hand, the wet conditions during 2010 growing season increased photosynthesis in the hummocks and the vegetated surface represented a net CO2 sink during the growing season.
Northeastern Canadian peatlands are mostly represented by oligotrophic patterned fens with a large pool coverage. Over the last 700 years, hydrologic changes leading to an increased water table have been registered in the region. This rise of the general water level in peatlands enhances peat decomposition on strings and degraded hummocks due to flooding and changes the pools/terrestrial surfaces ratio: 42 % of the studied peatland is covered by pools. Although mean pool CO2 fluxes are lower than those registered in the vegetated surface, they are unidirectional towards the atmosphere and hence play a greater role in the carbon budget of a peatland. In this study, we established that pool fluxes represent 38–40 % of the annual spatially extrapolated CO2 fluxes.
Undergoing a subarctic climate, Northeastern Canada is characterised by short growing seasons and long winters. During the cold season, photosynthesis is inhibited but respiration fluxes are still measured. Although mean winter fluxes are rather low, the cold season represents a long period of net C loss to the atmosphere in the region and play an important role in the annual carbon budget (37–55 % in this study).
With long winters, high pool coverage and vegetated surfaces sensitive to changing meteorological conditions during the growing season, we conclude that Northeastern Canadian peatlands influenced by a hydrologic disequilibrium are and will continue to be a source of carbon, the magnitude of which will mostly be controlled by environmental conditions during the growing season.
References
Alm J, Schulman L, Walden J, Nykänen H, Martikainen PJ, Silvola J (1999) Carbon balance of a boreal bog during a year with an exceptionally dry summer. Ecology 80:161–174
Arlen-Pouliot, Y., 2009. Développement holocène et dynamique récente des tourbières minérotrophes structurées du Haut-Boréal québécois. Thèse de doctorat, département de biologie. Université Laval, Québec
Aurela M, Lohila A, Tuovinen J-P, Hatakka J, Riutta T, Laurila T (2009) Carbon dioxide exchange on a northern boreal fen. Boreal Environ Res 14:699–710
Bubier JL, Crill PM, Moore TR, Savage K, Varner RK (1998) Seasonal patterns and controls on net ecosystem CO2 exchange in a boreal peatland complex. Global Biogeochem Cycles 12:703–714
Bubier JL, Bhatia G, Moore TR, Roulet NT, Lafleur PM (2003a) Spatial and temporal variability in growing-season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems 6:353–367
Bubier JL, Crill P, Mosedale A, Frolking S, Linder E (2003b) Peatland responses to varying interannual moisture conditions as measured by automatic CO2 chambers. Global Biogeochem Cycles 17:1066
Charman D (2002) Peatlands and environmental change. Wiley, England
Cole JJ, Caraco N (1998) Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol Oceanogr 43:647–656
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Crill PM, Bartlett KB, Harriss RC, Gorham E, Verry ES, Sebacher DI, Madzar L, Sanner W (1988) Methane flux from Minnesota peatlands. Global Biogeochem Cycles 2:371–384
Crusius J, Wanninkhof R (2003) Gas transfer velocities measured at low wind speed over a lake. Limnol Oceanogr 48:1010–1017
de Elia R, Côté H (2010) Climate and climate change sensitivity to model configuration in the Canadian RCM over North America. Meteorol Z 19:325–339
Demarty M, Bastien J, Tremblay A (2009) Carbon dioxide and methane annual emissions from two boreal reservoirs and nearby lakes in Quebec, Canada. Biogeosci Discuss 6:2939–2963
