Abstract
Migratory animals can alter ecosystem function via the provision of nutrient subsidies. These subsidies are heterogeneous in space and time, which may create hot spots or hot moments in biogeochemical transformations, in turn altering the ecosystem effect of the subsidy by changing the form of the nutrients. Annual migrations of Pacific salmon (Oncorhynchus spp.) transport nutrients from the marine environment to their natal freshwater ecosystems. Salmon subsidies provide high quality nutrients (e.g., nitrogen, phosphorus, carbon) that may also be large in quantity where salmon migrations are near historic levels. We hypothesized that the nutrient subsidy provided via the excretion of ammonium (NH4 +) by live salmon would stimulate microbially mediated nitrification rates in stream sediments and increase streamwater nitrate (NO3 −) concentrations. We quantified sediment nitrification in seven streams in Southeast Alaska before and during the salmon run in 2007 and 2008. Nitrification rates increased 3-fold from before to during the salmon run (mean ± SE = 0.07 ± 0.01 to 0.24 ± 0.02 mgN gAFDM−1 d−1, respectively). The variation in nitrification was explained by both streamwater and exchangeable NH4 + concentrations (R 2 = 0.50 and 0.71, respectively), which were low before salmon and increased relative to the size of the salmon run. To experimentally test the effect of salmon subsidies on nitrification rates, we staked senesced salmon carcasses on stream sediments for 3 weeks during the salmon run and then measured nitrification rates directly under the carcasses. Sediment nitrification was 2–5 times higher under the carcasses compared to nearby sediments without the direct carcass influence. Our results confirm that biogeochemical transformations alter the form of salmon-derived nitrogen, representing an overlooked aspect in the dynamics of this subsidy. Therefore, animal-derived nutrient subsidies are not passively retained or exported in recipient ecosystems, but also transformed, thereby influencing the form and incorporation of these nutrient subsidies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The animal-mediated flux of nutrients across ecosystem boundaries can significantly influence recipient ecosystems (Polis et al. 1997; Marcarelli et al. 2011). For instance, organisms that move frequently between ecosystems provide nutrient subsidies that can support primary production and have dramatic consequences for community structure (e.g., Maron et al. 2006). While the effect of animal-derived subsidies on food webs has been well documented, the roles of microbes on and biogeochemical transformations of these nutrients have largely been overlooked (Polis et al. 2004). Subsidies can create “hot spots” or “hot moments” (after McClain et al. 2003) in nutrient transformations, where and when reaction rates are greater than ambient rates, which may alter the influence of the nutrient on the ecosystem (e.g., McIntyre et al. 2008). As a result, biogeochemical transformations may (1) enhance the effect of the subsidy by providing multiple nutrient forms to meet biological demands, or (2) dampen the influence of the subsidy if the demand for the transformed form of the nutrient is less than the initial form. The fate of nutrients from animal-derived subsidies in recipient ecosystems, whether incorporated into the food web or lost from the ecosystem, may ultimately depend on the transformations of those nutrients.
Pacific salmon (Oncorhynchus spp.) may create hot spots and hot moments in biogeochemical transformations during their annual spawning runs by transporting a discrete pulse of nutrients from the marine environment to their natal freshwaters (Schindler et al. 2003). Salmon migrations provide a useful model for investigating the effect of biogeochemical transformations on nutrient subsidies, as they deliver inorganic nitrogen (N) to streams as ammonium (NH4 +) via excretion, often increasing this form of dissolved N by orders of magnitude above background concentrations (see meta-analysis by Janetski et al. 2009). Furthermore, several previous studies have documented concurrent increases in NH4 + and NO3 − during salmon runs, suggesting that microbially mediated nitrification in the sediments transforms salmon-derived NH4 + to NO3 − (Johnston et al. 2004; Levi et al. 2011; Tiegs et al. 2011). While research has demonstrated that resident fish can create hot spots of biogeochemical transformations (McIntyre et al. 2008), no previous study has investigated the potential hot moment in nutrient transformations associated with anadromous fish migrations.
Nitrogen transformations and cycling have been studied extensively in stream ecosystems (Mulholland et al. 2000), demonstrating that the chemical form of a nutrient (e.g., NH4 + vs NO3 −) influences retention, processing, and export (Peterson et al. 2001; Webster et al. 2003). However, these studies were often conducted in streams with low nitrogen (N) concentrations and not in the context of an animal-derived nutrient subsidy. Nitrification is the oxic transformation of NH4 +–N to NO3 −–N by chemoautotrophic bacteria in stream sediments and is controlled primarily by NH4 + availability and oxic sediment conditions (Kemp and Dodds 2001; Strauss et al. 2002). Salmon-derived NH4 + converted to NO3 − may produce a less favorable form of inorganic N for biotic assimilation due to the high energy costs of reducing NO3 − (Dortch 1990; Hall and Tank 2003). Consequently, nitrification may result in a higher loss of salmon-derived NH4 + from the recipient stream habitat because NO3 − often travels a longer distance downstream before being incorporated into the food web (Mulholland et al. 2000; Tank et al. 2008). To date, the transformation of nutrient subsidies and subsequent export of alternative nutrient forms has been overlooked in the mass-balance studies quantifying nutrient subsidies.
To study the role of biogeochemical transformations on salmon subsidies, we quantified sediment nitrification rates in multiple streams before and during an annual salmon migration. We predicted that salmon-derived NH4 + during the run would increase sediment nitrification rates, thereby altering the form of the nutrient subsidy and, consequently, increase streamwater NO3 − concentrations. We also conducted experimental NH4 + and carcass amendments to sediments to measure the response of nitrifying bacteria to pulsed N sources. The results of our study further our understanding of the effect and fate of salmon-derived nutrients in stream ecosystems, and the microbial transformation of nutrient subsidies in general.
Methods
Site description and salmon density
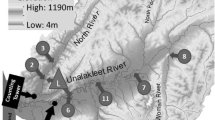
We studied seven streams on Prince of Wales Island in Southeast Alaska, which has a coastal temperate climate. As part of a larger project, we selected our study streams across a gradient in watershed timber harvest intensity, and within each stream we selected a representative 300-m study reach located 1–5 km upstream of the estuary (Table 1). The dominant salmon on Prince of Wales Island are pink (O. gorbuscha) and chum (O. keta) salmon, which migrate and spaw0n during a 6- to 8-week period from August to October and then die after spawning (Quinn 2005). We quantified salmon density by counting all individuals in a 4-m wide belt transect every 10 m in the reach (Tiegs et al. 2008). We scaled the counts to the 300-m reach and calculated density by the average width from measurements taken at every 10 m transect.
Sediment nitrification assay
In each stream, we collected sediment samples for nitrification assays at two time periods in 2007 and three time periods in 2008. In 2007 and 2008, we sampled all streams before the salmon run (before salmon) and near the end of the salmon run when <50 % of the peak number of live salmon remained and carcasses were present (during salmon + carcasses). Additionally, in 2008, we sampled near the peak of live salmon density when few to no carcasses were present (during salmon). During each time period in each stream, we collected sediments from both the thalweg and margin of the stream channel to examine spatial differences in nitrification rates. We collected 4 to 6 sediment cores (30 cm2 by 2 cm deep; ~250 mL total) and pooled them to create a composite sediment sample for each of the two stream habitats, replicating this along five transects in each stream reach, for a total of ten sampling locations in each stream. We sampled to 2 cm depth, and given the unconsolidated nature and low organic matter content of the sediments as well as the high dissolved oxygen concentrations in the overlying streamwater (PS Levi, unpublished data), we are confident that the sediments we sampled were generally oxic. We placed sediment samples in a cooler, returned them to the laboratory, and immediately began the nitrification assays.
We used the nitrapyrin-inhibition method to quantify sediment nitrification rates (Hall 1984; Kemp and Dodds 2001). In the laboratory, we placed 25 mL of sediment from each sample into each of two flasks, an “inhibited” and a “reference”, along with 50 mL of unfiltered stream water. In the inhibited flask, we added 20 μL of a 10 % solution of nitrapyrin dissolved in dimethyl sulfoxide (DMSO), which delivers the nitrapyrin across the cell membrane and blocks the conversion of NH4 + to NO3 −, thereby inhibiting nitrification. In the reference flask, we only added 20 μL DMSO, thereby allowing nitrification to occur. We incubated the flasks for 24 to 48 h on a rotary shaker at 175 rpm. After incubation, we sub-sampled 25 mL of the 75-mL sediment slurry, added 25 mL of 2 M KCl, and shook the samples for 1 h to flush NH4 + from cation exchange sites. We centrifuged each sample, filtered the supernatant into bottles, and froze the samples for subsequent NH4 + analysis (see below). To determine the nitrification rate, we calculated the difference in NH4 + between the inhibited and reference flasks and scaled these differences by assay duration and expressed the rates per unit sample area (mgN m−2 d−1) and per unit organic matter content (mgN gAFDM−1 d−1). Nitrification rates measured using the nitrapyrin-inhibition method are likely to be higher than in situ rates because redox conditions were optimized with slurries open to the atmosphere (e.g., well-oxygenated) and incubated at room temperature (Strauss et al. 2004). However, we did not amend the sediments with additional NH4 + to quantify maximum potential rates (e.g., Arango and Tank 2008). Therefore, the nitrification rates reported here represent an estimate between ambient rates and the maximum potential for nitrification.