Dissanska M, Bernier M, Payette S (2009) Object-based classification of very high resolution panchromatic images for evaluating recent change in the structure of patterned peatland. Canadian Journal of Remote Sensing 25:189–215
Dribault Y, Chokmani K, Bernier M (2012) Monitoring seasonal hydrological dynamics of minerotrophic peatlands using multi-date GeoEye-1 very high resolution imagery and object-based classification. Remote Sensing 4:1887–1912
Dyke AS, Prest VK (1987) Late Wisconsinan and Holocene history of the Laurentide ice sheet. Géog Phys Quatern 41:237–263
Frolking S, Roulet N (2007) Holocene radiative forcing impact of northern peatland carbon accumulation and methane emission. Glob Change Biol 13:1079–1088
Frolking S, Roulet NT, Moore TR, Lafleur PM, Bubier JL, Crill PM (2002) Modeling seasonal to annual carbon balance of Mer Bleue Bog, Ontario, Canada. Global Biogeochemical Cycles 16:1030
Griffis, T.J., Rouse, W.R., Waddington, J.M., 2000. Interannual variability of net ecosystem CO2 exchange at a subarctic fen. Global Biogeochem Cycles 14
Hamilton JD, Kelly CA, Rudd JWM, Hesslein RH, Roulet NT (1994) Flux to the atmosphere of CH4 and CO2 from wetland ponds on the Hudson Bay lowlands (HBLs). J Geophys Res 99:1495–1510
Heikkinen JEP, Elsakov V, Martikainen PJ (2002) Carbon dioxide and methane dynamics and annual carbon balance in tundra wetland in NE Europe, Russia. Global Biogeochem Cycles 16:1115
Hutchinson MF, McKenney DW, Lawrence K, Pedlar JH, Hopkinson RF, Milewska E, Papadopol P (2009) Development and testing of Canada-wide interpolated spatial models of daily minimum–maximum temperature and precipitation for 1961–2003. J Appl Meteorol Climatol 48:725–741
Joiner DW, Lafleur PM, McCaughey JH, Bartlett PA (1999) Interannual variability in carbon dioxide exchanges at a boreal wetland in the BOREAS northern study area. J Geophys Res 104:27663–27672
Kling GW, Kipphut GW, Millet MC (1991) Arctic lakes and streams as gas conduits to the atmosphere: implications for tundra carbon budgets. Science 251:298–301
Lafleur PM, Griffis TJ, Rouse WR (2001) Interannual variability in net ecosystem CO2 exchange at the arctic treeline. Arct Antarct Alp Res 33:149–157
MacDonald GM, Beilman DW, Kremenetski KV, Sheng Y, Smith LC, Velichko AA (2006) Rapid early development of circumarctic peatlands and atmospheric CH4 and CO2 variations. Science 314:285–288
Macrae ML, Bello RL, Molot LA (2004) Long-term carbon storage and hydrological control of CO2 exchange in tundra ponds in the Hudson Bay Lowland. Hydrol Process 18:2051–2069
McEnroe NA, Roulet NT, Moore TR, Garneau M (2009) Do pool surface area and depth control CO2 and CH4 fluxes from an ombrotrophic raised bog, James Bay, Canada? J Geophys Res 114:G01001
Moore TR, Dalva M (1993) The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. Eur J Soil Sci 44:651–664
Moore TR, Bubier JL, Frolking SE, Lafleur PM, Roulet NT (2002) Plant biomass and production and CO2 exchange in an ombrotrophic bog. J Ecol 90:25–36
Payette S, Rochefort L (2001) Écologie des tourbières du Québec et du Labrador. Les Presses de l’Université Laval, Ste-Foy
Payette S, Bhiry N, Delwaide A, Simard M (2000) Origin of the lichen woodland at its southern range limit in eastern Canada : the catastrophic impact of insect defoliators and fire on the spruce-moss forest. Can J For Res 30:288–305
Pelletier L, Garneau M, Moore TR (2011) Interrannual variation in net ecosystem exchange at the microform scale in a boreal bog, Eastmain region, Quebec. Canada. Journal of Geophysical Research. doi:10.1029/2011JG001657