To simulate the effect of an NH4 + subsidy on sediment nitrification, we conducted an amendment study on sediments in two streams (IND, TWE) prior to the salmon migration. We followed the same method as described above, and for each pooled sediment sample, designated “control” (CTL) flasks that received stream water only and “nitrogen-amended” (+N) flasks that received stream water amended with NH4Cl to 4 mg NH4 +–N L−1, a doubling of the highest exchangeable NH4 + concentrations observed in 2007 (Bruesewitz et al. 2006). Furthermore, to estimate the small-scale, localized effect that salmon carcasses may have on nitrification, we measured sediment nitrification directly under carcasses in two streams in 2008 (IND, TWE). We staked 8–10 naturally senesced pink salmon carcasses in the stream margin during our second sampling period (during salmon). When we returned for the third sampling period 19 to 22 days later (during salmon + carcasses), we sampled sediment directly underneath the carcasses (“treatment”) and from nearby, non-carcass-amended sediments (“control”). We conducted the nitrification assay on these samples as described above.
Stream sediment characterization
From each sediment sample collected for the nitrification assays, we also measured exchangeable (i.e., porewater) NH4 + and NO3 − and sediment organic matter content. We sub-sampled 30 g of sediment from each pooled sediment sample, added 30 mL of 2 M KCl, and shook these samples to flush all NH4 + and NO3 − from ion exchange sites. After 1 h, we centrifuged the samples, filtered the supernatant, and froze the samples for NH4 + and NO3 − analysis as described below. To determine sediment organic matter content, we sub-sampled 5 mL of sediment from each pooled sample, dried the samples at 60 °C for 24 h, weighed them for dry mass, ashed them at 550 °C for 4 h, and re-weighed the samples. We calculated percent organic matter (%OM) as one minus the ratio of ash-free dry mass (AFDM) to dry mass multiplied by 100. In addition, we also estimated median sediment size for each stream by measuring the medial diameter of 5 random particles collected along transects every 10 m over our 300-m study reach (n = 150) (Bunte and Abt 2001).
Water chemistry and stream discharge
From June to October in 2007 and 2008, we collected 3 replicate water samples for dissolved inorganic nutrient analyses during each sampling visit (range = 6 to 43 samples per stream per year). We filtered the water through a 0.7 μm glass fiber filter and froze the samples for laboratory analysis. We analyzed the samples for NH4 +–N using the sodium-hypochlorite method (APHA 2005) on a Shimadzu UV-1601 spectrophotometer (Shimadzu, Columbia, MD, USA). To determine NO3 −–N, we used the cadmium-reduction method on a Lachat QC-8500 Flow Injection Autoanalyzer (Lachat Instruments, Loveland, CO, USA; APHA 2005). To determine stream discharge, we measured stream water velocity at fixed transects using a flow meter on multiple visits to the study reaches (n = 5 to 15 per stream per year).
Statistical analyses
To test for differences in inorganic N concentrations in stream water, we used a 2-way randomized-block analysis of variance (2-way RB-ANOVA, α = 0.05, Zar 2009) with stream and time period (i.e., before versus during the salmon run) as the factors, and year as the block. We also used a 2-way RB-ANOVA to test for differences among streams and time periods in exchangeable NH4 + and NO3 − concentrations during the nitrification assays (i.e., before salmon, during salmon, during salmon + carcasses), using habitat (i.e., channel vs. margin) as the block. Similarly, we used a 2-way RB-ANOVA to test for differences in sediment nitrification rates with stream and time period as the factors and blocked by habitat. Since we measured exchangeable N and nitrification rates for different numbers of time periods during each year (2 in 2007; 3 in 2008), we ran these analyses separately for each year, and used a Bonferroni correction to determine statistical significance (α = 0.02, Zar 2009). To determine whether an N amendment altered nitrification in the two streams before salmon, we used a 2-way ANOVA with treatment and stream as the factors. We used a Friedman’s non-parametric test in lieu of ANOVA (Zar 2009) to determine whether nitrification rates in sediments beneath carcasses were different than control sediments due to an unbalanced number of samples between the two streams. We used simple linear regression (SLR, α = 0.05, Zar 2009) to determine if exchangeable and streamwater NH4 + explained significant variation in nitrification rates. Furthermore, we compared our data to previously published nitrification data from reference streams, which we defined as streams with NH4 + ≤ 20 μgN L−1 and in watersheds dominated by native vegetation, as well as rates from streams in agricultural and urban landscapes (Kemp and Dodds 2001; Bernhardt et al. 2002; Webster et al. 2003; Arango and Tank 2008). We ran SLRs with our data and these previous studies to determine if streamwater NH4 + predicted nitrification rates and, secondly, if these rates predicted streamwater NO3 − concentrations. We transformed the data with log or square-root transformations when statistical assumptions were violated. We performed all statistical analyses using SYSTAT 12 (Systat Corp., Chicago, IL, USA).
Results
Salmon nutrients increase stream water N and sediment nitrification
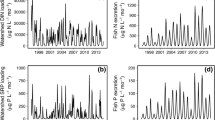
The concentration of both streamwater NH4 + and NO3 − increased during the salmon run (Fig. 1). Prior to salmon, inorganic N concentrations were low in all streams in both years (NH4 + < 15 μgN L−1; NO3 − < 50 μgN L−1, except for IND in 2007). In contrast, NH4 + and NO3 − were higher during the salmon run (2-way ANOVA, p < 0.001). The magnitude of change in N varied by stream (p < 0.001), with some streams changing little between time periods and others increasing orders of magnitude during the salmon run above background concentrations (median magnitude of increase for NH4 + = 8.9 times higher; NO3 − = 2.6). The time period-stream interaction was significant for NH4 + (p < 0.001), but not for NO3 − (p = 0.07). Similar to dissolved inorganic N, soluble reactive phosphorus (SRP) also significantly increased in all the study streams during the salmon run (see Levi et al. 2011).
Patterns in exchangeable NH4 + and NO3 − from our study reflected differences among streams, time periods, and habitats. In general, exchangeable N was higher in the time periods during the salmon run in 2007 and 2008 (2-way RB-ANOVA, p < 0.006; except exchangeable NO3 − in 2008, p > 0.05; Table 2). In both years, exchangeable NO3 − varied among the study streams (2-way RB-ANOVA, p < 0.001), while exchangeable NH4 + varied among streams in 2008 only (p < 0.001). Finally, exchangeable NH4 + was higher in the channel margins relative to the channel thalweg (2-way RB-ANOVA, p < 0.002; Table 2). In contrast, exchangeable NO3 − was higher in the channel thalweg relative to the channel margin in 2008 (p = 0.004), but the habitats did not differ in 2007 (p = 0.5).
Ambient sediment nitrification rates were low before salmon, but increased during the salmon run (Fig. 2). In both years, the differences in nitrification rate varied significantly by time period (2-way RB-ANOVA, p < 0.001) and mean (±SE) sediment nitrification rates tripled from before salmon to during salmon + carcasses (0.07 ± 0.01 to 0.24 ± 0.02 mgN gAFDM−1 d−1, respectively; Fig. 2). In 2008, the mean rate during salmon fell between the mean before salmon and during salmon + carcasses rates (0.14 ± 0.01 mgN gAFDM−1 d−1). In addition, differences in nitrification rate also varied significantly by stream (p < 0.001), but we could not identify pairwise differences between specific time periods or streams (time period-stream interaction p < 0.01). Finally, nitrification rates did not differ among the two stream habitats in 2007 or 2008 (2-way RB-ANOVA p = 0.4 and 0.5, respectively; Table 2), suggesting that sediment nitrification potential is comparable across the entire wetted width of the stream channel.
Stream sediment nitrification rates (mean + SE) during the three time periods, before salmon, during salmon, and during salmon + carcasses, in a 2007 and b 2008. In 2007, rates were not measured in the during salmon period. The open circles represent the peak salmon density in each stream, which are arranged along the x-axis by increasing density. Note that the stream ordering differs in 2007 and 2008 due to interannual variation in peak salmon density
The NH4 + amendment confirmed that stream sediments have the potential for greater nitrification rates when provided with additional NH4 +. Prior to the salmon migration, IND and TWE sediments amended with inorganic NH4 + had significantly higher nitrification rates than unamended sediments (2-way ANOVA, p < 0.001; Fig. 3a). The response to the N amendment also varied by stream (p < 0.001) and the treatment-stream interaction was significant (p < 0.001). The carcass addition experiment conducted during the salmon run confirmed the localized effect of salmon-derived NH4 +; sediment nitrification rates were up to 100-fold higher under salmon carcasses, compared to sediments without carcasses (Friedman’s test, p = 0.014; Fig. 3b). Interestingly, both amendments in TWE and the carcass amendment in IND increased nitrification similarly (up to ~1.5 mgN gAFDM−1 d−1; Fig. 3), which may represent the maximum potential for nitrification in these sediments given the high NH4 + conditions of the treatments.