Proulx-Mc Innis, S., 2010. Caractérisation hydrologique, topographique et géomorphologique d’un bassin versant incluant une tourbière minérotrophe fortement aqualysée, Baie-de-James, Québec. Mémoire de maîtrise, Science de l’eau. INRS, Québec
Proulx-McInnis, S., St-Hilaire, A., Rousseau, A.N., Jutras, S., Carrer, G., Levrel, G., 2012. Seasonal and monthly hydrological budgets of a fen-dominated forested watershed, James Bay region, Quebec. Hydrol Process 27:1365–1378
Roulet NT, Lafleur PM, Richard PJH, Moore TR, Humphreys ER, Bubier J (2007) Contemporary carbon balance and late Holocene carbon accumulation in a northern peatland. Glob Change Biol 13:397–411
Shurpali NJ, Verma SB, Kim J, Arkebauer TJ (1995) Carbon dioxide exchange in a peatland ecosystem. J Geophys Res 100:14319–14326
Silvola J, Alm J, Ahlholm U, Nykanen H, Martikainen PJ (1996) CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J Ecol 84:219–228
Strack M, Price JS (2009) Moisture controls on carbon dioxide dynamics of peat-Sphagnum monolith. Ecohydrology 2:34–41
Strack M, Waddington JM, Rochefort L, Tuittila ES (2006) Response of vegetation and net ecosystem carbon dioxide exchange at different peatland microforms following water table drawdown. J Geophys Res 111:G02006
Strack M, Waddington JM, Lucchese MC, Cagampan JP (2009) Moisture controls on CO2 exchange in a Sphagnum-dominated peatland: results from an extreme drought field experiment. Ecohydrology 2:454–461
Tardif S, St-Hilaire A, Roy R, Bernier M, Payette S (2009) Statistical properties of hydrographs in minerotrophic fens and small lakes in mid-latitude Québec, Canada. Canadien Water Resour J 34:365–380
Tarnocai C, Kettles IM, Lacelle B (2002) Peatlands of Canada database. Natural Resources Canada, Ottawa
Trudeau N, Garneau M, Pelletier L (2013) Methane fluxes from a patterned fen of the northeastern part of the La Grande river watershed, James Bay, Canada. Biogeochemistry 113:409–422
Ullah S, Frasier R, Pelletier L, Moore TR (2009) Greenhouse gas fluxes from boreal forest soils during the snow-free period in Quebec, Canada. Can J For Res 3:666–680
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11:311–326
Van Bellen S, Garneau M, Ali AA., Lamarre A., Robert EC, Magnan G, Asnong H, Pratte S (2013) Poor fen succession over ombrotrophic peat related to late-Holocen increased surface wetness in subarctic Quebec, Canada. J Quat Sci 28:748–760
Waddington JM, Roulet NT (1996) Atmosphere-wetland carbon exchanges: scale dependency of CO2 and CH4 exchange on the developmental topography of a peatland. Global Biogeochem Cycles 10:233–245
Waddington JM, Griffis TJ, Rouse WR (1998) Northern canadian wetlands: net ecosystem CO2 exchange and climatic change. Climatic Change 40:267–275
Yu Z, Loisel J, Brosseau DP, Beilman DW, Hunt SJ (2010) Global peatland dynamics since the Last Glacial Maximum. Geophys Res Lett 37:L13402
Acknowledgments
The authors would like to thank NSERC, FQRNT, Hydro-Québec and Consortium Ouranos for financial support. We gratefully thank Hans Asnong, Éric Rosa and Jean-François Hélie for judicious advices and laboratory assistance. We also thank Yan Bilodeau, Antoine Thibault, Robin Beauséjour, Sébastien Lacoste and Sylvain Jutras for field and laboratory assistance. Special thanks to Gwenael Carrer, Yann Dribault and Sandra Proulx McInnis for sharing data and results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: James Sickman
Rights and permissions
About this article
Cite this article
Cliche Trudeau, N., Garneau, M. & Pelletier, L. Interannual variability in the CO2 balance of a boreal patterned fen, James Bay, Canada. Biogeochemistry 118, 371–387 (2014). https://doi.org/10.1007/s10533-013-9939-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9939-9