Stream sediment nitrification rates (mean + SE) resulting from a an inorganic NH4 + amendment before salmon and b a natural carcass amendment during salmon + carcasses period in IND and TWE. Control bars represent rates of unamended sediments at the same time period (before salmon for (a) and during salmon + carcasses for (b))
Predictors of stream nitrification rates
Sediment nitrification rates were positively related to exchangeable and streamwater NH4 + (SLR, exchangeable NH4 + R 2 = 0.71, p = 0.001; streamwater NH4 + R 2 = 0.50, p < 0.001; Fig. 4a, b), which both increased during the salmon run. Furthermore, nitrification rates weakly predicted streamwater NO3 − concentrations (R 2 = 0.19, p = 0.008). Although nitrification rates were higher during the salmon run, the rates were not related to our measure of salmon density (SLR, p = 0.12).
We scaled our data to areal rates (mgN m−2 d−1) to compare nitrification in Southeast Alaska streams with previously published rates from reference, agricultural, and urban streams. Ambient NH4 + concentrations and sediment nitrification rates were similar between our streams before salmon and other reference streams (e.g., NH4 + ≤ 20 μgN L−1, nitrification rates <100 mgN m−2 d−1; Fig. 5a), although no previous study had been conducted in coastal temperate rainforest streams. In contrast, NH4 + concentrations (range = 4–151 μgN L−1) and nitrification rates (range = 26–300 mgN m−2 d−1) during the salmon run were up to 10-fold higher than previously reported data in reference streams. Areal nitrification rates were predicted by NH4 + concentrations in both our study and studies in other reference streams (SLR; R 2 = 0.64 and 0.21, respectively; p < 0.001 for both; Fig. 5a). Furthermore, nitrification rates predicted stream NO3 − concentrations in both the salmon-bearing streams and reference streams (R 2 = 0.40 and 0.35, respectively; p < 0.001 for both; Fig. 5b). When pooling all the data from our study and previously published rates from streams draining watersheds of varying land-use (e.g., reference, agriculture, urban), streamwater NH4 + predicted nitrification rates (SLR; R 2 = 0.35; p < 0.001; Fig. 5c) and, consequently, nitrification rates predicted stream NO3 − (R 2 = 0.34; p < 0.001; Fig. 5d).
Linear regressions of a, c streamwater NH4 + and sediment nitrification rates and b, d sediment nitrification rates and streamwater NO3 −. Closed circles represent data from this study and open symbols represent data from previous studies of sediment nitrification from reference, agricultural, and urban streams (circle, triangle, and square, respectively; Bernhardt et al. 2002; Webster et al. 2003; Arango and Tank 2008, LINX, unpublished data)
Discussion
Salmon-derived NH4 + subsidy increases sediment nitrification in streams
Our research provides multiple lines of evidence that salmon-derived NH4 + subsidies can create hot spots and hot moments of sediment nitrification (sensu McClain et al. 2003). First, nitrification during the salmon run was positively related to exchangeable and streamwater NH4 +, the substrate required for nitrification (Strauss et al. 2002). Second, the experimental amendment of inorganic NH4 + before salmon consistently increased sediment nitrification rates, evidence a hot moment occurs when NH4 + concentrations are elevated, such as during the salmon run. Third, nitrification was higher directly beneath carcasses relative to sediments without carcasses, which suggests that salmon carcasses created a hot spot on a localized scale. Together, these data demonstrate that sediments in salmon-bearing streams have the capacity to rapidly process additional sources of NH4 +, which may mediate the ecosystem effects of the salmon-derived nutrient subsidy.
While other studies have demonstrated the importance of resident fish on stream nutrient cycling (e.g., McIntyre et al. 2008), no previous study has investigated the role of migratory fish on stream nutrient transformations. Salmon-derived NH4 + increased sediment nitrification rates, which in turn altered the form of the subsidized nutrient from NH4 +–N to NO3 −–N. This biogeochemical transformation, leading to increased stream water NO3 −, has potentially important ecological consequences. Often, NO3 − is less preferred by aquatic autotrophs and heterotrophs than NH4 + (e.g., Raven et al. 1992), because the reduction of NO3 − to NH4 + requires additional energy (Dortch 1990). Therefore, as the by-product of nitrification, the temporarily elevated NO3 − concentrations may alter the community composition of autotrophs in the benthic biofilm (Raven et al. 1992). Furthermore, the transformation may dampen the local enrichment of stream food webs and extend the effects of the subsidy to downstream ecosystems (e.g., Cak et al. 2008), such as estuaries that may be N-limited (Howarth and Marino 2006). Food web responses to the biogeochemical transformations of nutrient subsidies warrants further study in order to better understand the ecological consequences of these transformations.
Our finding that salmon-derived NH4 + creates a hot moment in biogeochemical transformation rates is consistent with studies that have reported concurrent increases in NH4 + and NO3 − during salmon runs (Johnston et al. 2004; Levi et al. 2011; Tiegs et al. 2011). However, not all salmon-derived nutrient studies have reported increases in NO3 − (Janetski et al. 2009). For example, NO3 − dynamics in other Southeast Alaska streams were similar above and below salmon barriers before and during the run (Chaloner et al. 2004; Mitchell and Lamberti 2005), suggesting that patterns in NO3 − were linked to watershed processes. In our study, mean daily discharge did not differ significantly before and during the salmon run across streams, and NO3 − concentrations did not vary significantly within a stream before the salmon run (Levi et al. 2011); therefore, increases in NO3 − were likely a result of in-stream nitrification. The contrasting results between studies may be due to localized habitat conditions that could vary from stream to stream, and, as a result, could augment or inhibit nitrification (e.g., sediment organic matter content, pH; Strauss et al. 2002). In addition, variation in watershed and regional characteristics (e.g., riparian vegetation, climate; Compton et al. 2003) may also influence streamwater N concentrations and nitrification rates.
While salmon-derived NH4 + increased nitrification rates in temperate rainforest streams, salmon subsidies may not have consistent effects across all salmon-bearing streams. In addition to their role as a nutrient subsidy, salmon act as ecosystem engineers and disturb sediments and benthic biofilm during their migration and spawning (Moore et al. 2004), the magnitude of which depends on physical stream characteristics (e.g., Tiegs et al. 2008). Although sediment size varied among our streams, benthic disturbance by spawning salmon did not appear to negatively influence nitrifying bacteria. However, in sand-bottom or fine-sediment streams, benthic disturbance may disrupt the bacterial assemblage and alter the biogeochemical transformations associated with the salmon nutrient subsidy.
Nitrification of salmon NH4 + in context: dissimilatory transformation versus assimilatory uptake
Nitrification rates have been quantified in streams located in eight biomes of North America as part of the Lotic Intersite Nitrogen eXperiment (LINX; Webster et al. 2003), as well as in several other studies (Kemp and Dodds 2001; Bernhardt et al. 2002; Arango and Tank 2008). The areal nitrification rates we quantified before the salmon run (mean ± SE = 31.9 ± 7.6 mgN m−2 d−1) were similar to rates in other reference streams (19.6 ± 4.1 mgN m−2 d−1), despite differences in geomorphology, geology, and climate. In contrast, the rates we measured during the salmon run were, on average, 6 times higher (134.4 ± 31.9 mgN m−2 d−1) and more similar to rates measured in streams in agricultural and urban watersheds (Webster et al. 2003, LINX II, unpublished data; Fig. 5c, d). The higher rates we quantified during the salmon run extend both the range of streamwater NH4 + and nitrification rates previously measured in reference streams, defined as streams with low background NH4 + and in watersheds dominated by natural vegetation. Furthermore, NH4 + concentrations and nitrification rates in our Southeast Alaska study streams, where watershed development is low, were comparable to concentrations and rates in streams draining agricultural or urban watersheds, demonstrating that streams in relatively pristine landscapes have the capacity to transform and process elevated inorganic N loads.
Both assimilatory uptake and dissimilatory processes, such as nitrification, contribute to the removal of dissolved NH4 + from the water column. To place our nitrification rates in the context of total NH4 + demand, which we did not measure directly, we used NH4 + uptake velocities (Vf) from two previously published studies—one conducted in Alaska tundra streams (Webster et al. 2003) and the other in a coastal temperate stream in Oregon (Ashkenas et al. 2004). The Vf estimates from both studies were similar (mean ± SE = 13.3 ± 1.6 mm min−1), suggesting the NH4 + Vf in the coastal temperate streams of Southeast Alaska, located midway between those two sites, may be similar. Using this estimate of Vf from the literature, we calculated the NH4 + uptake per area in our streams (U; μgNH4 +–N m−2 min−1) using the relationship between Vf and U (U = Vf * [NH4 +–N]; Stream Solute Workshop 1990). Our calculations suggest that approximately 25 % of the NH4 + removed from the water column may be attributed to nitrification, both before and during the salmon run. In salmon-bearing streams, sediment nitrification is likely a significant component of NH4 + uptake, especially given the elevated concentrations of NH4 + during the salmon run. In the one study of N uptake in a forested stream in the Pacific Northwest, Ashkenas et al. (2004) reported that nitrification accounted for 40–50 % of NH4 + uptake, similar to the percentage we estimated for Southeast Alaskan streams. One caveat to our calculations is that we assumed that NH4 + demand is not saturated during the salmon run, such that Vf would remain similar through time. We cannot confirm constant NH4 + demand during elevated conditions because no studies have examined NH4 + Vf in relation to salmon subsidies. However, we have evidence from our carcass addition experiment that nitrification demand for NH4 + was not saturated as the nitrifying bacteria were able to further process even higher concentrations of NH4 + (range of exchangeable NH4 + = 229–23,900 μgN L−1).
Biogeochemical transformations alter nutrient subsidies
Nitrogen subsidies may undergo diverse, but largely overlooked transformations in their recipient ecosystems. In a study of riparian biogeochemical transformations and salmon nutrient subsidies, Holtgrieve et al. (2009) found that bears foraging on salmon increased NH4 + availability and fluxes of nitrous oxide (N2O) and carbon dioxide (CO2). Other studies have examined the influence of land use or anthropogenic nutrient inputs (e.g., agricultural run-off) on the N-cycle in streams. For example, Mulholland et al. (2008) reported a strong relationship with NO3 − concentration and denitrification in streams across multiple biomes and land uses. In our study, nitrifying bacteria responded to a pulsed subsidy of N, both from the natural subsidy during the salmon run and our artificial NH4 + amendment (i.e., hot moment) as well as the experimental under-carcass sampling (i.e., hot spot). While natural and anthropogenic nitrogen subsidies have been studied to some extent, less is known about the transformations of other nutrient subsidies, such as carbon and phosphorus.
In summary, biogeochemical transformations can fundamentally alter nutrient subsidies. These transformations may extend or dampen the subsidy’s ecosystem effects by diversifying the nutrient form. Such transformations may lead to biogeochemical hot spots or hot moments in the recipient ecosystems (McClain et al. 2003). In our study, we present evidence that an organism’s life history can stimulate biogeochemical transformations via the transfer of nutrient subsidies across ecosystem boundaries. A simple mass balance approach to subsidies does not sufficiently explain the dynamics of the subsidized nutrients in recipient ecosystems, because nutrients are not passively retained or exported in their original form. Rather, the nutrients provided by migratory and highly mobile animals also stimulate biogeochemical transformations, further altering the structure and function of the recipient ecosystem.
References
APHA (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Arango CP, Tank JL (2008) Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. J N Am Benthol Soc 27:90–107
Ashkenas LR, Johnson SL, Gregory SV, Tank JL, Wollheim WM (2004) A stable isotope tracer study of nitrogen uptake and transformation in an old-growth forest stream. Ecology 85:1725–1739
Bernhardt ES, Hall RO, Likens GE (2002) Whole-system estimates of nitrification and nitrate uptake in streams of the Hubbard Brook Experimental Forest. Ecosystems 5:419–430
Bruesewitz DA, Tank JL, Bernot MJ, Richardson WB, Strauss EA (2006) Seasonal effects of the zebra mussel (Dreissena polymorpha) on sediment denitrification rates in Pool 8 of the Upper Mississippi River. Can J Fish Aquat Sci 63:957–969
Bunte K, Abt SR (2001) Sampling frame for improving pebble count accuracy in coarse gravel-bed streams. J Am Water Resour As 37:1001–1014
Cak AD, Chaloner DT, Lamberti GA (2008) Effects of spawning salmon on dissolved nutrients and epilithon in coupled stream-estuary systems of southeastern Alaska. Aquat Sci 70:169–178
Chaloner DT, Lamberti GA, Merritt RW, Mitchell NL, Ostrom PH, Wipfli MS (2004) Variation in responses to spawning Pacific salmon among three south-eastern Alaska streams. Freshwater Biol 49:587–599
Compton JE, Church MR, Larned ST, Hogsett WE (2003) Nitrogen export from forested watersheds in the Oregon Coast Range: the role of N2-fixing red alder. Ecosystems 6:773–785
Dortch Q (1990) The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol-Prog Ser 61:183–201
Hall GH (1984) Measurement of nitrification rates in lake sediments: comparison of the nitrification inhibitors nitrapyrin and allylthiourea. Microb Ecol 10:25–36
Hall RO, Tank JL (2003) Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol Oceanogr 48:1120–1128
Holtgrieve GW, Schindler DE, Jewett PK (2009) Large predators and biogeochemical hotspots: brown bear (Ursus arctos) predation on salmon alters nitrogen cycling in riparian soils. Ecol Res 24:1125–1135
Howarth RW, Marino R (2006) Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnol Oceanogr 51:364–376
Janetski DJ, Chaloner DT, Tiegs SD, Lamberti GA (2009) Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159:583–595
Johnston NT, MacIsaac EA, Tschaplinski PJ, Hall KJ (2004) Effects of the abundance of spawning sockeye salmon (Oncorhynchus nerka) on nutrients and algal biomass in forested streams. Can J Fish Aquat Sci 61:384–403
Kemp MJ, Dodds WK (2001) Spatial and temporal patterns of nitrogen concentrations in pristine and agriculturally-influenced prairie streams. Biogeochemistry 53:125–141
Levi PS, Tank JL, Tiegs SD, Rüegg J, Chaloner DT, Lamberti GA (2011) Does timber harvest influence the dynamics of marine-derived nutrients in Southeast Alaska streams? Can J Fish Aquat Sci 68:1316–1329
Marcarelli AM, Baxter CV, Mineau MM, Hall RO (2011) Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–1225
Maron JL, Estes JA, Croll DA, Danner EM, Elmendorf SC, Buckelew SL (2006) An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecol Monogr 76:3–24
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, Thomas SA (2008) Fish distributions and nutrient cycling in streams: can fish create biogeochemical hotspots? Ecology 89:2335–2346
Mitchell NL, Lamberti GA (2005) Responses in dissolved nutrients and epilithon abundance to spawning salmon in Southeast Alaska streams. Limnol Oceanogr 50:217–227
Moore JW, Schindler DE, Scheuerell MD (2004) Disturbance of freshwater habitats by anadromous salmon in Alaska. Oecologia 139:298–308
Mulholland PJ, Tank JL, Sanzone DM, Wollheim WM, Peterson BJ, Webster JR, Meyer JL (2000) Nitrogen cycling in a forest stream determined by a N-15 tracer addition. Ecol Monogr 70:471–493
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202–255
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Marti E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory SV, Morrall DD (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Polis GA, Power ME, Huxel GR (eds) (2004) Food webs at the landscape level. University of Chicago Press, Chicago
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout. University of Washington Press, Seattle
Raven JA, Wollenweber B, Handley LL (1992) A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytol 121:19–32
Schindler DE, Scheuerell MD, Moore JW, Gende SM, Francis TB, Palen WJ (2003) Pacific salmon and the ecology of coastal ecosystems. Front Ecol Environ 1:31–37
Strauss EA, Mitchell NL, Lamberti GA (2002) Factors regulating nitrification in aquatic sediments: effects of organic carbon, nitrogen availability, and pH. Can J Fish Aquat Sci 59:554–563
Strauss EA, Richardson WB, Bartsch LA, Cavanaugh JC, Bruesewitz DA, Imker H, Heinz JA, Soballe DM (2004) Nitrification in the Upper Mississippi River: patterns, controls, and contribution to the NO3 − budget. J N Am Benthol Soc 23:1–14
Stream Solute Workshop (1990) Concepts and methods for assessing solute dynamics in stream ecosystems. J N Am Benthol Soc 9:95–119
SYSTAT 12 (2007) Systat Software, Inc, Chicago
Tank JL, Rosi-Marshall EJ, Baker MA, Hall RO (2008) Are rivers just big streams? A pulse method to quantify nitrogen demand in a large river. Ecology 89:2935–2945
Tiegs SD, Chaloner DT, Levi P, Rüegg J, Tank JL, Lamberti GA (2008) Timber harvest transforms ecological roles of salmon in southeast Alaska rain forest streams. Ecol Appl 18:4–11
Tiegs SD, Levi PS, Rüegg J, Chaloner DT, Tank JL, Lamberti GA (2011) Ecological effects of live salmon exceed those of carcasses during an annual spawning migration. Ecosystems 14:598–614
Webster JR, Mulholland PJ, Tank JL, Valett HM, Dodds WK, Peterson BJ, Bowden WB, Dahm CN, Findlay S, Gregory SV, Grimm NB, Hamilton SK, Johnson SL, Marti E, McDowell WH, Meyer JL, Morrall DD, Thomas SA, Wollheim WM (2003) Factors affecting ammonium uptake in streams—an inter-biome perspective. Freshwater Biol 48:1329–1352
Zar JH (2009) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River, NJ
Acknowledgments
Our research was possible because of the field and technical support provided by J. Berkowitz, N. Bonzey, M. Brueseke, E. Campbell, S. Hebbeler, J. Junker, A. M. Larquier, S. Meyer, J. Rüegg, M. Stephen, C. Turner, and S. Winikoff. We are grateful to B. Hall, D. Janetski, S. Roley, J. Rüegg, and M. Vanni for insightful comments on earlier versions of this manuscript. We also thank the Craig and Thorne Bay Ranger Districts for logistical support on Prince of Wales Island, and the support of the USDA Forest Service Pacific Northwest Research Station Aquatic-Land Interactions Program. Funding for this research was provided by USDA-CSREES National Research Initiative (Managed Ecosystems Program 2006-35101-16566) and NSF-IGERT training grant (DGE-0504495).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levi, P.S., Tank, J.L., Tiegs, S.D. et al. Biogeochemical transformation of a nutrient subsidy: salmon, streams, and nitrification. Biogeochemistry 113, 643–655 (2013). https://doi.org/10.1007/s10533-012-9794-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9794-0